Abstract
Oral mucositis (OM) is among the most common, painful, and debilitating toxicities of cancer regimen–related treatment, resulting in the formation of ulcers, which are susceptible to increased colonization of microorganisms. Novel discoveries in OM have focused on understanding the host-microbial interactions, because current pathways have shown that major virulence factors from microorganisms have the potential to contribute to the development of OM and may even prolong the existence of already established ulcerations, affecting tissue healing. Additional comprehensive and disciplined clinical investigation is needed to carefully characterize the relationship between the clinical trajectory of OM, the local levels of inflammatory changes (both clinical and molecular), and the ebb and flow of the oral microbiota. Answering such questions will increase our knowledge of the mechanisms engaged by the oral immune system in response to mucositis, facilitating their translation into novel therapeutic approaches. In doing so, directed clinical strategies can be developed that specifically target those times and tissues that are most susceptible to intervention.
Keywords: cancer, oral microbiome, Toll-like receptor, pathogen-associated molecular pattern, damage-associated molecular pattern, cancer complications
Introduction
Oral mucositis (OM) is among the most common, painful, and debilitating toxicities of cancer regimen–related treatment. In its most clinically significant form, OM presents as large, irregular, deep ulcers of the movable mucosa, often covered by a pseudomembrane (Elting et al. 2008; Sonis 2011; Villa and Sonis 2015) (Fig. 1). Among patients receiving aggressive regimens of myeloablative chemotherapy or conditioning regimens prior to a hematopoietic stem cell transplant (HSCT), severe mucositis occurs in approximately 40% of patients. For patients being treated with conventional chemoradiation of the head and neck, severe mucositis affects more than two-thirds of patients (Sonis et al. 2004; Villa and Sonis 2015). The loss of mucosal integrity universally results in levels of pain for which even opioids may not be effective. When asked to rank their worst intratreatment side effects, cohorts of patients receiving either chemotherapy or radiotherapy were in agreement that mucositis was at the top of the list. Furthermore, pain is frequently accompanied by loss of function (Elting et al. 2008). Patients are unable to eat normally and must rely on gastrostomy feeding or total parenteral nutrition (Elting et al. 2008; Sonis 2011). The loss of an intact epithelial barrier in the oral environment places myelosuppressed patients at risk for focal secondary infections, bacteremias, and sepsis (Sonis 2004; Wang et al. 2013; Villa and Sonis 2015). In addition to its physiological and symptomatic costs, mucositis has significant health and economic burdens, largely driven by increased resource use including hospitalization, office and emergency room visits, and increased diagnostic testing and medication use. In fact, the incremental cost of OM in patients with head and neck or non–small cell lung cancer exceeds $17,000 (Nonzee et al. 2008; Villa and Sonis 2015).
Figure 1.
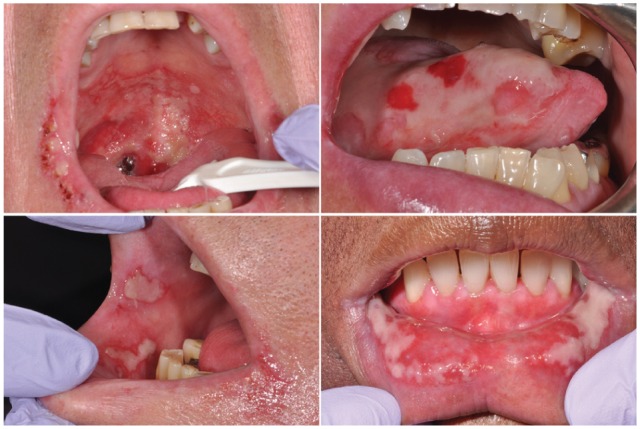
Manifestation of oral mucositis in its severe form presents ulcerative lesions, which penetrate the submucosa. Loss of mucosal layer integrity represents a clinically significant risk factor for bacteremia, fungemia, and sepsis.
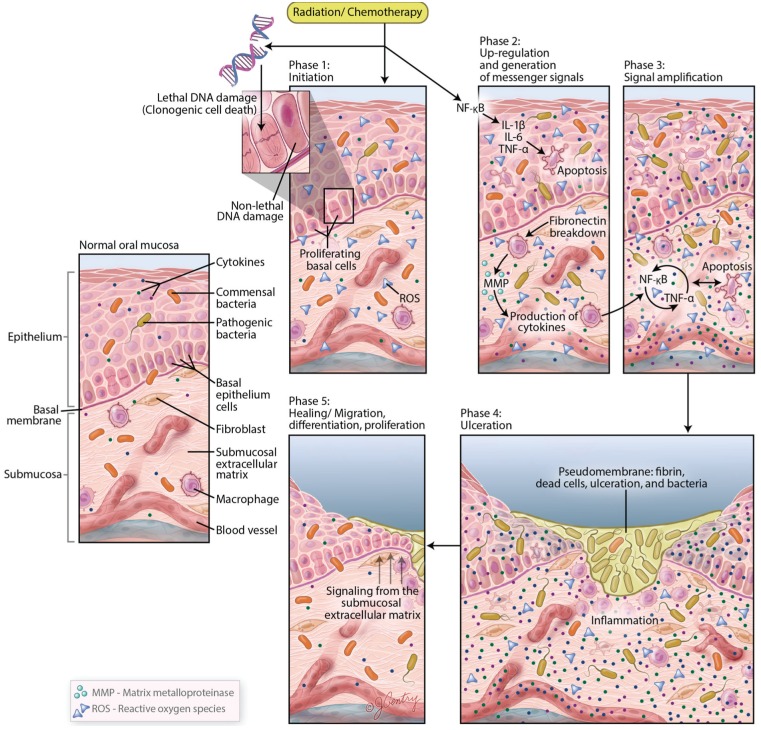
Historically, the pathogenesis of chemotherapy- or radiation-induced toxicities was attributed to clonogenic cell death directly induced on basal epithelial stem cells. Although direct injury surely plays a role in OM development, it has become increasingly clear that the pathogenesis of the condition is much more complex. A 5-stage schema to explain the biological trajectory of mucositis (Sonis 2004) has been proposed and studied in some detail (Fig. 2). Although the basal epithelium is the “target” of the destructive biological events, most of the activity is mediated by the cells and tissues of the submucosa. The first stage of mucositis is initiated by a surge of oxidative stress (reactive oxygen species [ROS]) and activation of the innate immune response. It seems likely that elements of the inflammasome are activated, followed by key proinflammatory transcription factors including nuclear factor-κB (NF-κB), with consequent expression of many of the genes associated with inflammatory pathways. In fact, a total of at least 14 canonical pathways have been associated with mucositis progression. As a consequence, key proinflammatory cytokines (e.g., tumor necrosis factor-α [TNF-α], interleukin [IL]-1β, and IL-6) are produced, the ceramide pathway is activated, and connective tissue breakdown results in a profusion of matrix metalloproteinases. Local increases in tissue-damaging kinases