Abstract
Cementum is a mineralized tissue covering the tooth root that functions in tooth attachment and posteruptive adjustment of tooth position. During formation of the apically located cellular cementum, some cementoblasts become embedded in the cementoid matrix and become cementocytes. As apparently terminally differentiated cells embedded in a mineralized extracellular matrix, cementocytes are part of a select group of specialized cells, also including osteocytes, hypertrophic chondrocytes, and odontoblasts. The differentiation and potential function(s) of cementocytes are virtually unknown, and the question may be posed whether the cementocyte is a dynamic actor in cementum in comparable fashion with the osteocyte in the skeleton, responding to changing tooth functions and endocrine signals and actively directing local cementum metabolism. This review summarizes the literature with regard to cementocytes, comparing them to their closest “cousins,” the osteocytes, where insights gained from osteocyte studies serve to inform the critical examination of cementocytes. The review identifies important unanswered questions about these cells regarding their origins, differentiation, morphology and lacuno-canalicular system, selective markers, and potential functions.
Keywords: cementum, bone, extracellular matrix, periodontium, odontogenesis, cementoblasts
Introduction
Cementum is a mineralized tissue covering tooth root dentin. Two major types of cementum exist in human teeth, distinct in location and function (Foster and Somerman 2012). Acellular cementum (acellular extrinsic fiber cementum, or primary cementum) is located on the cervical portions of roots and anchors the primary collagen fibers of the periodontal ligament (PDL), providing a strong attachment between the tooth and surrounding alveolar bone. Cellular cementum (cellular intrinsic fiber cementum, or secondary cementum) is located on apical roots and in furcation regions and has a role in posteruptive adjustment of tooth position. During formation of cellular cementum, cementoblasts secrete a layer of unmineralized extracellular matrix (ECM), the cementoid. As cementoid deposition progresses, some cementoblasts are embedded in the ECM and become cementocytes. While similar to bone in composition, cementum is avascular and does not undergo physiological remodeling but grows by continuous apposition throughout life.
As apparently terminally differentiated cells embedded in a mineralized ECM, cementocytes are part of a select group of specialized cells, also including osteocytes, hypertrophic chondrocytes, and odontoblasts. Although osteocytes comprise more than 95% of skeletal cells, they were long thought to be passive residents. More than 2 decades of intense study of osteocytes in vivo and in vitro overturned this paradigm, revealing that osteocytes modulate bone homeostasis and remodeling, act as mechanosensors, and direct endocrine regulation of mineral metabolism (as summarized in Bonewald 2011; Dallas et al. 2013). The question may be posed whether the cementocyte is a dynamic actor in cementum in comparable fashion with the osteocyte in the skeleton, responding to changing tooth functions and endocrine signals, as well as actively directing local cementum metabolism.
The aim of this review is not only to summarize the literature to date with regard to cementocytes but also to identify important unanswered questions about these cells. These fundamental questions are reflected in the organization of the review, addressing the origin and differentiation process of cementocytes, their lacuno-canalicular system allowing cell-cell communication, selective markers expressed, and what potential functions the cells may have. As the closest “cousins” to cementocytes, osteocytes will serve as relevant comparisons, and insights gained from osteocyte studies will serve to inform the critical examination of cementocytes.
Where Do Cementocytes Come from and How Do They Differentiate?
Osteoblasts come from mesodermal or ectomesenchymal (in the craniofacial region) progenitors. Following their differentiation and secretory activity, osteoblasts have 3 potential fates: becoming bone lining cells, undergoing apoptosis, or transitioning to osteocytes within the bone. The process of osteocyte formation, or osteocytogenesis, is an active transition that involves not only the embedding of an osteoblast in the ECM but also changes in cell morphology and cytoskeletal organization, formation of dendritic processes and connection to other cells to make a lacuno-canalicular network, changes in gene expression, local ECM remodeling, and regulation of ECM mineralization (Bonewald 2011; Dallas et al. 2013).
The origin of cementoblasts remains controversial, with a classic hypothesis pointing to ectomesenchymal cells of the dental follicle and a more recently proposed alternative hypothesis suggesting that Hertwig’s epithelial root sheath (HERS) cells undergo epithelial-mesenchymal transformation and contribute to the pool of cementoblasts (Bosshardt 2005; Foster and Somerman 2012). Despite this uncertainty, it is widely assumed that cementoblasts producing the cellular cementum become the cementocytes embedded in the ECM. Morphological studies of cementoblasts of the cellular cementum describe large, cuboidal cells with euchromatin-rich nuclei (suggesting active gene transcription) and abundant rough endoplasmic reticulum (ER) and Golgi complex (suggesting active protein synthesis) (Furseth 1969; Jande and Bélanger 1970; Bosshardt and Schroeder 1990, 1992). Notably, this description is entirely consistent with osteoblasts. Some studies have pointed out differences in cementoblasts versus osteoblasts in vitro. For example, Grzesik et al. (2000) noted differences in the composition and organization of ECM produced by human cementum-associated cells versus bone marrow stromal-derived osteoblasts in an ex vivo ossicle transplant model. However, direct comparison of the regulation and differentiation of cementoblasts and osteoblasts in vivo, as well as composition of their respective matrices, reveals they have much in common (Bosshardt 2005). For example, common elements in the differentiation of osteoblasts and cellular cementum cementoblasts are indicated by effects of gene mutation and knockout of key osteoblast regulators such as Runx2 and Osterix (Camilleri and McDonald 2006; Cao et al. 2012).
