Abstract
Methamphetamine (MA) users are assumed to have a high burden of tooth decay. Less clear is how the distribution and severity of dental caries in MA users differ from the general population. Using a covariate-balancing propensity score strategy, we investigated the differential effects of MA use on dental caries by comparing the patterns of decayed, missing, and filled teeth in a community sample of 571 MA users with a subset of 2,755 demographically similar control individuals selected from a National Health and Nutrition Examination Survey (NHANES) cohort. Recruited over a 2-y period with a stratified sampling protocol, the MA users underwent comprehensive dental examinations by 3 trained and calibrated dentists using NHANES protocols. Propensity scores were estimated with logistic regression based on background characteristics, and a subset of closely matched subjects was stratified into quintiles for comparisons. MA users were twice as likely to have untreated caries (odds ratio [OR] = 2.08; 95% confidence interval [95% CI]: 1.55 to 2.78) and 4 times more likely to have caries experience (OR = 4.06; 95% CI: 2.24 to 7.34) than the control group of NHANES participants. Additionally, MA users were twice as likely to have 2 more decayed, missing, or filled teeth (OR = 2.08; 95% CI: 1.29 to 2.79) than the NHANES participants. The differential involvement of the teeth surfaces in MA users was quite distinctive, with carious surface involvement being highest for the maxillary central incisors, followed by maxillary posterior premolars and molars. Users injecting MA had significantly higher rates of tooth decay compared with noninjectors (P = 0.04). Although MA users experienced decayed and missing dental surfaces more frequently than NHANES participants, NHANES participants had more restored surfaces, especially on molars. The high rates and distinctive patterns of dental caries observed could be used 1) to alert dentists to covert MA use in their patients and 2) as the basis for comprehensive management strategies.
Keywords: methamphetamine use, high rates, distinctive patterns, propensity score matching, NHANES controls, epidemiology
Introduction
Since its description in the early 21st century (Richards and Brofeldt 2000; Shaner 2002), the phenomenon of “meth mouth” has captured the public’s imagination and the attention of dental and substance abuse specialists. Anchored in clinical encounters with methamphetamine (MA) users, a substantial body of case reports, case series, and small cohort studies describe the rampant dental caries associated with MA use (Shaner 2002; Mallatt 2005; Saini et al. 2005; Shaner et al. 2006; Goodchild et al. 2007; Cretzmeyer et al. 2007; Padilla and Ritter 2008). Several reports have utilized the attention-grabbing imagery of extreme examples to support the “meth mouth” moniker: narratives that allow the reader to vicariously learn about the phenomenon but that perpetuate an exaggerated impression not reflective of the entire range of the condition (Diago 2003; Donaldson and Goodchild 2006; Linnemann and Wall 2013). The small sample sizes, lack of methodological rigor, and the inherent reporting biases all raise questions about the reliability, validity, and representativeness of the existing meth mouth literature. Because of the weak evidentiary basis, some (Shafer 2005; Murakawa 2011) have even questioned the depiction of the meth mouth phenomenon, labeling it more an exaggerated claim rather than a scientific fact.
The relative lack of scientifically rigorous investigations can be attributed, in large part, to the complexities of conducting clinical studies in substance-using populations. MA users are prone to chaotic lifestyles, and much effort is required to recruit an adequate sample size within a reasonable timescale and budget. Furthermore, longitudinal investigations of oral health in MA users require specialized personnel and settings as well as resource-intensive tracking strategies. In such populations, cross-sectional studies offer an appealing alternative: a strategy that we have utilized successfully to verify the high burden of dental disease in a broad spectrum of MA users (Shetty et al. 2010; Shetty et al. 2015). However, left unanswered was the following question: How do the patterns and rates of dental disease in MA users differ from the general population? Inferences from cross-sectional studies are notoriously prone to confounding and selection bias arising from fundamental differences in baseline characteristics between MA users and nonusers. Advances in statistical methodology, such as propensity scoring, now offer pathways to address the covariate imbalance between the groups and minimize the effects of confounding (Rosenbaum and Rubin 1983, 1984).
The primary goal of this study was to use a covariate-balancing propensity score strategy (Harrell et al. 2016) to examine the differential effects of MA use on dental caries. Specifically, we compared the rates and patterns of dental caries in a large group of MA users with a comparable group of presumptive nonusers chosen from the National Health and Nutrition Examination Survey (NHANES). This large health survey examines a nationally representative sample of the U.S. population at regular 2-y intervals without being selected on any preexisting condition or risk indicator. Using data from NHANES participants as a comparison cohort, we addressed the following questions: 1) Do MA users have higher rates of dental disease (decayed, missing, and filled teeth) compared with nonusers? 2) Are different teeth and tooth surfaces affected differentially in MA users? We hypothesized that MA users would manifest greater rates and different patterns of dental disease when compared with demographically similar individuals from a nationally representative sample of the U.S. population.