are noted. Importantly, all of this biological havoc occurs before the patient is symptomatic and before there is any clinical evidence of tissue injury. Feedback provides a continuous loop, which amplifies a sequence resulting in progressive tissue injury, ultimately leading to the clinical manifestation of ulceration. Importantly, early in the process, the integrity of the epithelial tight junctions is threatened and breached, leading to increases in mucosal permeability (Sonis 2004, 2009, 2010; Sonis et al. 2004). Bacterial colonization of the ulcerated tissue results in an active lesion in which cell wall products activate macrophages to stimulate an inflammatory response. As noted above, it is during this phase, particularly in myeloablated patients, that the risk of bacteremia and sepsis increases. But even in cases in which bacteria do not violate the mucosa, pyogenic cell wall products often result in fever (Laheij et al. 2012). Mucositis uncomplicated by local or systemic circumstances typically resolves spontaneously from a few days to a few weeks after the cessation of treatment (Sonis 2004; Sonis et al. 2004).
Figure 2.
Biological complexities underline the mucosal injury that is initiated by cytotoxic cancer therapy. This figure illustrates the pathogenesis of oral mucositis, which encompasses a series of biological events coupled with the influence of the oral microbiota and overall oral environment. In an oral ecosystem, a host-microbiota homeostasis is maintained under normal health conditions. In patients with cancer undergoing radiation therapy and chemotherapy, a dramatic change in the oral environment occurs, which causes an imbalance of the oral microorganisms and influences the modification of oral mucositis barrier function, innate immunity, and cellular mechanisms. The progression of oral mucositis can be summarized in 5 stages: initiation, messaging and signaling, amplification, ulceration, and healing. Based on this model, inflammation, together with apoptosis, leads to the loss of integrity of the mucosal barrier, thereby promoting bacteria translocation. Adapted from Sonis (2004). IL, interleukin; MMP, matrix metalloproteinase; NF-κB, nuclear factor-κB; ROS, reactive oxygen species; TNF, tumor necrosis factor.
Altogether, in a complex stochastic pathway, these specific transcription factors, inflammatory mediators, and physiologic molecules contribute to modify mucosal response to chemoradiation challenges, and ultimately to epithelial basal-cell death and injury (hereafter referred to as OM ulcers).
The role of bacteria in the pathogenesis of mucositis has been an area of interest for some time (Wang et al. 2013; Laheij and de Soet 2014; Stringer and Logan 2015; Vanhoecke et al. 2015). For many years, the importance of the oral microbiome was relegated to it being a frequently identified source of bacteremia and sepsis in myelosuppressed patients in whom mucositis served as a convenient systemic portal of entry for orally dwelling bacteria. However, given its richness and diversity and its role in local inflammatory diseases, the potential of oral microflora to affect the course of mucositis seemed likely. Adding to this thinking was the finding over 50 y ago of shifts in the oral flora in response to chemotherapy (Peterson et al. 1987; Reynolds et al. 1989; Peterson 1990; Spijkervet et al. 1991; Ruescher et al. 1998; Stokman et al. 2003; Napenas et al. 2010; de Mendonca et al. 2012; Laheij et al. 2012). Consequently, numerous antimicrobial strategies have been studied as interventions for mucositis. Some have included the administration of systemic antibiotics (selective decontamination), whereas others have tested topical antibacterial therapies (Wijers et al. 2001; Stokman et al. 2003; Giles et al. 2004). None have been successful. Despite the seeming futility of strict antimicrobial approaches in preventing mucositis, data derived from chemotherapy-induced enteritis (gastrointestinal mucositis) suggest that bacteria could modify the course of the condition (van Vliet et al. 2010).
The Role of the Oral Microbiota in the Putative Etiology of OM
Although clonogenic cell death of mucosal stem cells (crypt cells, in the case of the intestinal tract) is a direct result of damage from chemotherapy and/or radiation therapy, the initiation of mucositis is the consequence of at least 2 other elements: oxidative stress and activation of the innate immune response. As the cascade of biological events that lead to injury follow, shifts in microflora have been noted in both the intestine and the oral cavity. The nature of these shifts varies depending on the cancer treatment regimen (chemotherapy selection, extent of myelosuppression, concomitant xerostomia, and a range of patient- and treatment-related variables) (Napenas et al. 2010; van Vliet et al. 2010; Jenq et al. 2012; Hu et al. 2013; Stringer 2013; Touchefeu et al. 2014; Vanhoecke et al. 2015).