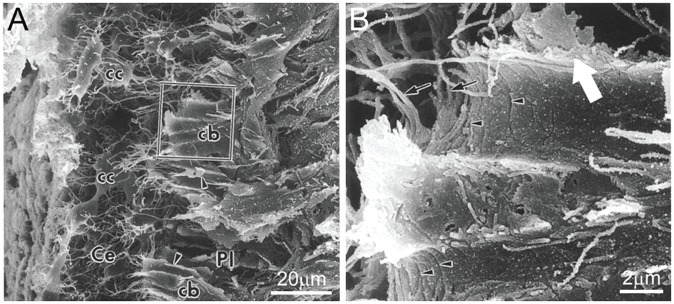
The process of cementocytogenesis is not well understood, and to date, there have been no detailed studies to define the molecular sequence of events. Entrapment of cementoblasts within the cementoid has been associated with the rapid rate and multipolar mode of ECM secretion during cellular cementum formation (Bosshardt and Schroeder 1990, 1992). In some of the few studies considering cementocytogenesis, cementoblasts near the cementoid surface that appear to be precementocytes are described as having wing-like cell processes that may have a secretory function and that transition into more slender and branching cementocyte processes upon engulfment (Fig. 1) (Yamamoto and Hinrichsen 1993; Yamamoto et al. 1996). Cellular changes in newly embedded cementoid cementoblasts parallel those observed in osteoid osteocytes—namely, alteration of cell shape and size, as well as decrease in cytoplasmic organelles indicative of declining secretory activity (Frank and Steuer 1977; Yamamoto et al. 1996). With aging, further changes are reported in more deeply buried cementocytes, including decreases in cell size, nucleus, and organelles; increased numbers of vacuoles and lysosomes; and less endocytic activity (Furseth 1967, 1969; Jande and Bélanger 1970; Ayasaka et al. 1992). Signs of cell stress can be observed in some deeply buried cementocytes, including hyperchromatic nuclei, large vacuoles, and disrupted mitochondria. Empty lacunae (and some featuring nuclear remnants or fibrillary materials) are observed in deep cellular cementum, suggesting cell death via an unknown mechanism. The life span of an osteocyte is thought to be dictated by the rate of bone turnover and, as such, may continue for decades. In cellular cementum, where there is no significant physiological remodeling, cementocytes are embedded for life and, with time and continued cementum apposition, may exceed the distance for effective nutrient exchange.
Figure 1.
Cementocytogenesis. (A) Scanning electron micrograph showing cementoblasts (cb) at the border of cellular cementum (Ce) and the periodontal ligament (Pl). Once cementoblasts are embedded in the cementum extracellular matrix, they transition to cementocytes (cc), with smaller cell size and numerous dendrites. The boxed cementoblast in (A) is shown at higher magnification in (B), where wing-like cell processes (large white arrow) are evident and may be involved with protein secretion. Other visible features include segmentations (arrowheads) and finger-like processes (small arrows). Panels A and B are adapted and reproduced with permission from Yamamoto et al., Anat Embryol, 1996, copyright Springer International Publisher.
In summary, cementocyte differentiation remains poorly understood, with no detailed insights on underlying mechanisms and with competing hypotheses about the cell lineage giving rise to the cementoblasts of the cellular cementum.
Do Cementocytes Communicate with Each Other and Surface Cells?
A defining feature of osteocytes that allows them to survive while entombed within the mineralized ECM is their complex dendritic network (Fig. 2) that branches out through a lacuno-canalicular system (Bonewald 2011; Dallas et al. 2013). Osteocyte cell processes (dendrites) extend through tiny tunnels (canaliculi) that connect the cells to each other (to communicate), to the vasculature (to receive nutrients and send/receive endocrine signals), and to the bone surface (to exchange signals with external cells). The network is filled with a canalicular fluid thought to provide oxygen and nutrients. Importantly, the fluid flow produces shear stress and potentially hydrostatic pressure within the network, providing mechanical cues from bone loading and unloading; therefore, osteocytes function as mechanosensory cells based on this feedback system.
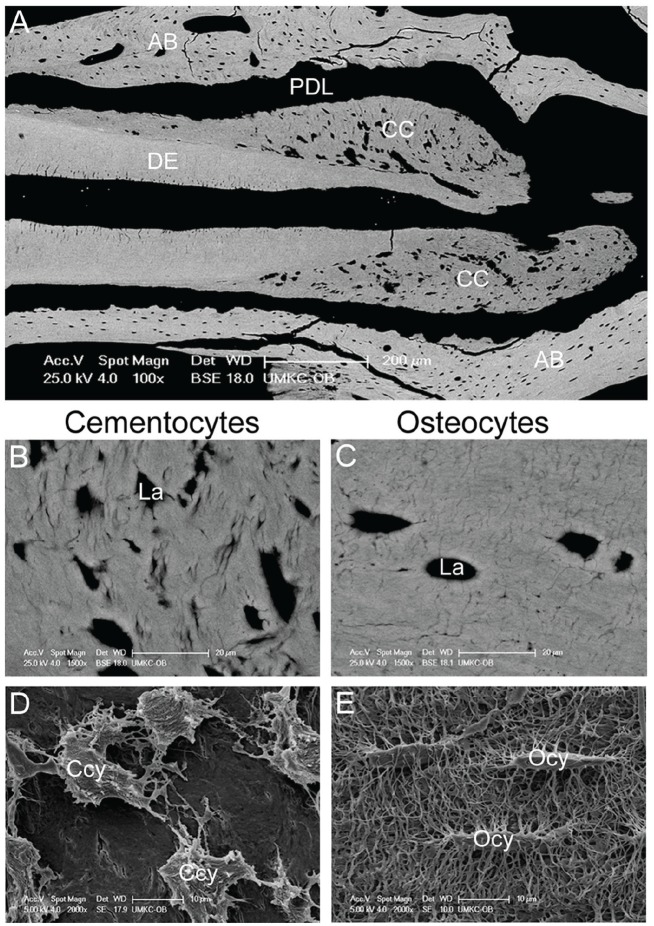
Figure 2.