Materials and Methods
Details of the study design and settings have been described (Shetty et al. 2015). Briefly, a broad community sample of 571 MA users from Los Angeles County was recruited over a 2-y period through a stratified sampling protocol that balanced the subjects across substance use patterns (mild, moderate, or heavy use). To be eligible, subjects had to be ≥18 y, speak English or Spanish, have used MA in the past 30 d, and be able to undergo a detailed dental examination and psychosocial assessments. Written informed consent was obtained through procedures approved by the University of California, Los Angeles, Institutional Review Board. An initial target sample size of 500 subjects was based on the need to offer substantial power for distinguishing MA users from nonusers while providing sufficient power to relate MA use behaviors to the patterns of dental disease.
Data Collection
Standardized intraoral examinations were conducted by 3 experienced dentists who were trained, calibrated, and evaluated by the national trainer and reference examiner (B.A.D.) for the NHANES studies (Dye et al. 2015). To maximize comparability with national data sets, assessments for dental caries and periodontal status adhered to NHANES examination protocols (Dye et al. 2008; Dye et al. 2011). The presence and absence of teeth were recorded via NHANES guidelines as well. Missing permanent teeth were identified as missing because of either dental disease (caries or periodontal disease) or other reasons (e.g., trauma). Dental caries were assessed at the surface level through Radike criteria (Radike 1972) with evidence of dental caries experience visually assessed with a dental explorer for each surface of each tooth.
Participants also completed a comprehensive set of interviewer-facilitated questionnaires covering various psychological and dietary attributes, as well as drug use behaviors and medications, linked to the development of dental disease. Extensive information was collected on MA use over the past 30 d, such as quantity, frequency, mode, and duration of use. The veracity of the drug use self-reports was verified by random urine drug tests carried out in a subset of the participants. Data were also collected on key sociodemographic and behavioral covariates, including age, sex, race/ethnicity, education, smoking history, toothbrushing frequency, and consumption of sugary sodas. All data were collected directly on a laptop computer with a web-based data-management system. Built-in logic and data range checks allowed real-time data verification to protect against invalid data and to ensure data completeness.
Key Variables
The main outcome variables were tooth retention and the prevalence of dental caries. The number of permanent teeth present (excluding the third molars) was analyzed as a continuous variable (1 to 28) along with a dichotomous summary indicating ≥10 versus <10 permanent teeth present. Dental caries experience (DMFT) was calculated as the number of decayed (D), missing (M), and filled (F) teeth (T). As customary in the calculation of the DMFT, the number of missing teeth represents teeth lost due to dental disease alone. The extent of untreated dental caries was determined by the number of decayed surfaces. A subcategory of the decayed component was also calculated to indicate the severity of the decay (i.e., whether only coronal fragments or residual root tips remained). The main exposure variable was MA use. On the basis of the self-reported drug history and patterns of MA use over the past 30 d, participants were classified as either “light” MA users (<10 d of use over the past month) or “moderate/heavy” MA users (≥10 d of use over the past month).