A potential link between the intestinal flora and chemotherapy-induced mucositis has been suggested by a series of animal studies. The recent findings of Pedroso et al. (2015), in which irinotecan-induced mucositis was studied in a germ-free and selectively colonized mouse model, implicate bacteria as modifiers of mucositis progression. Of course, it would be naïve to fail to recognize the differences in epithelial anatomy, microbiome–soft tissue impact, and the course of mucositis seen between the mouth and intestine. Or the fact that the oral cavity harbors distinct species of organisms that colonize specific anatomic sites (teeth, gingiva, interproximal sites, tongue, and movable mucosa) (Corby et al. 2008; Vanhoecke et al. 2015), which are different from those throughout the gastrointestinal tract (Laheij and de Soet 2014). Nonetheless, although perhaps not as direct or dramatic, it would be equally naïve to disregard the oral flora as being biologically complacent in the face of mucosal injury.
We have known for over 30 y that chemotherapy-induced myelosuppression is followed by microbial dysbiosis. Radiation-induced xerostomia gives way to shifts in the oral microbiome. Given its ability to affect the innate immune response, a known stimulator of the mucositis pathway, the oral microflora could serve to exacerbate or extend mucositis (Laheij and de Soet 2014; Stringer and Logan 2015). Because the pain associated with OM likely affects patients’ ability to perform conventional oral hygiene procedures, supplemental antibacterial oral rinses such as chlorhexidine or povidone iodine have been studied in the context of OM prevention (Yoneda et al. 2007; Choi and Kim 2012; McGuire et al. 2013). The inconsistent results of such trials are perplexing and reinforce the need for additional study. It seems clear that the intestinal microbiome is actively involved in the pathogenesis of mucositis. However, it is unclear how the intestinal microbiome is involved in its pathoetiology. As noted above, although the environmental, immunological, biological, and structural differences between the mouth and intestine cannot be ignored, the oral microflora could behave in a similar way to accelerate or facilitate different phases of OM development. It would seem possible that the past failures of antimicrobials to impact oral mucositis might be attributable to the nature of the agents studied and, critically, their failure to specifically target the cluster of bacteria which impact OM’s pathogenesis. This would also support the potential for synergism between successful, mechanistically-based interventions and targeted antibiotic therapy.
Host-Microbiome Cross-talk in OM
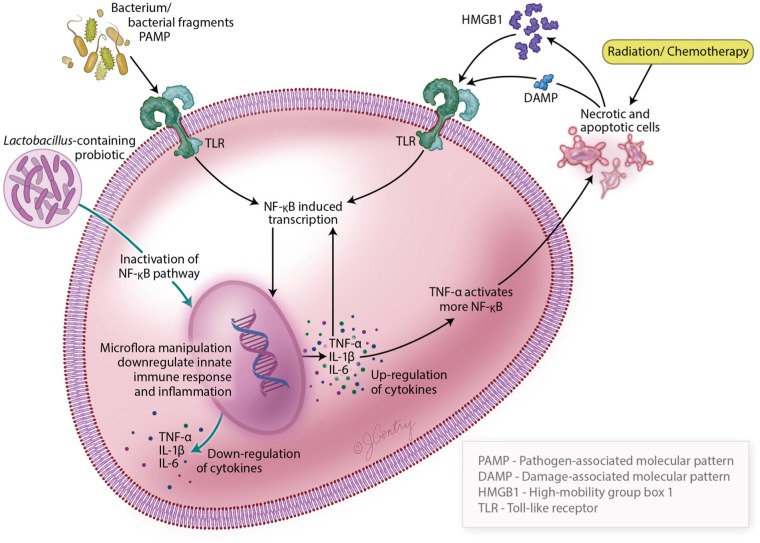
Activation of the innate immune response is a key component in the initiation of mucositis (Fig. 3). At this phase of mucositis, the epithelial barrier is intact and drivers of the response are likely derived from damage-associated molecular patterns (DAMPs) such as alarmin high-mobility group box-1 (HMGB1) released from apoptotic or necrotic cells caused by the initial wave of clonogenic cell targeting by radiotherapy or chemotherapy. Subsequently, binding to pathogen recognition receptors, such as Toll-like receptors (TLRs), occurs on epithelial and endothelial cells and fibroblasts and an inflammatory cascade ensues (Srikrishna and Freeze 2009; Sonis 2010). At this stage, it seems unlikely that the local microbiome is not a driving force.
Figure 3.
This figure describes the molecular pathways involved in microbiota-host interactions and the development of oral mucositis. The detection of microbial components (PAMPs) and endogenous damage-associated molecular patterns (DAMPs and HMGB1) by pattern recognition receptors such as Toll-like receptors triggers a cascade of cellular signals, resulting in activation of NF-κB (among other pathways) that contribute to amplify proinflammatory cytokines and apoptosis. The use of probiotic bacteria has the ability to activate pathways that are involved in the reduction of inflammatory signaling and apoptosis through the downregulation of the innate immune response of the epithelial cells by way of inactivation of the NF-κB pathway. DAMP, damage-associated molecular pattern; HMGB1, high-mobility group box-1; IL, interleukin; NF-κB, nuclear factor-κB; PAMP, pathogen-associated molecular pattern; TNF, tumor necrosis factor.