The cementocyte and its lacuno-canalicular network. (A) Backscatter scanning electron microscopy (SEM) showing a mouse molar tooth featuring numerous cementocyte lacunae within the cellular cementum (CC) surrounding the dentin (DE). The surrounding alveolar bone (AB) exhibits typical osteocyte lacunae. PDL, periodontal ligament. (B) Cementocyte lacunae (La) are more irregular in size and shape than (C) the typical ellipsoid osteocyte lacunae. (D, E) SEM of acid-etched resin-embedded mouse mandible allows for visualization of resin-filled lacunae in cellular cementum and alveolar bone. Note that cementocytes exhibit larger and more irregularly shaped lacunae and fewer canaliculi compared with osteocytes. Ccy, cementocytes; Ocy, osteocytes. Panels A to E are adapted and reproduced with permission from Zhao et al., J Bone Mineral Res, 2016, copyright John Wiley & Sons.
Cementocytes also occupy lacunae and display dendrites within a lacuno-canalicular network (Fig. 2), based on studies of cellular cementum in mice (Cao et al. 2012; Zhao et al. 2016), rats (Ayasaka et al. 1992; Yamamoto and Hinrichsen 1993; Yamamoto et al. 1996; Kagayama et al. 1997), and humans (Bosshardt and Schroeder 1992; Scivetti et al. 2007). This indicates that, like osteocytes, they are adapted to reside within a mineralized ECM and are potentially capable of communicating with one another and with outside cells. However, studies to date offer important distinctions between cementocytes and osteocytes, in terms of dendrites, lacunae, and canaliculi. Three-dimensional analysis of human cementocytes by confocal laser scanning microscopy (Scivetti et al. 2007) indicates an average cell body length (9–17 µm) similar to that estimated for chick calvarial osteocytes (10–15 µm), although the number of dendrites per cell differs significantly between the cell types. Whereas cementocytes feature about 8 to 20 dendrites per cell, osteocytes extend an estimated number of dendrites from 40 to more than 100, depending on species (Beno et al. 2006; Dallas et al. 2013).
While osteocyte lacunae feature a regular ellipsoid shape, cementocyte lacunae are described as larger and more irregular in size and shape (from oval to tubular) and with rougher walls (Fig. 2A–C) (Kagayama et al. 1997; Zhao et al. 2016). Occasionally, lacunae containing more than 1 cell are observed in cellular cementum, an event not typically observed in osteocyte lacunae (Jande and Bélanger 1970; Zhao et al. 2016). However, there is some suggestion that epithelial rests of Malassez (ERM), a network of cell remnants from fenestration of HERS, may occasionally be incorporated into cellular cementum, although morphology is distinct from cementocytes (Lester 1969; Jande and Bélanger 1970; Frank and Steuer 1977; Yamamoto and Hinrichsen 1993).
In agreement with cementocytes harboring fewer dendrites than osteocytes, their canalicular network is also much less dense and appears less organized, leading to areas of cementum sparsely populated with canaliculi (Figure 2D, E) (Kagayama et al. 1997; Zhao et al. 2016). Cementocyte dendrites have been reported to be predominantly directed toward the cementum surface (rather than toward the dentin), but orientation and plane of sectioning must be kept in mind when considering this 3-dimensional arrangement (Bosshardt and Selvig 1997). Osteocyte dendrites feature gap junctions, transmembrane intercellular channels allowing exchange of small signaling molecules between adjacent cells. Gap junctions have been shown to be present between the cytoplasmic processes of adjacent cementocytes (Frank and Steuer 1977). While gap junctions indicate the potential for interconnectivity and cell-cell communication, nothing is known of the molecular components (e.g., specific connexin proteins) present in cementocytes.
An important question is whether the lacuno-canalicular network is actually functional for cementocytes to maintain their connectivity, in order to receive and send signals. Key studies have provided important insights. In vivo intravenous injection of microperoxidase tracer into rats confirmed a functional transport pathway into cementum (Ayasaka et al. 1992). Whereas osteocyte lacunae exhibited homogeneous tracer distribution, the pericellular spaces around cementocytes were disproportionately labeled. Interestingly, lacunae of deeply buried cementocytes were more consistently positive than those of superficial cells, although tracer was detected intracellularly in most cementocytes regardless of position. While this study suggests an uneven circulation within the cementocyte lacuno-canalicular system, it does indicate the existence of a cementum fluid equivalent to canalicular fluid in bone and confirms that cementocytes can remain connected, even in the deep cementum. Fluorochrome labeling of rat cementocyte lacuno-canalicular systems (Kagayama et al. 1997) and fluorescein isothiocyanate (FITC) penetration into deep cementocyte lacunae and canaliculi in extracted human teeth (Suda et al. 1989) also support these findings.