Statistical Methods
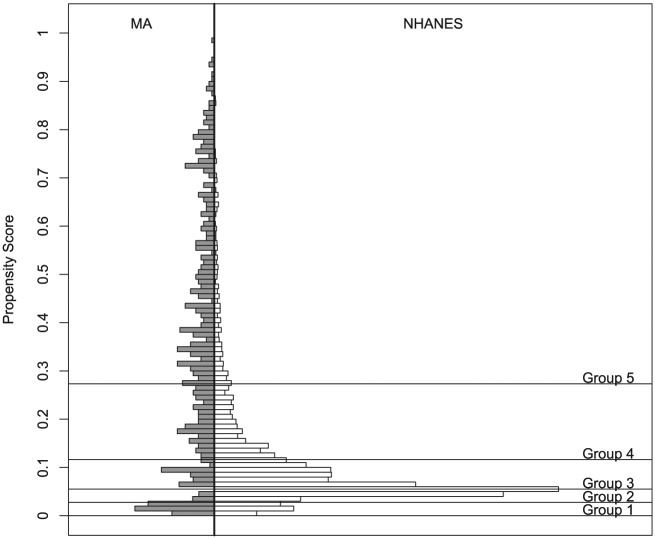
Statistical analyses were carried out with SAS 9 (SAS Institute Inc.) and publicly available R software. Descriptive summaries were used to summarize the sociodemographics of the MA users. To mimic the particular characteristics of a randomized controlled trial and create a control group of comparable non-MA users, we utilized the propensity score framework described by Rosenbaum and Rubin (1983, 1984) and implemented by Ho et al. (2011). Specifically, using a propensity method described previously (Harrell et al. 2016), we compared the cross-sectional sample of 552 dentate MA users with a subset of 2,755 sociodemographically similar control individuals selected from the NHANES 1999-2004 cohort. A combination of modeling and odds ratios (ORs) was used to stratify the sample into 5 propensity score subgroups within which the distributions of all observed background characteristics were balanced. Within groups, continuously scaled outcomes were compared with independent-sample t tests, and categorical outcomes were investigated with chi-square tests of independence and Fisher exact tests. Figure 1 shows the overlap in the propensity scores of the 2 groups, and Table 1 summarizes the distribution of demographic characteristics across each propensity score subgroup. Regression analysis predicting each characteristic with indicators for propensity score subgroup and MA use (vs. selection from the NHANES sample) was conducted to check for balance of the distributions of characteristics. After controlling for propensity score subgroup, there was no significant difference between MA users and NHANES subjects for any of the modeled characteristics.
Figure 1.
Mirrored histogram of propensity score distribution for methamphetamine (MA) and National Health and Nutrition Examination Survey (NHANES) subjects.
Table 1.
Proportion of Cases (MA Users)a and Controls (NHANES Participants)b by Sociodemographic Factors Distributed among 5 Propensity Score Subgroups.
| Subgroup 1 | Subgroup 2 | Subgroup 3 | Subgroup 4 | Subgroup 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Propensity score range | 0.001 to 0.027 | 0.027 to 0.054 | 0.054 to 0.114 | 0.114 to 0.268 | 0.268 to 0.971 | |||||
| MA | NHANES | MA | NHANES | MA | NHANES | MA | NHANES | MA | NHANES | |
| Sample, n | 31 | 630 | 29 | 632 | 34 | 628 | 129 | 532 | 328 | 333 |
| Male | 54.8 | 46.0 | 37.9 | 61.6 | 52.9 | 61.0 | 76.0 | 70.1 | 92.1 | 89.2 |
| Female | 45.2 | 54.0 | 62.1 | 38.5 | 47.1 | 39.0 | 24.0 | 29.9 | 7.9 | 10.8 |
| Born in the U.S. | 74.2 | 74.2 | 86.2 | 86.6 | 91.2 | 85.0 | 76.7 | 79.5 | 87.2 | 83.8 |
| Born in Mexico | 5.1 | 11.9 | 3.5 | 5.5 | 2.9 | 9.1 | 15.5 | 11.5 | 6.4 | 8.4 |
| Born outside of U.S. or Mexico | 5.1 | 14.0 | 5.7 | 7.9 | 5.9 | 5.9 | 7.8 | 9.0 | 6.4 | 7.8 |
| White | 38.7 | 36.5 | 24.1 | 32.1 | 55.9 | 41.4 | 26.4 | 25.8 | 9.6 | 13.8 |
| African American | 22.6 | 17.0 | 24.1 | 30.4 | 29.4 | 30.6 | 27.9 | 33.3 | 53.1 | 42.6 |
| Hispanic | 32.3 | 42.2 | 48.3 | 33.1 | 11.8 | 23.3 | 42.6 | 36.3 | 28.4 | 38.7 |
| Other race | 6.5 | 4.3 | 3.5 | 4.4 | 2.9 | 4.8 | 3.1 | 4.7 | 9.2 | 4.8 |
| High school graduate | 77.4 | 64.0 | 72.4 | 66.6 | 64.7 | 71.5 | 54.3 | 61.7 | 77.4 | 66.4 |
| Bachelor’s degree | 19.4 | 6.8 | 3.5 | 15.0 | 8.8 | 11.3 | 12.4 | 8.8 | 4.0 | 6.6 |
| Married / cohabiting | 71.0 | 54.4 | 27.6 | 34.0 | 23.5 | 15.8 | 0.8 | 1.7 | 0 | 0 |
| Single / divorced / separated | 29.0 | 45.6 | 72.4 | 66.0 | 76.5 | 84.2 | 99.2 | 98.3 | 100 | 100 |
| Former smoker | 22.6 | 12.7 | 17.2 | 16.9 | 11.8 | 12.6 | 11.6 | 12.6 | 6.7 | 9.6 |
| Current smoker | 24.8 | 31.6 | 37.9 | 34.5 | 61.8 | 53.7 | 49.6 | 57.7 | 83.2 | 71.2 |
| Mean age (SE), y | 42.4 (1.74) | 42.1 (0.65) | 38.7 (1.86) | 36.6 (0.58) | 39.9 (1.7) | 36.8 (0.53) | 41.0 (0.86) | 38.3 (0.58) | 46.5 (0.47) | 47.3 (0.68) |
Values presented in percentages, except where noted.