Bacterial colonization increases as mucosal injury progresses and, simultaneously, epithelial tight junctions are damaged and breakdown results in increased permeability and a conduit for bacterial cell wall products. Consequently, a second opportunity for involvement of the innate immune response could be possible and could serve a role in amplifying the severity or duration of mucositis. Infiltrating natural killer cells, mast cells, macrophages, and dendritic cells of the innate immune system recognize a ubiquitous conserved molecular pattern called pathogen-associated molecular patterns (PAMPs) (Sonis 2009, 2010; Srikrishna and Freeze 2009). PAMPs are expressed by the oral microflora (Sonis 2010). Although it has been recognized for decades that the composition of the oral flora shifts in response to myeloablative chemotherapy, changes have also been noted in response to radiation. Whereas the former are most likely associated with the host’s immune status, the latter (i.e., radiotherapy) may be more directly related to the local oral environment (Vanhoecke et al. 2015). Nonetheless, the effects on the innate immune response are similar. The immune system responds to PAMPs, now in addition to DAMPs and chemotherapy radiation-induced damage-associated patterns, in signaling pathway interactions described above to bind to pattern recognition receptors such as TLRs to active NF-κB, activate up to 200 genes, and facilitate proinflammatory cytokine production (Srikrishna and Freeze 2009; Sonis 2010; Villa and Sonis 2015).
Understanding the role of the oral microbiome in the pathogenesis of mucositis has been challenging. There is little doubt that patients who have good oral care during cancer therapy (either radiation or chemotherapy) have better outcomes. Contrastingly, prophylactic antimicrobial strategies using antibiotics or antifungals have consistently failed to be efficacious in preventing the development of mucositis or in attenuating its course (Wijers et al. 2001; Stokman et al. 2003; Giles et al. 2004). Likewise efforts to decontaminate the mouth or reduce its bacterial load have been inconsistent and conflicting in their efficacy (Laheij and de Soet 2014). Finally, there are no data to suggest that either the risk or course of mucositis is different between dentulous and edentulous patients.
Clinical Studies
A few clinical studies have assessed microbial changes in patients receiving anticancer therapy. A study performed in an outpatient population with breast cancer used molecular techniques to identify microbial species before and after chemotherapy. Results of this study revealed an increase in the number of species within the microbial community, suggesting an alteration in the nature of oral bacterial flora after treatment. A total of 41 species were detected, with a predominance of Gemella haemolysans and Streptococcus mitis. More than 60% of the species identified in buccal mucosal sites were exclusively present after therapy, suggesting an alteration in the profile of the oral microflora after cancer treatment (Napenas et al. 2010).
The relationship between yeasts, bacteria associated with periodontitis, and oral ulcerations was evaluated in allogeneic HSCT recipients. The authors reported a direct relationship between overabundance of Porphyromonas gingivalis in particular, but also Parvimonas micra, Treponema denticola, Fusobacterium nucleatum, Candida glabrata, and Candida kefyr, and mucositis ulcerations in more severe cases (Laheij et al. 2012). Ames et al. (2012) also looked at the effects of an allogeneic HSCT on the oral microbiota and its implications on the development of respiratory complications. The common core bacteria such as species of Streptococcus, Gemella, and Veillonella in patients’ oral cavity remained stable before and after transplantation. In this study, although the profile of the oral microbiome was changed minimally by the transplantation process, the development of respiratory complications after transplantation was found to be associated with changes in the oral microbiome (Ames et al. 2012). In an adult population, Belazi et al. (2004) showed that 77% of patients with oral squamous cell carcinoma who were undergoing radiation and were affected by OM had a significant increase in Candida spp. at the end stages of radiation therapy (Belazi et al. 2004). In an attempt to profile the core microbiome of the oral microbiota in patients with head and neck cancers undergoing radiation therapy, high-throughput pyrosequencing was used to profile the supragingival plaque samples collected from 8 patients before and after radiation therapy at different time intervals. A representation of 4 phyla (Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria) and 11 genera (Streptococcus, Actinomyces, Veillonella, Capnocytophaga, Derxia, Neisseria, Rothia, Prevotella, Granulicatella, Luteococcus, and Gemella) were found in all subjects. Changes in the relative abundance of microbial species before and after radiation therapy were also observed, as well as a negative correlation between the number of operational taxonomic units and radiation dose, supporting the hypothesis that exposure to ionizing radiation has the potential to disturb the microbial community of the oral environment (Hu et al. 2013) (Table).
Table.
Oral Microbiome Changes due to Anticancer Therapy.