In summary, cementocytes feature dendritic processes and a lacuno-canalicular network, making an osteocyte-like network of communication and mechanosensory function possible. However, irregular cell spacing and lacunar shape, as well as fewer dendritic connections compared with osteocytes, suggest fundamental differences in how this network develops and/or functions. Does this arrangement suggest less communication between, to, and from cementocytes compared with osteocytes? It is worth noting that these differences may reflect physiological differences in bone versus cementum. Bone is highly vascular and continuously remodeling, and we now know that osteocytes play a central role in orchestrating these processes. On the other hand, cellular cementum is avascular and grows by apposition, with no apparent physiological turnover. In this context, cementocytes are not so densely connected to the vasculature and may not require a high degree of interconnectivity with one another to function.
Are There Selective Markers for Cementocytes?
In osteocytogenesis, cells transition through several stages, including osteoid osteocyte, mineralizing osteocyte, and mature osteocyte (Bonewald 2011; Dallas et al. 2013). During each stage, cells alter expression of key functionally important genes, and these serve as useful markers to define cells and understand their biology. Osteoid osteocytes express E11, which functions in dendrite development, and membrane type 1 matrix metalloproteinase (MT1-MMP), which functions in ECM remodeling to develop the lacuno-canalicular system, as well as mineralization regulators phosphate regulating endopeptidase homolog, X-linked (PHEX), and matrix extracellular phosphoglycoprotein (MEPE). Mineralizing osteocytes increase expression of the ECM protein, dentin matrix protein 1 (DMP1). Mature osteocytes are marked by Wnt signaling regulator sclerostin, endocrine signal fibroblast growth factor 23 (FGF23), and oxygen-related protein ORP150, a cell survival factor.
The expression and functional importance for most of these osteocyte markers have not been well explored in cementocytes in vivo. A key development in understanding cementocyte biology was the recent establishment of the first immortalized cementocyte cell line, IDG-CM6 (Zhao et al. 2016). Cells from the cellular cementum were harvested from Immortomouse+/–; Dmp1-GFP+/– mice, followed by the use of interferon (IFN)–γ to induce immortalization by activating the IFN-γ promoter to drive expression of a thermolabile large T antigen. The same strategy was used to establish an immortalized osteocyte line, IDG-SW3 (Woo et al. 2011). The expression profile of IDG-CM6 cementocytes was consistent with observed patterns in vivo and paralleled IDG-SW3 cells for key osteocyte markers, including Dmp1/DMP1, E11/gp38, and Sost/sclerostin.
DMP1 was initially discovered in dentin but was later found to be more widely expressed and most highly expressed in osteocytes. DMP1 is a secreted ECM phosphoprotein that may regulate mineralization. DMP1 is expressed in mouse cementocytes in vivo and in vitro (Foster 2012; Zhao et al. 2016). In macaques, DMP1 was immunolocalized to cementocyte lacunae (Sawada et al. 2012). In hypophosphatemic Dmp1 null mice, cellular cementum was reduced and hypomineralized, cementocyte morphology was altered, and the lacuno-canalicular system was abnormal (Fig. 3), all changes that paralleled those observed in osteocytes and bone (Ye et al. 2008). Hyp mutant mice, harboring a mutation in another early osteocyte marker, Phex, also exhibit hypophosphatemic rickets and similar cellular cementum phenotype compared with Dmp1 null mice (Foster et al. 2014).
Figure 3.
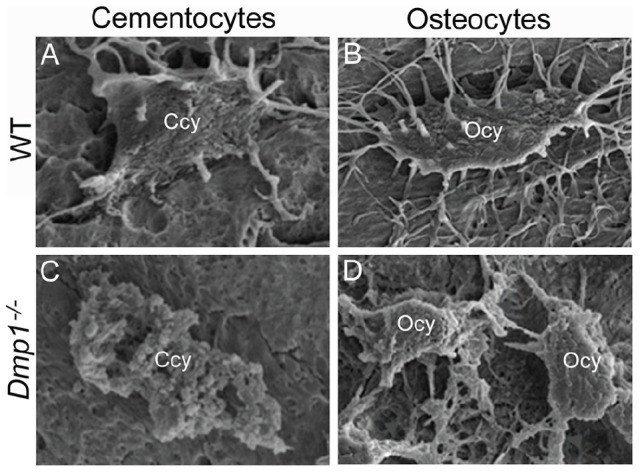
Defective cementocytes and osteocytes in Dmp1 null mice. Compared with normal wild-type (WT) (A) cementocytes (Ccy) and (B) osteocytes (Ocy), with typical cell morphology and numerous dendritic processes, Dmp1–/– mice feature defective cell morphology and deformation and reduced numbers of dendrites in both (C) cementocytes and (D) osteocytes, suggesting conservation of physiologic function between the cells. Panels A to D adapted and reproduced with permission from Ye et al., J Dent Res, 2008, copyright Sage Publications.