Data from the study of oral health of methamphetamine (MA) users.
Data from the matched subsample from the National Health and Nutrition Examination Survey (NHANES), 1999 to 2004.
Within-subgroup propensity score comparisons were performed between NHANES and MA subjects. Separate t tests and ORs based on logistic regression modeling were computed for continuously scaled and dichotomous variables, respectively, within each propensity score subgroup. Additionally, an overall mean difference was calculated between NHANES and MA groups by summing the weighted subgroup differences by the proportion of MA users in that subgroup. Overall ORs were calculated for binary outcomes by combining weighted estimates of the log ORs for each subgroup and then exponentiating them.
Results
Of the 571 study participants, 19 were completely edentulous, thus leaving 552 dentate subjects. Participants were predominantly male, African American and Hispanic (42.2% and 31.2%, respectively), and >30 y old (mean ± SD: age, 44.4 ± 9.5 y), and most had completed high school (years of education, 12.5 ± 1.6). Many of the MA users were current smokers (68.9%). According to the patterns of MA use over the past month, over half the participants could be classified as moderate/heavy MA users. On average, participants reported MA use on 4.5 ± 8.6 d of the preceding 30 d, and the preferred route of MA administration was by smoking (64.2%, n = 190). Most subjects (75%) self-rated the conditions of their teeth and gums as fair or poor; nearly 40% indicated that they were often self-conscious or embarrassed because of the condition of their teeth or dentures.
Table 1 summarizes the counts and sociodemographic characteristics of the MA-using subjects and their NHANES controls. Within the propensity score subgroups, MA users represented a higher proportion of the subgroups with higher propensity scores and only a small fraction of the subgroups with lower propensity scores. High propensity scores indicate an elevated likelihood of a study participant being in the MA-using sample given his or her background characteristics. Study participants with higher propensity scores were more likely to be male, unmarried, current smokers, and African American on the basis of the estimated propensity score model; however, there were no significant imbalances on background characteristics after initial matching and subsequent subclassification into 5 propensity score subgroups. Results from regression analyses are presented in Table 2. In logistic models adjusting for sociodemographic and behavioral risk indicators, MA users were found to be roughly twice as likely to have untreated caries (OR = 2.04; 95% CI: 1.55 to 2.78) and 4 times more likely to have caries experience (OR = 4.06; 95% CI: 2.24 to 7.34) compared with NHANES participants. Moreover, MA users were 40% less likely to have all teeth present than the NHANES participants (OR = 0.59; 95% CI: 0.45 to 0.76), but there was no significant difference in the likelihood of having <10 teeth present. Results from linear regression modeling show that MA users had an average of 2 more decayed, missing, or filled teeth (OR = 2.08; 95% CI: 1.29 to 2.79) than the NHANES participants after adjusting for sociodemographic and behavioral risk indicators. Additionally, NHANES participants had an average of 0.68 more teeth present than MA users (OR = −0.68; 95% CI: −1.29 to −0.0065). Also, users who injected MA had significantly higher rates of dental disease as compared with noninjectors (P = 0.04).
Table 2.
Differences between MA Users and NHANES Subjects for Dental Caries and Missing Teeth.
| Dichotomous Outcome | Log OR (SE) | OR (95% CI) |
|---|---|---|
| Has all teeth | −0.53 (0.13) | 0.59 (0.45 to 0.76) |
| <10 teeth | 0.06 (0.24) | 1.06 (0.65 to 1.7) |
| Has untreated cariesa | 0.73 (0.15) | 2.08 (1.55 to 2.78) |
| Caries experienceb | 1.4 (0.3) | 4.06 (2.24 to 7.34) |
| Continuous Outcome | Average Mean Difference (SE) | 95% CI |
| Decayed, missing, or filled teeth, n | 2.04 (0.38) | 1.29 to 2.79 |
| Teeth present, nc | −0.68 (0.31) | −1.29 to −0.065 |
| Decayed or filled teeth, n | 1.35 (0.27) | 0.82 to 1.88 |
95% CI, 95% confidence interval; MA, methamphetamine; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio.