| Reference | Study Population | Cancer Treatment | Time of Sample Collection | Sample Type | Findings |
|---|---|---|---|---|---|
| Belazi et al. (2004) | 39 patients with head and neck cancer | RT | During | Swabs (lesion) | Candida albicans, Candida glabrata, Candida krusei, Candida tropicalis, and Candida kefyr were most common |
| Napenas et al. (2010) | 9 patients with breast cancer | CT | Before/during | Buccal mucosa swabs | The total number of bacterial species per patient increased and a shift to a more complex oral bacterial was found during CTGemella haemolysans and Streptococcus mitis were the most predominant species |
| Sonalika et al. (2012) | 61 patients with squamous cell carcinoma and 72 controls | RT | Before/after | Saliva | Significant increase in Candida spp. was promoted by RT Increase in Escherichia coli, Pseudomonas aeruginosa, Enterobacter sp., and Klebsiella pneumonia contributed to exacerbation of mucositis |
| Panghal et al. (2012) | 186 patients with squamous cell carcinoma | RT or CT or chemoRT | During | Oral swab blood | P. aeruginosa was isolated from the blood of RT patients and K. pneumonia was isolated from the oral cavity of CT patients.Staphylococcus aureus and Staphylococcus epidermidis were observed in the blood of CT and chemoRT patients and in the oral cavity of patients undergoing RTC. albicans was the most significant oral cavity pathogen in RT and chemoRT casesAnaerobic species such as Parvimonasmicra, Fusobacterium nucleatum, Treponema denticola, C. glabrata, and C. kefyr were also associated with ulcerative OM |
| Laheij et al. (2012) | 49 patients with hematological malignancies | HSCT | Before/during | Oral rinse | Porphyromonas gingivalis was a prediction factor for OM |
| Hu et al. (2013) | 8 patients with head and neck cancer | RT | Before/during | Dental plaque | 4 phyla (Actinobacteria, Bacteroidetes, Firmecutes, and Proteobacteteria) and 11 genera (Streptococcus, Actinomyces, Veillonella, Capnocytophaga, Derxia, Neisseria, Rothia, Prevotella, Granulicatella, Luteococcus, and Gemella) were found in all subjects |
| Ames et al. (2012) | 45 patients who underwent allogeneic transplantation | HSCT | Before/after | Saliva, dental plaque, buccal and tongue brush | Many common bacterial genera such as Streptococcus, Veillonella, Gemella, Granulicatella, and Campylobacter were identified as being present before and after transplantation |
| Chavan et al. (2013) | 11 children with hematological malignancies | HSCT | After | Blood, cerebrospinal fluid, tissue |
Rothia mucilaginosa infections was clinically significant in neutropenic children undergoing HSCT |
chemoRT, chemoradiation; CT, chemotherapy; HSCT, hematopoietic stem cell transplant; OM, oral mucositis; RT, radiation therapy.
Blijlevens et al. (2009) suggested that the severity of OM may be directly associated with critical cases of febrile neutropenia in HSCT patients. In this review, critical cases of febrile neutropenia were often associated with the release of proinflammatory cytokines involved with OM, which preceded microbial translocation. Chavan et al. (2013) reported that invasive bacterial infections in a subset of HSCT patients after transplantation evolved from a predominance of gram-negative to gram-positive bacteria (Blijlevens et al. 2009; Chavan et al. 2013). Similarly, HSCT patients affected by graft versus host disease frequently develop a massive and progressive involvement of the oral and gastrointestinal mucosa. These specific pathologic and immunologic clinical manifestations are induced by the transplantation in a body with a compromised immune system. Thus, disruption of intestinal flora in these patients may contribute to gut inflammation by compromising epithelial barrier integrity and stimulating cytokine production (Eriguchi et al. 2012; Taur et al. 2012). Holler et al. (2014) and Jenq et al. (2012) evaluated the intestinal microbiota in this population and observed a relative microbial shift toward Enterococcus, which was more pronounced under antibiotic prophylaxis and treatment of neutropenic infections after transplantation. This may be explained by the use of many antibiotics to treat the various infections, allowing the spectrum of several bacterial pathogens to become overabundant, including opportunistic organisms that are usually of low virulence and benign in the immunocompetent host (Jenq et al. 2012; Holler et al. 2014).
Insights on Probiotics Host Communication
A detailed review of the signaling pathways associated with probiotics and mucositis is beyond the scope of this article, and the following description represents only a brief summary.
The use of probiotics represent a novel approach to the treatment of mucositis in patients undergoing anticancer treatment, primarily through the prevention of gastrointestinal toxicity through the modification of intestinal barrier function, innate immunity, and intestinal repair mechanisms. Probiotics can be defined as live bacteria that, when administered in abundant numbers, are able to exert beneficial physiologic or therapeutic activities (Touchefeu et al. 2014). Beneficial effects of probiotics include enhancing intestinal epithelial cell function, protecting against physiologic stress, modulating cytokine secretion profiles, influencing T-lymphocyte populations, and enhancing antibody secretion (Thomas and Versalovic 2010). The augmented immune functions would be helpful for mucositis prevention, which it focuses on the benefits of microflora manipulation with the aim of modulating host immune and inflammatory response and restoring the intestinal barrier after injury (Andrade et al. 2015). Probiotic bacteria have the ability to activate pathways in epithelial cells, including induction of ROS signaling, displacement of pathogenic bacteria, and interaction with signaling pathways involved in mucosal integrity and immune cell activity. Probiotics communicate with the host by modulating key signaling pathways, such as NF-κB and mitogen-activated protein kinase, to either enhance or suppress activation and influence downstream pathways (Thomas and Versalovic 2010). The NF-κB pathway is key to this cross-talk between the microbiota and the host responsible for activation of immune responses. This mechanism is believed to prevent or reverse the adverse effects of pathogens by inducing changes in the intestinal epithelial cell signaling pathway and modulating cell survival, cytokine secretion, and consequently activating an immune response (van Vliet et al. 2010). Thus, the use of probiotics is believed to prevent the activation of NF-κB and influence downstream cytokine secretion. A brief illustration of this pathway is also represented in Figure 3.
A few clinical trials of varying design, patient populations, and probiotic products have been reported. However, despite the evidence, no single probiotic strain or product has been approved through human clinical trials for mucositis management. A recent review of probiotic use in cytotoxic therapy-associated gastrointestinal mucositis concluded that both clinical and preclinical studies support the idea that Lactobacillus probiotics have the potential to reduce gastrointestinal toxicity when administered prophylactically and an adjunct treatment (Ciorba et al. 2015). However, few other probiotics have demonstrated efficacy in clinical trials. A recent randomized clinical trial proposed the use of Lactobacillus brevis CD2 lozenges as a potential approach for the treatment of mucositis in patients with head and neck cancer undergoing chemotherapy or radiation therapy. L. brevis CD2 lozenges reduced the incidence of grade III and IV OM and were associated with a lower overall rate of mucositis as well as a higher rate of anticancer treatment completion in this population (Sharma et al. 2012). A phase 1b study in patients with head and neck cancer receiving induction chemotherapy examined the use of an oral rinse (AG013) containing recombinant Lactococcus lactis, which was genetically engineered to secrete the mucosal protectant human trefoil factor 1 (a family of peptides that play important roles in the protection and repair of epithelial surfaces, including the gastrointestinal tract). The use of AG013 resulted in a 35% reduction in the number of days with ulcerative OM compared with placebo (Limaye et al. 2013).