E11/gp38/podoplanin is an early osteocyte-selective protein thought to play a role in dendrite formation (Wetterwald et al. 1996; Schulze et al. 1999). E11 protein has been identified in rat cementocytes (Tenorio et al. 1993). In mouse cementocytes, E11 messenger RNA (mRNA) is expressed in vivo, and E11 protein localizes to cementoid cementoblasts (Zhao et al. 2016). IDG-CM6 cells exhibit peak E11/gp38 expression at early stages, consistent with osteocytes. Deletion of MT1-MMP, another early osteocyte marker, results in reduced and defective dendrites due to loss of ability to remodel osteoid ECM (Holmbeck et al. 2005). Conditional ablation of MT1-MMP has caused abnormal cellular cementum morphology and distribution (Xu et al. 2016).
Sclerostin/SOST is expressed by mature osteocytes and negatively regulates bone formation by antagonizing canonical Wnt signaling in osteoblasts. Cementocytes express Sost/sclerostin in vivo and in vitro (van Bezooijen et al. 2009; Jäger et al. 2010; Lehnen et al. 2012; Zhao et al. 2016), suggesting they may regulate cementoblast activity. Deletion of Sost in mice resulted in 1.5-fold increased deposition of cellular cementum (Fig. 4), in parallel to increased alveolar bone deposition (Kuchler et al. 2014).
Figure 4.
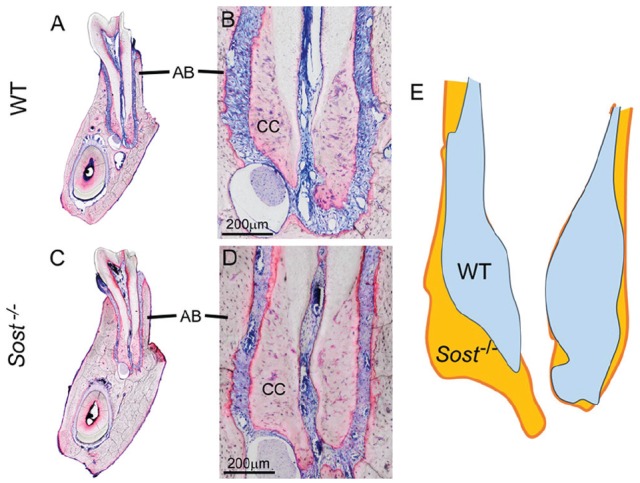
Increased cellular cementum and bone in Sost null mice. Compared with (A, B) normal cellular cementum (CC) and alveolar bone (AB), (C, D) Sost–/– mouse molar teeth at 4 mo exhibit 1.5-fold significantly greater cellular cementum area (including lingual and buccal aspects combined), paralleling increased size of alveolar bone. (E) The 1.5-fold significantly greater cellular cementum area is shown in an overlay of Sost–/– (orange) and wild-type (WT) (blue) cellular cementum profiles. Panels A to D adapted and reproduced with permission from Kuchler et al., Int J Oral Sci, 2014.
The compositions of cellular cementum and bone ECM have been previously reviewed in detail (Bosshardt 2005; Foster and Somerman 2012) and present overwhelmingly similar profiles of collagens, proteoglycans, phosphoproteins, and other components. Some factors have been described as cementum selective or enriched compared with bone (Salmon et al. 2013), but these so far have not provided novel insights into the physiology of cellular cementum versus bone in vivo or clues about the origin or functions of cementocytes. Primary markers for cementoblasts and cementum, including collagen type I, bone sialoprotein (BSP), osteopontin (OPN), and tissue nonspecific alkaline phosphatase (TNAP), are also expressed in osteoblasts and bone. These types of early, synthetic ECM factors are downregulated during osteocytogenesis (Dallas et al. 2013), but little is known of their expression during cementocytogenesis or in cementocytes. ECM proteins, including BSP, OPN, and others, may be involved in directing mineralization and responsible for differences in hydroxyapatite crystal habit (size and shape) between the 2 (McKee et al. 2013).
In summary, cementocytes in vivo and in vitro express key markers known to be important in osteocyte differentiation, including Dmp1/DMP1, E11/gp38, and Sost/sclerostin. Loss-of-function studies in mice produced cellular cementum phenotypes resembling those in bone, supporting that these factors operate in similar fashion in the 2 tissues. However, until the functions of cementocytes are better understood, it remains unclear whether cementocytes play a causative role in the observed phenotypes.
What Are the Functions (if Any) for Cementocytes?
Osteocytes have critical functions at both systemic and local levels (Dallas et al. 2013). Osteocytes express regulators of systemic mineral metabolism, including DMP1, sclerostin, MEPE, PHEX, and FGF23. Loss of function of DMP1 or PHEX causes hypophosphatemic rickets in humans and mice, in part through upregulation of FGF23, an endocrine factor highly expressed by osteocytes that controls systemic phosphate metabolism (Foster et al. 2014).
Cementocytes in rats have also been reported to express FGF23 (Yoshiko et al. 2007). Are cementocytes, like osteocytes, linked into the endocrine system and able to receive and respond to signals or even secrete endocrine factors themselves? While some work has been done on cementoblast receptors, little is known about receptors expressed by cementocytes. However, cementocytes are reported to express receptors for endothelins, neurokinins, and calcitonin gene-related peptide 1 (Vandevska-Radunovic et al. 2003; Neuhaus and Byers 2007). It remains unclear whether cementocytes play any role in directing systemic mineral metabolism and seems unlikely that they could have a significant effect in comparison to the multitudes of osteocytes expressing FGF23 and other factors.