Untreated caries is defined as decayed teeth >0.
Caries experience is defined as decayed, missing, or filled teeth >0.
Out of 28 teeth, excluding third molars.
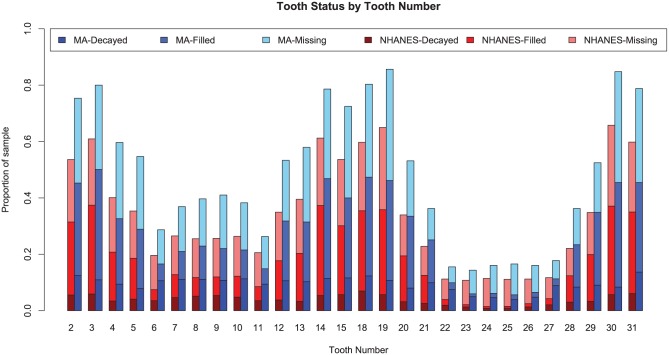
Figure 2 displays the distribution of decayed, missing, and filled teeth for MA users and matched NHANES participants for each tooth. Overall, the teeth of MA users were more likely to be decayed, missing, or filled for each tooth when compared with NHANES participants. The proportion of untreated dental caries is higher for all teeth of MA users than NHANES participants. For maxillary anterior teeth (6 to 11), the proportion of untreated dental caries in MA users is comparable to the combined proportion of untreated and filled teeth for NHANES participants. Although the proportion of untreated caries and filled teeth is very low in NHANES participants’ lower anterior incisors (23 to 26), the proportion of untreated caries in MA users’ lower anterior incisors is comparable to the proportion of untreated caries in MA users’ upper anterior incisors (7 to 10). Almost 80% MA users have experienced caries on their molars.
Figure 2.
Proportion of decayed, missing, and filled teeth for methamphetamine (MA) users and National Health and Nutrition Examination Survey (NHANES) participants.
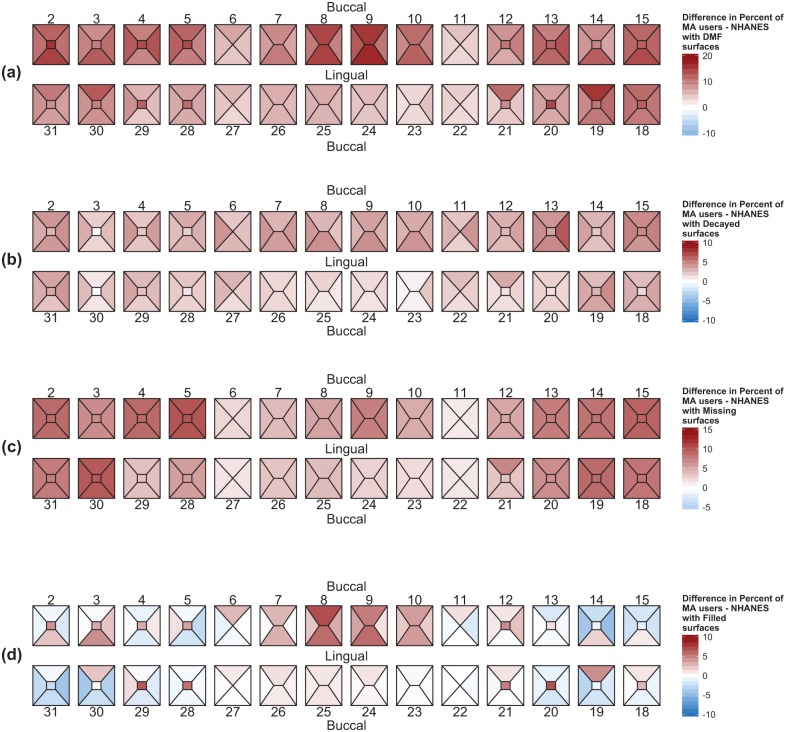
Weighted differences in the proportion of decayed, missing, and filled surfaces between MA users and NHANES subjects are shown in Figure 3. Caries experience (Fig. 3a) shows that there are more dental surfaces affected by caries (darker red color) for MA users than for NHANES participants. This difference is most pronounced for the buccal and lingual surfaces of teeth 8 and 9, where this difference approaches 20%. Simply stated, close to 20% more MA users have decayed, missing, or filled dental surfaces at teeth 8 and 9 when compared with NHANES participants. Although MA users have more dental surfaces affected by caries, for the maxillary teeth in particular, this difference ranges from 5% to 10% (Fig. 3b). MA users have more dental surfaces that are missing, and this is most noticeable for upper and lower posterior teeth (Fig. 3c). Figure 3d shows the difference of restored dental surfaces between MA users and NHANES participants. MA users have more maxillary anterior dental surfaces restored (darker red color) than do NHANES participants, whereas NHANES participants tend to have more dental surfaces restored in their posterior teeth (more blue color) than do MA users.