Key concerns with the utilization of a bacterial vehicle stem from the potential risk of the development of clinically relevant infections. Completely restoring homeostasis might be a clinical problem, because whole live bacteria used as probiotics have already been described as causing invasive infections in immunocompromised patients and were associated with increased mortality in patients with severe pancreatitis (Sturm et al. 2005; Kwon et al. 2010). However, it is possible to substitute the probiotic vehicle by utilizing bacterial parts instead of whole live bacteria; this approach might be sufficient to attenuate local and systemic inflammation without the risk of invasive infections (Carol et al. 2006; Fujiya et al. 2007; Reiff et al. 2009). In summary, evidence supports the idea that probiotics could potentially be used as prophylactic treatments targeted to inhibit the development of OM or as a post-treatment to facilitate the recovery process.
Future Directions
It has been suggested that microflora dysbiosis, invasion, and colonization of oral cavity mucosal tissues might contribute to the pathophysiology of ulcerative OM. Nevertheless, to advance this hypothesis, it is important to both address the clinical failures and successes of past antimicrobial strategies and to explain the apparent successes of therapeutic approaches that do not knowingly target specific microflora. It has already been shown that topical and systemic antimicrobial approaches aimed at selective elimination of specific oral microflora (Wijers et al. 2001; Stokman et al. 2003) or to prevent and treat ulcerative OM (Giles et al. 2004; Elad et al. 2012) do not support the hypothesis that topical administration of an antimicrobial agent can reduce the severity of ulcerative OM. Although mucositis causes dysfunction and debilitating distress in patients with cancer, controlled clinical trials evaluating the efficacy of novel treatments for OM rarely assess the treatment’s ability to modulate the oral microflora by understanding the structural of biofilm conformation that occurs on tooth and mucosal surfaces in vivo rather than on its composition. This approach may lead to designing potent new inhibitors and improved strategies to combat the formation of pathogenic oral biofilms.
Overall, it is not clear that OM is directly caused by bacterial infection. Most likely, the complex mechanism involved in host-microbiome cross-talk in OM is that anticancer treatment “damages” the host (and consequently cells), making it more susceptible to infection. Therefore, this perturbation triggers a cascade of events, including bacterial and subsequent yeast infections. The bacteria and yeast will exacerbate the perturbation, and an ensuing infection rages in a compromised host.
Further longitudinal clinical investigation is needed to carefully characterize the relationship between the host and the clinical trajectory of OM, the clinical and molecular levels of inflammatory changes, and characteristics of the oral microbiota. Answering such questions will increase our knowledge of the mechanisms engaged by the oral immune system in response to mucositis, facilitating their translation into novel therapeutic approaches. In doing so, directed clinical strategies can be developed that specifically target those times and tissues that are most susceptible to intervention.
Author Contributions
R.M. Vasconcelos, contributed to conception and design, drafted the manuscript; N. Sanfilippo, B.J. Paster, S.T. Sonis, contributed to data interpretation, critically revised the manuscript; A.R. Kerr, Y. Li, L. Ramalho, contributed to conception, critically revised the manuscript; E.L. Queiroz, B. Smith, contributed to conception, drafted the manuscript; P.M. Corby, contributed to conception and design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This study was supported by the New York University (NYU) Provost’s Mega-Grants Initiative, a Philanthropic Gift from Marty and Connie Silver (to study effects of radiation therapy in patients with head and neck cancer), the National Institutes of Health National Center for Advancing Translational Sciences (CTSA grant 1UL1 TR001445 to NYU), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - 9162-13-5).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ames NJ, Sulima P, Ngo T, Barb J, Munson PJ, Paster BJ, Hart TC. 2012. A characterization of the oral microbiome in allogeneic stem cell transplant patients. PLoS One. 7(10):e47628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade ME, Araujo RS, de Barros PA, Soares AD, Abrantes FA, Generoso SV, Fernandes SO, Cardoso VN. 2015. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Cin Nutr. 34(6):1080–1087. [DOI] [PubMed] [Google Scholar]
- Belazi M, Velegraki A, Koussidou-Eremondi T, Andreadis D, Hini S, Arsenis G, Eliopoulou C, Destouni E, Antoniades D. 2004. Oral Candida isolates in patients undergoing radiotherapy for head and neck cancer: prevalence, azole susceptibility profiles and response to antifungal treatment. Oral Microbiol Immunol. 19(6):347–351. [DOI] [PubMed] [Google Scholar]
- Blijlevens NM, Logan RM, Netea MG. 2009. The changing face of febrile neutropenia-from monotherapy to moulds to mucositis. Mucositis: from febrile neutropenia to febrile mucositis. J Antimicrob Chemother. 63(Suppl 1):i36–i40. [DOI] [PubMed] [Google Scholar]