There is increasing evidence for the importance for local regulation of bone mineralization through direct actions of factors including OPN, DMP1, regulators of pyrophosphate, and others (McKee et al. 2013; Yuan et al. 2014). Osteocytes have been shown to direct mineralization and remodeling of their perilacunar bone (Bonewald 2011; Dallas et al. 2013). Like osteocytes in vivo and the IDG-SW3 cell line, IDG-CM6 cementocytes express TNAP and promote mineralization in vitro, supporting in vivo studies where vital dye labeling of cementocyte lacunae and canaliculi suggested their participation in secondary calcification of cementoid matrix (Kagayama et al. 1997). In parallel to bone osteocytes, cellular cementum exhibits delayed and reduced mineralization with altered cementocytes in Dmp1 null mice (Fig. 3) (Ye et al. 2008; Foster et al. 2014).
A small number of studies suggest that cementocytes participate in “cementolysis,” or resorption of perilacunar cementum. Administration of parathyroid extract or low-calcium and/or low–vitamin D diets to rats resulted in apparent cementolysis and disturbed cellular cementum mineralization (Belanger 1968; Rasmussen 1977; Bielaczyc and Gołebiewska 1997). Osteocytes perform perilacunar remodeling under mineral stress (e.g., lactation, hibernation) or pathological conditions (e.g., rickets). In support of this concept, osteocytes can express osteoclastic factors such as tartrate-resistant acid phosphatase and cathepsin K, and lacunar area increases and decreases in size in response to stimuli (Qing et al. 2012; Dallas et al. 2013).
Osteocytes are mechanosensory cells responsive to changes in bone loading by virtue of their location within the bone, interconnected dendritic processes, and ability to sense and respond to hydrostatic changes in fluid flow within the lacuno-canalicular system. Osteocyte mechanotransduction is strongly influenced by the material properties of the perilacunar bone, which surrounds the cell body and dendrites (Stern and Nicolella 2013). Perilacunar bone is hypomineralized (compared with surrounding bulk bone) and rich in ECM proteins, including OPN and DMP1 (McKee et al. 1993; Feng et al. 2006), which are responsive to mechanical loading and thought to regulate perilacunar mineralization and/or influence cell function via integrin signaling (Harris et al. 2007). The presence of proteoglycans in perilacunar bone is also thought to regulate mineralization and possibly contribute to cell membrane adhesion (Smith et al. 1997). It remains unknown whether cementocytes regulate their perilacunar cementum composition or mineralization in a similar fashion and whether these affect potential mechanotransduction functions of the cells.
In response to changes in bone loading, osteocytes direct osteoblastic and osteoclastic activities at the bone surface to orchestrate local bone remodeling (Bonewald 2011; Dallas et al. 2013). Osteoclast differentiation and activity are promoted by the cytokine, receptor activator of nuclear factor κB ligand (RANKL), and inhibited by the decoy receptor, osteoprotegerin (OPG). Conditional ablation of Tnfs11 (gene for RANKL) in osteocytes significantly decreased osteoclast numbers and increased bone mass, a key finding demonstrating the significant role of osteocytes in directing local bone remodeling (Xiong et al. 2015).
In vivo regulation of RANKL and OPG expression in either cementoblasts or cementocytes has not been extensively studied. Cementum is commonly attributed an “antiosteoclast” or “antiresorptive” property because of the lack of physiological remodeling, in addition to anecdotal observations that alveolar bone is more prone to resorption in pathological conditions such as periodontitis. It is not clear whether this putative antiresorptive property resides in the tissue (e.g., an ECM component), is within the associated cells (e.g., active antiosteoclastic signaling), or is derived from some other circumstance, such as the greater distance of cementum from the vascular elements that supply osteoclast precursors.
In vitro studies indicate that cementoblasts may directly inhibit osteoclast differentiation (Boabaid et al. 2004), but additional experiments suggested cementoblasts may recruit osteoclasts under proinflammatory conditions (Nemoto et al. 2006). Compared with either alveolar or long bone, cellular cementum featured significantly higher Tnfrsf11b (gene for OPG) and lower Tnfsf11a mRNA, resulting in a higher OPG/RANKL ratio that should inhibit osteoclast differentiation (Zhao et al. 2016). Furthermore, in vitro studies showed that IDG-CM6 cementocytes maintained a significantly higher OPG/RANKL ratio than IDG-SW3 osteocytes. More important, experiments subjecting cementocytes and osteocytes to fluid flow shear stress (FFSS) to mimic mechanical stimuli revealed fundamentally different responses. IDG-CM6 cells significantly increased Tnfrsf11b (no change in IDG-SW3) and significantly reduced Tnfsf11, in contrast to increased expression by IDG-SW3 cells. The higher OPG/RANKL ratio in IDG-CM6 cementocytes under FFSS suggests a potential mechanism for lack of cementum resorption observed during physiological use, as well as during orthodontic tooth movement and altered function (Kagayama et al. 1994; Walker et al. 2010). However, it must be noted that cementum is not immune to resorption, and osteoclastic activity on the cementum can be induced by heavy orthodontic forces (Cheng et al. 2010), hyperocclusion (Walker et al. 2008), or unknown etiology. The participation of cementocytes in RANKL/OPG signaling to osteoclasts at the root surface provides an opportunity for better understanding cervical versus apical patterns of root resorption.