Figure 3.
Graphical summary of diseased, missing, and filled tooth surfaces. Polygons for each tooth surface characterize the differences in the proportions of DMF (a), decayed (b), missing (c), and filled (d) surfaces between MA users and NHANES subjects. The range and intensity of the color map reflect the magnitude and direction of the differences in proportions of afflicted surface in the MA users (red) or NHANES subjects (blue).
Discussion
With use of novel statistical methods to achieve covariate balance in a large sample of MA users and NHANES controls, our study established that MA users have distinct patterns and much higher rates of dental disease (decayed, missing, and filled teeth) when compared with presumptive nonusers. MA users were roughly 4 times more likely to have teeth affected by dental caries and roughly twice as likely to have untreated dental caries when compared with demographically comparable NHANES participants. Furthermore, MA users were more likely to have a greater number of missing teeth than their matched NHANES controls. These findings conclusively substantiate our initial findings of quantifiably higher rates of dental disease and oral health problems in a cohort of 301 MA-dependent subjects who underwent comprehensive physician-conducted health assessments (Shetty et al. 2010). Our findings echo and reinforce those of Morio and colleagues (2008), who examined a group of 18 MA users and determined that they had fewer teeth and more dental caries than a corresponding group of age- and sex-matched nonusers (average age, 31 ± 6 y). More recently, Rommel et al. (2015) studied a sample of 100 chronic MA users and 100 matched-pair controls to establish that MA users have significantly higher rates of caries (P < 0.001) and higher DMFT scores (P < 0.001). Our propensity score and quintile stratification approach confirmed these findings while minimizing the biases inherent to the simple matching approach used by Rommel et al. (2015).
The patterns of tooth surface involvement in the MA users were also very distinctive. In addition to having more surfaces affected by dental caries, the surface involvement in MA users was most pronounced for the maxillary central incisors, followed by maxillary posterior premolars and molars. The finding is consistent with reports by Morio et al. (2008) and others (Saini et al. 2005; Shaner et al. 2006; Goodchild et al. 2007) who reported that MA users tend to manifest greater percentages of anterior teeth, premolars, and molars with gross decay versus nonusers. MA users had more restored maxillary anterior teeth than NHANES participants. Whereas MA users experienced decayed and missing dental surfaces more frequently than NHANES participants, NHANES participants manifested proportionately more restored surfaces, especially on molars. Our findings suggest that MA users may choose to have their posterior teeth extracted rather than restored. This may be a result of the cost of restorative procedures for posterior teeth, especially if caries has significantly compromised the coronal integrity of the tooth. Beyond the higher rates of tooth loss, 19 MA subjects were completely edentulous—a noteworthy finding given the relative youth of our MA cohort (age, 44.4 ± 9.5 y). Overall, our findings do suggest that the prevalence and patterns of dental caries are distinctively different in MA users as compared with a sample selected from the U.S. general population. In a different setting, utilizing a large convenience sample of 308 MA users presenting to substance abuse treatment centers in South Africa, Smit and Naidoo (2015) found even higher rates of dental caries and tooth loss. Although younger (age, 28 ± 6.7 y), 14% of their sample had ≥10 teeth missing, and 1% were completely edentulous. On average, each subject had 5 decayed permanent teeth, and most (89.29%) were untreated.
When oral health professionals generally think of how “meth mouth” presents, most envision rampant caries, with the maxillary anterior teeth most affected. Although the patterns of dental decay across the spectrum of MA users are not consistently as extreme as depicted in earlier reports, our findings show that extensive caries is more likely in MA users than comparable adults drawn from the U.S. population. The dental consequences of MA use were more pronounced in injection MA users, a finding similar to that of Brown et al. (2012), who found that 18% of the MA users in their small cohort of injection drug users had ≥7 residual roots and the mean number of decayed surfaces was 28.8. The reasons for the differential impact by mode of MA use are unclear but might be related to the level of addiction. Those who use MA are believed to make the transition from noninjection MA (smoking or snorting) to injection MA as their MA dependence becomes more severe (Wood et al. 2008). The subsequent poor diet, increased consumption of sugary sodas, and fewer toothbrushing behaviors contribute to the rampant caries seen in MA users in general and injection MA users in particular (Smit and Naidoo 2015; Murphy et al. 2016).