- Carol M, Borruel N, Antolin M, Llopis M, Casellas F, Guarner F, Malagelada JR. 2006. Modulation of apoptosis in intestinal lymphocytes by a probiotic bacteria in Crohn’s disease. J Leukoc Biol. 79(5):917–922. [DOI] [PubMed] [Google Scholar]
- Chavan RS, Pannaraj PS, Luna RA, Szabo S, Adesina A, Versalovic J, Krance RA, Kennedy-Nasser AA. 2013. Significant morbidity and mortality attributable to Rothia mucilaginosa infections in children with hematological malignancies or following hematopoietic stem cell transplantation. Pediatr Hematol Oncol. 30(5):445–454. [DOI] [PubMed] [Google Scholar]
- Choi SE, Kim HS. 2012. Sodium bicarbonate solution versus chlorhexidine mouthwash in oral care of acute leukemia patients undergoing induction chemotherapy: a randomized controlled trial. Asian Nurs Res (Korean Soc Nurs Sci). 6(2):60–66. [DOI] [PubMed] [Google Scholar]
- Ciorba MA, Hallemeier CL, Stenson WF, Parikh PJ. 2015. Probiotics to prevent gastrointestinal toxicity from cancer therapy: an interpretive review and call to action. Curr Opin Support Palliat Care. 9(2):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby PM, Biesbrock A, Bartizek R, Corby AL, Monteverde R, Ceschin R, Bretz WA. 2008. Treatment outcomes of dental flossing in twins: molecular analysis of the interproximal microflora. J Periodontol. 79(8):1426–1433. [DOI] [PubMed] [Google Scholar]
- de Mendonca RM, de Araujo M, Levy CE, Morari J, Silva RA, Yunes JA, Brandalise SR. 2012. Prospective evaluation of HSV, Candida spp., and oral bacteria on the severity of oral mucositis in pediatric acute lymphoblastic leukemia. Support Care Cancer. 20(5):1101–1107. [DOI] [PubMed] [Google Scholar]
- Elad S, Epstein JB, Raber-Durlacher J, Donnelly P, Strahilevitz J. 2012. The antimicrobial effect of iseganan HCl oral solution in patients receiving stomatotoxic chemotherapy: analysis from a multicenter, double-blind, placebo-controlled, randomized, phase III clinical trial. J Oral Pathol Med. 41(3):229–234. [DOI] [PubMed] [Google Scholar]
- Elting LS, Keefe DM, Sonis ST, Garden AS, Spijkervet FK, Barasch A, Tishler RB, Canty TP, Kudrimoti MK, Vera-Llonch M, et al. 2008. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer. 113(10):2704–2713. [DOI] [PubMed] [Google Scholar]
- Eriguchi Y, Takashima S, Oka H, Shimoji S, Nakamura K, Uryu H, Shimoda S, Iwasaki H, Shimono N, Ayabe T, et al. 2012. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting paneth cell production of alpha-defensins. Blood. 120(1):223–231. [DOI] [PubMed] [Google Scholar]
- Fujiya M, Musch MW, Nakagawa Y, Hu S, Alverdy J, Kohgo Y, Schneewind O, Jabri B, Chang EB. 2007. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe. 1(4):299–308. [DOI] [PubMed] [Google Scholar]
- Giles FJ, Rodriguez R, Weisdorf D, Wingard JR, Martin PJ, Fleming TR, Goldberg SL, Anaissie EJ, Bolwell BJ, Chao NJ, et al. 2004. A phase III, randomized, double-blind, placebo-controlled, study of iseganan for the reduction of stomatitis in patients receiving stomatotoxic chemotherapy. Leuk Res. 28(6):559–565. [DOI] [PubMed] [Google Scholar]
- Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, Zhu W, Sporrer D, Hehlgans T, Kreutz M, et al. 2014. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 20(5):640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YJ, Shao ZY, Wang Q, Jiang YT, Ma R, Tang ZS, Liu Z, Liang JP, Huang ZW. 2013. Exploring the dynamic core microbiome of plaque microbiota during head-and-neck radiotherapy using pyrosequencing. PLoS One. 8(2):e56343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ, et al. 2012. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 209(5):903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. 2010. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. 107(5):2159–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laheij AM, de Soet JJ. 2014. Can the oral microflora affect oral ulcerative mucositis? Curr Opin Support Palliat Care. 8(2):180–187. [DOI] [PubMed] [Google Scholar]
- Laheij AM, de Soet JJ, von dem Borne PA, Kuijper EJ, Kraneveld EA, van Loveren C, Raber-Durlacher JE. 2012. Oral bacteria and yeasts in relationship to oral ulcerations in hematopoietic stem cell transplant recipients. Support Care Cancer. 20(12):3231–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye SA, Haddad RI, Cilli F, Sonis ST, Colevas AD, Brennan MT, Hu KS, Murphy BA. 2013. Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer. 119(24):4268–4276. [DOI] [PubMed] [Google Scholar]
- McGuire DB, Fulton JS, Park J, Brown CG, Correa ME, Eilers J, Elad S, Gibson F, Oberle-Edwards LK, Bowen J, et al. 2013. Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer. 21(11):3165–3177. [DOI] [PubMed] [Google Scholar]
- Napenas JJ, Brennan MT, Coleman S, Kent ML, Noll J, Frenette G, Nussbaum ML, Mougeot JL, Paster BJ, Lockhart PB, et al. 2010. Molecular methodology to assess the impact of cancer chemotherapy on the oral bacterial flora: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 109(4):554–560. [DOI] [PubMed] [Google Scholar]
- Nonzee NJ, Dandade NA, Patel U, Markossian T, Agulnik M, Argiris A, Patel JD, Kern RC, Munshi HG, Calhoun EA, et al. 2008. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer. 113(6):1446–1452. [DOI] [PubMed] [Google Scholar]