In addition to directing osteoclastic bone resorptive activity, osteocytes signal to osteoblasts to direct bone formation, such as by modulating Wnt activity through sclerostin secretion. In parallel to osteocytes and bone, cementocytes express Sost/sclerostin, and increased cellular cementum results from ablation of Sost in mice (as described above; Fig. 4). In vitro experiments show that like osteocytes, cementocytes decrease Sost mRNA in response to FFSS (Zhao et al. 2016). This raises the intriguing question of whether cementocytes can direct new cellular cementum formation in response to mechanical stimuli. While there is no clear answer at present, there are physiological circumstances associated with changes in tooth loading and function, where cellular cementum formation is stimulated. For example, cellular cementum formation begins around the time the tooth enters occlusion, and these mechanical forces have been speculated to direct the switch from acellular to cellular cementogenesis, although other evidence such as cellular cementogenesis in the furcation prior to eruption would argue against this (Bosshardt 2005). Second, continued apposition of cellular cementum is thought to compensate for the slow attrition of enamel throughout life, maintaining proper occlusion of teeth. Increased cellular cementum production has been induced by unopposed super-eruption of mouse teeth (Walker et al. 2010), as well as in response to physiological tooth movement in rats, whereas alveolar bone has been resorbed (Kagayama et al. 1994). After small resorptions on the root surface, cellular “reparative” cementum is often formed in the course of healing, even when the site is on the cervical root (Bosshardt and Sculean 2009). In all these scenarios, it is currently unclear whether the cementocyte is an active regulator of cementum formation.
Conclusions
Fundamental questions remain on cementum biology and the potential role for cementocytes. While differences exist between cementocytes and osteocytes, there are clearly important commonalities between them (Table). However, functions of cementocytes in cementum biology remain to be demonstrated. Ultimately, the question that must be answered is whether cementocyte functions are important to cellular cementum development, homeostasis, adaptation, and/or regeneration.
Table.
Comparison of Osteocytes and Cementocytes.a
| Feature | Osteocytes | Cementocytes |
|---|---|---|
| Location | Bone | Cellular cementum of the apical portion of the tooth |
| Origin | Differentiation from mesodermal or ectomesenchymal osteoblasts embedded in the osteoid matrix | Differentiation from ectomesenchymal cementoblasts embedded in the cementoid matrix |
| Transition to mature cell type | Alteration of shape and size, development of dendritic processes, and declining secretory activity indicated by reduction in secretory organelles | Alteration of shape and size, development of dendritic processes, and declining secretory activity indicated by reduction in secretory organelles |
| Cell morphology | Ellipsoid (cortical bone) or rounded (trabecular, calvarial bone) shape with dense network of dendritic cell processes (40–100 or more) | Erratic size and shape with less dense network of dendritic cell processes (estimated at 8–20) |
| Communication | A complex lacuno-canalicular network connects osteocytes to each other, to vascular elements, and to surface cells such as osteoblasts; evidence for canalicular fluid flow in bone | A less complex lacuno-canalicular network connects cementocytes to each other and to surface cells such as cementoblasts; evidence for canalicular fluid flow in cementum |
| Markers | Stage-specific expression of E11, MT1-MMP, PHEX, MEPE, DMP1, sclerostin, FGF23, and ORP150 | Stage-specific expression of E11, PHEX, DMP1, and sclerostin (other osteocyte markers not tested to date) |
| Life span | Dictated by the rate of bone turnover in the region | Because cementum is not thought to remodel, cementocytes are embedded for life; more deeply buried cementocytes show signs of stress and degradation, and empty lacunae may indicate cell death |
| Functions | Act as mechanosensors, regulate local bone homeostasis and remodeling, and contribute to endocrine control of systemic mineral metabolism | Unknown |
See text for definitions and relevant references.
Author Contributions
N. Zhao, B.L. Foster, L.F. Bonewald, contributed to conception and design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by grant AR046758 (to L.F.B.) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH), National Natural Science Funds of China (Grant No. 81000420; to N.Z.), and grant AR066110 (NIAMS/NIH) and a seed grant from The Ohio State University College of Dentistry (to B.L.F.). The authors thank Dr. Reinhard Gruber (University of Bern, Switzerland) for supplying Sost–/– and control mouse images and Dr. Jian Q. Feng (Baylor College of Dentistry) for providing Dmp1–/– and control mouse images.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ayasaka N, Kondo T, Goto T, Kido M, Nagata E, Tanaka T. 1992. Differences in the transport systems between cementocytes and osteocytes in rats using microperoxidase as a tracer. Arch Oral Biol. 37(5):363–369. [DOI] [PubMed] [Google Scholar]
- Belanger LF. 1968. Resorption of cementum by cementocyte activity (“cementolysis”). Calcif Tissue Res. 2(3):229–236. [DOI] [PubMed] [Google Scholar]
- Beno T, Yoon YJ, Cowin SC, Fritton SP. 2006. Estimation of bone permeability using accurate microstructural measurements. J Biomech. 39(13):2378–2387. [DOI] [PubMed] [Google Scholar]