In addition to our propensity score adjustment methods that corrected for observed imbalances between the groups, our study benefits from several other strengths—namely, access to a large community sample of MA users with a range of MA use behaviors, the utilization of an oral health data set from a large national study that does not select participants based on any preexisting condition or risk factor, and the use of trained dentists and a calibrated measurement protocol to catalogue the nature and extent of dental disease in the MA users. Despite the strengths, some of the limitations merit discussion. First, there was a temporal difference due to the use of data from an earlier NHANES cohort (i.e., 1999 to 2004). Propensity score models provide a basis for adjusting for observed covariates but rely on the assumption of strongly ignorable treatment assignment (Rosenbaum and Rubin 1983) and leave open the possibility of hidden bias due to unobserved characteristics. However, given the magnitude of the observed differences between the MA users and NHANES subjects, it would require a substantial amount of hidden bias to overturn the observed differences in our study. Additionally, our screening questions focused on establishing MA as the primary drug of abuse in the past 30 d, and we did not collect a history of polydrug use. It is conceivable that a history of polydrug use may have contributed to the dental disease patterns in some of the subjects.
In summary, we have conclusively established that the use of MA is associated with high rates of dental disease and that the proportions of decayed, missing, and filled teeth in MA users are significantly greater than that encountered in demographically comparable adults from the general U.S. population. MA users have more untreated dental caries and a greater number of missing teeth when compared with matched NHANES controls. The differential involvement of the teeth and teeth surfaces in MA users is quite distinctive, with the surface involvement being highest for the maxillary central incisors, followed by maxillary posterior premolars and molars. The high rates and distinctive patterns of caries could be used to alert dentists to covert MA use in their patients, which could lead to a more comprehensive management strategy, such as implementing “screening, brief interventions, and referral for treatment” strategies (Agerwala and McCance-Katz 2012) adapted to the dental setting.
Author Contributions
V. Shetty, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript; L. Harrell, contributed to design, data acquisition, analysis, and interpretation, critically revised the manuscript; J. Clague, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; D.A. Murphy, contributed to conception, design, and data interpretation, critically revised the manuscript; B.A. Dye, contributed to data acquisition and analysis, critically revised the manuscript; T.R. Belin, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We wish to acknowledge the efforts of Peter Cebezas (interviewer) and Rachel Fintzy (project director), who were responsible for recruitment, study coordination, and data collection. We gratefully acknowledge the participation and support of the subjects as well as the administrative and clinical staff of the clinics that participated in the study.
Footnotes
This study was funded by the National Institutes of Health / National Institute on Drug Abuse (5R01DA25680: V. Shetty), which had no role in the design of the study; in the collection, analysis, and interpretation of data; or in the writing of the report. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute on Drug Abuse, the National Institutes of Health, or the Centers for Disease Control and Prevention.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Agerwala SM, McCance-Katz EF. 2012. Integrating screening, brief intervention, and referral to treatment (SBIRT) into clinical practice settings: a brief review. J Psychoactive Drugs. 44(4):307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Krishnan S, Hursh K, Yu M, Johnson P, Page K, Shiboski CH. 2012. Dental disease prevalence among methamphetamine and heroin users in an urban setting: a pilot study. J Am Dent Assoc. 143(9):992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretzmeyer M, Walker J, Hall JA, Arndt S. 2007. Methamphetamine use and dental disease: results of a pilot study. J Dent Child. 74(2):85–92. [PubMed] [Google Scholar]
- Diago S. 2003. When your patient is an addict. AGD Impact. 33(9):12–16. [Google Scholar]
- Donaldson M, Goodchild JH. 2006. Oral health of the methamphetamine abuser. Am J Health Syst Pharm. 63(21):2078–2082. [DOI] [PubMed] [Google Scholar]