- Pedroso SH, Vieira AT, Bastos RW, Oliveira JS, Cartelle CT, Arantes RM, Soares PM, Generoso SV, Cardoso VN, Teixeira MM, et al. 2015. Evaluation of mucositis induced by irinotecan after microbial colonization in germ-free mice. Microbiology. 161(10):1950–1960. [DOI] [PubMed] [Google Scholar]
- Peterson DE. 1990. Pretreatment strategies for infection prevention in chemotherapy patients. NCI Monogr. 9(9):61–71. [PubMed] [Google Scholar]
- Peterson DE, Minah GE, Overholser CD, Suzuki JB, DePaola LG, Stansbury DM, Williams LT, Schimpff SC. 1987. Microbiology of acute periodontal infection in myelosuppressed cancer patients. J Clin Oncol. 5(9):1461–1468. [DOI] [PubMed] [Google Scholar]
- Reiff C, Delday M, Rucklidge G, Reid M, Duncan G, Wohlgemuth S, Hormannsperger G, Loh G, Blaut M, Collie-Duguid E, et al. 2009. Balancing inflammatory, lipid, and xenobiotic signaling pathways by VSL#3, a biotherapeutic agent, in the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 15(11):1721–1736. [DOI] [PubMed] [Google Scholar]
- Reynolds MA, Minah GE, Peterson DE, Weikel DS, Williams LT, Overholser CD, DePaola LG, Suzuki JB. 1989. Periodontal disease and oral microbial successions during myelosuppressive cancer chemotherapy. J Clin Periodontol. 16(3):185–189. [DOI] [PubMed] [Google Scholar]
- Ruescher TJ, Sodeifi A, Scrivani SJ, Kaban LB, Sonis ST. 1998. The impact of mucositis on alpha-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer. 82(11):2275–2281. [PubMed] [Google Scholar]
- Sharma A, Rath GK, Chaudhary SP, Thakar A, Mohanti BK, Bahadur S. 2012. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: a randomized double-blind placebo-controlled study. Eur J Cancer. 48(6):875–881. [DOI] [PubMed] [Google Scholar]
- Sonis ST. 2004. The pathobiology of mucositis. Nat Rev Cancer. 4(4):277–284. [DOI] [PubMed] [Google Scholar]
- Sonis ST. 2009. Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 45(12):1015–1020. [DOI] [PubMed] [Google Scholar]
- Sonis ST. 2010. New thoughts on the initiation of mucositis. Oral Dis. 16(7):597–600. [DOI] [PubMed] [Google Scholar]
- Sonis ST. 2011. Oral mucositis. Anticancer Drugs. 22(7):607–612. [DOI] [PubMed] [Google Scholar]
- Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB, et al. 2004. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 100(9 Suppl):1995–2025. [DOI] [PubMed] [Google Scholar]
- Spijkervet FK, Van Saene HK, Van Saene JJ, Panders AK, Vermey A, Mehta DM, Fidler V. 1991. Effect of selective elimination of the oral flora on mucositis in irradiated head and neck cancer patients. J Surg Oncol. 46(3):167–173. [DOI] [PubMed] [Google Scholar]
- Srikrishna G, Freeze HH. 2009. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 11(7):615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokman MA, Spijkervet FK, Burlage FR, Dijkstra PU, Manson WL, de Vries EG, Roodenburg JL. 2003. Oral mucositis and selective elimination of oral flora in head and neck cancer patients receiving radiotherapy: a double-blind randomised clinical trial. Br J Cancer. 88(7):1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer AM. 2013. Interaction between host cells and microbes in chemotherapy-induced mucositis. Nutrients. 5(5):1488–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer AM, Logan RM. 2015. The role of oral flora in the developmentof chemotherapy-induced oral mucositis. J Oral Pathol Med. 44(2):81–87. [DOI] [PubMed] [Google Scholar]
- Sturm A, Rilling K, Baumgart DC, Gargas K, Abou-Ghazale T, Raupach B, Eckert J, Schumann RR, Enders C, Sonnenborn U, et al. 2005. Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling. 73(3):1452–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin KA, Socci ND, Viale A, et al. 2012. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 55(7):905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CM, Versalovic J. 2010. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes. 1(3):148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetiere MF. 2014. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—current evidence and potential clinical applications. Aliment Pharmacol Ther. 40(5):409–421. [DOI] [PubMed] [Google Scholar]
- van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. 2010. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 6(5):e1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoecke B, De Ryck T, Stringer A, Van de, Wiele T, Keefe D. 2015. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis. 21(1):17–30. [DOI] [PubMed] [Google Scholar]
- Villa A, Sonis ST. 2015. Mucositis: pathobiology and management. Curr Opin Oncol. 27(3):159–164. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou X, Xu X. 2013. Oral microbiota: an overlooked etiology for chemotherapy-induced oral mucositis? J Formos Med Assoc. 114(4):297–299. [DOI] [PubMed] [Google Scholar]
- Wijers OB, Levendag PC, Harms ER, Gan-Teng AM, Schmitz PI, Hendriks WD, Wilims EB, van der Est H, Visch LL. 2001. Mucositis reduction by selective elimination of oral flora in irradiated cancers of the head and neck: a placebo-controlled double-blind randomized study. Int J Radiat Oncol Biol Phys. 50(2):343–352. [DOI] [PubMed] [Google Scholar]
- Yoneda S, Imai S, Hanada N, Yamazaki T, Senpuku H, Ota Y, Uematsu H. 2007. Effects of oral care on development of oral mucositis and microorganisms in patients with esophageal cancer. Jpn J Infect Dis. 60(1):23–28. [PubMed] [Google Scholar]