- Dye BA, Barker LK, Li X, Lewis BG, Beltrán-Aguilar ED. 2011. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2005-08. J Public Health Dent. 71(1):54–61. [DOI] [PubMed] [Google Scholar]
- Dye BA, Harrell L, Murphy DA, Belin T, Shetty V. 2015. Performance of a quality assurance program for assessing dental health in methamphetamine users. BMC Oral Health. 15:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BA, Nowjack-Raymer R, Barker LK, Nunn JH, Steele JG, Tan S, Lewis BG, Beltran-Aguilar ED. 2008. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2003-04. J Public Health Dent. 68(4):218–226. [DOI] [PubMed] [Google Scholar]
- Goodchild JH, Donaldson M, Mangini DJ. 2007. Methamphetamine abuse and the impact on dental health. Dent Today. 26(5):124–126. [PubMed] [Google Scholar]
- Harrell L, Shetty V, Belin T. 2016. Developing a propensity-score framework for evaluating the oral health consequences of methamphetamine use. Communications in Statistics: Case Studies and Applications. 1(3):125–135. [Google Scholar]
- Ho DE, Imai K, King G, Stuart E. 2011. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 42(8):1–28. [Google Scholar]
- Linnemann T, Wall T. 2013. “This is your face on meth”: the punitive spectacle of “white trash” in the rural war on drugs. Theor Criminol. 17(3):315–334. [Google Scholar]
- Mallatt ME. 2005. Meth mouth: a national scourge. J Indiana Dent Assoc. 84(3):28–29. [PubMed] [Google Scholar]
- Morio KA, Marshall TA, Qian F, Morgan TA. 2008. Comparing diet, oral hygiene and caries status of adult methamphetamine users and nonusers: a pilot study. J Am Dent Assoc. 139(2):171–176. [DOI] [PubMed] [Google Scholar]
- Murakawa N. 2011. TOOTHLESS: the methamphetamine “epidemic,” “meth mouth,” and the racial construction of drug scares. Du Bois Rev: Soc Sci Res Race. 8(1):219–228. [Google Scholar]
- Murphy DA, Harrell L, Fintzy R, Vitero SJ, Gutierrez A, Shetty V. 2016. Soda consumption among methamphetamine users being seen for a dental exam. Oral Health Prev Dent [epub ahead of print 11 February 2016] in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla R, Ritter AV. 2008. Meth mouth: methamphetamine and oral health. J Esthet Restor Dent. 20(2):148–149. [DOI] [PubMed] [Google Scholar]
- Radike A. 1972. Criteria for diagnosis of dental caries. In: Proceedings of the Conference on the Clinical Testing of Cariostatic Agents Chicago (IL): American Dental Association; p. 87–88. [Google Scholar]
- Richards JR, Brofeldt BT. 2000. Patterns of tooth wear associated with methamphetamine use. J Periodontol. 71:1371–1374. [DOI] [PubMed] [Google Scholar]
- Rommel N, Rohleder N, Wagenpfeil S, Härtel-Petr R, Jacob F, Wolff K-D, Kesting M. 2015. The impact of the new scene drug “crystal meth” on oral health: a case-control study. Clin Oral Investig [epub ahead of print 15 July 2015]. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. 1983. The central role of the propensity score in observational studies for causal effects. Biometrika. 70:41–55. [Google Scholar]
- Rosenbaum PR, Rubin DB. 1984. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 79(387):516–524. [Google Scholar]
- Saini T, Edwards PC, Kimmes NS, Carroll LR, Shaner JW, Dowd FJ. 2005. Etiology of xerostomia and dental caries among methamphetamine abusers. Oral Health Prev Dent. 3(3):189–195. [PubMed] [Google Scholar]
- Shafer J. 2005. Meth mouth, our latest moral panic. Slate Mag [accessed 2016 Mar 2]. http://www.slate.com/articles/news_and_politics/press_box/2005/08/the_methmouth_myth.html.
- Shaner J. 2002. Caries associated with methamphetamine abuse. J Mich Dent Assoc. 84(9):42–47. [PubMed] [Google Scholar]
- Shaner JW, Kimmes N, Saini T, Edwards P. 2006. “Meth mouth”: rampant caries in methamphetamine abusers. AIDS Patient Care STDS. 20(3):146–150. [DOI] [PubMed] [Google Scholar]
- Shetty V, Harrell L, Murphy DA, Vitero S, Gutierrez A, Belin TR, Dye BA, Spolsky VW. 2015. Dental disease patterns in methamphetamine users: findings in a large urban sample. J Am Dent Assoc. 145(12):875–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty V, Mooney LJ, Zigler CM, Belin TR, Murphy D, Rawson R. 2010. The relationship between methamphetamine use and increased dental disease. J Am Dent Assoc. 141(3):307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DA, Naidoo S. 2015. Oral health effects, brushing habits and management of methamphetamine users for the general dental practitioner. Br Dent J. 218(9):531–536. [DOI] [PubMed] [Google Scholar]
- Wood E, Stoltz JA, Zhang R, Strathdee SA, Montaner JS, Kerr T. 2008. Circumstances of first crystal methamphetamine use and initiation of injection drug use among high-risk youth. Drug Alcohol Rev. 27(3):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]