- Bielaczyc A, Gołebiewska M. 1997. Ultrastructural changes of a tooth root in young rats fed a low calcium and vitamin D–deficient diet. Rocz Akad Med Bialymst. 42(Suppl 2):153–158. [PubMed] [Google Scholar]
- Boabaid F, Berry J, Koh A, Somerman M, McCcauley L. 2004. The role of parathyroid hormone–related protein in the regulation of osteoclastogenesis by cementoblasts. J Periodontol. 75(9):1247–1254. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. 2011. The amazing osteocyte. J Bone Miner Res. 26(2):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshardt DD. 2005. Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J Dent Res. 84(5):390–406. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, Schroeder HE. 1990. Evidence for rapid multipolar and slow unipolar production of human cellular and acellular cementum matrix with intrinsic fibers. J Clin Periodontol. 17(9):663–668. [PubMed] [Google Scholar]
- Bosshardt DD, Schroeder HE. 1992. Initial formation of cellular intrinsic fiber cementum in developing human teeth: a light- and electron-microscopic study. Cell Tissue Res. 267(2):321–335. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, Sculean A. 2009. Does periodontal tissue regeneration really work? Periodontol 2000. 51:208–219. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, Selvig KA. 1997. Dental cementum: the dynamic tissue covering of the root. Periodontol 2000. 13:41–75. [DOI] [PubMed] [Google Scholar]
- Camilleri S, McDonald F. 2006. RUNX2 and dental development. Eur J Oral Sci. 114(5):361–373. [DOI] [PubMed] [Google Scholar]
- Cao Z, Zhang H, Zhou X, Han X, Ren Y, Gao T, Xiao Y, de Crombrugghe B, Somerman MJ, Feng JQ. 2012. Genetic evidence for the vital function of osterix in cementogenesis. J Bone Miner Res. 27(5):1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LL, Turk T, Elekdag-Turk S, Jones AS, Yu Y, Darendeliler MA. 2010. Repair of root resorption 4 and 8 weeks after application of continuous light and heavy forces on premolars for 4 weeks: a histology study. Am J Orthod Dentofacial Orthop. 138(6):727–734. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Prideaux M, Bonewald LF. 2013. The osteocyte: an endocrine cell . . . and more. Endocr Rev. 34(5):658–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, et al. 2006. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 38(11):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL. 2012. Methods for studying tooth root cementum by light microscopy. Int J Oral Sci. 4(3):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Nociti FH, Jr, Somerman MJ. 2014. The rachitic tooth. Endocr Rev. 35(1):1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Somerman MJ. 2012. Cementum. In: McCauley LK, Somerman MJ, editors. Mineralized tissues in oral and craniofacial science: biological principles and clinical correlates. Ames, IA: Wiley-Blackwell; p. 169–192. [Google Scholar]
- Frank RM, Steuer P. 1977. Ultrastructural study of the cellular cementum in rats [in French]. J Biol Buccale. 5(2):121–135. [PubMed] [Google Scholar]
- Furseth R. 1967. A microradiographic and electron microscopic study of the cementum of human deciduous teeth. Acta Odontol Scand. 25(6):613–645. [DOI] [PubMed] [Google Scholar]
- Furseth R. 1969. The fine structure of the cellular cementum of young human teeth. Arch Oral Biol. 14(10):1147–1158. [DOI] [PubMed] [Google Scholar]
- Grzesik W, Cheng H, Oh J, Kuznetsov S, Mankani M, Uzawa K, Robey P, Yamauchi M. 2000. Cementum-forming cells are phenotypically distinct from bone-forming cells. J Bone Miner Res. 15(1):52–59. [DOI] [PubMed] [Google Scholar]
- Harris S, Gluhak-Heinrich J, Harris M, Yang W, Bonewald L, Riha D, Rowe P, Robling A, Turner C, Feng J, et al. 2007. DMP1 and MEPE expression are elevated in osteocytes after mechanical loading in vivo: theoretical role in controlling mineral quality in the perilacunar matrix. J Musculoskelet Neuronal Interact. 7(4):313–315. [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, Chrysovergis K, Yamada S, Birkedal-Hansen H, Poole AR. 2005. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 118(Pt 1):147–156. [DOI] [PubMed] [Google Scholar]
- Jäger A, Götz W, Lossdörfer S, Rath-Deschner B. 2010. Localization of Sost/sclerostin in cementocytes in vivo and in mineralizing periodontal ligament cells in vitro. J Periodontal Res. 45(2):246–254. [DOI] [PubMed] [Google Scholar]
- Jande SS, Bélanger LF. 1970. Fine structural study of rat molar cementum. Anat Rec. 167(4):439–463. [DOI] [PubMed] [Google Scholar]
- Kagayama M, Akita H, Sasano Y, Kindaichi K. 1994. Localization of uncalcified cementum in adult rat molar roots and its relation to physiological tooth movement. Arch Oral Biol. 39(10):829–832. [DOI] [PubMed] [Google Scholar]
- Kagayama M, Sasano Y, Mizoguchi I, Takahashi I. 1997. Confocal microscopy of cementocytes and their lacunae and canaliculi in rat molars. Anat Embryol (Berl). 195(6):491–496. [DOI] [PubMed] [Google Scholar]
- Kuchler U, Schwarze UY, Dobsak T, Heimel P, Bosshardt DD, Kneissel M, Gruber R. 2014. Dental and periodontal phenotype in sclerostin knockout mice. Int J Oral Sci. 6(2):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnen SD, Götz W, Baxmann M, Jäger A. 2012. Immunohistochemical evidence for sclerostin during cementogenesis in mice. Ann Anat. 194(5):415–421. [DOI] [PubMed] [Google Scholar]
- Lester KS. 1969. The incorporation of epithelial cells by cementum. J Ultrastruct Res. 27(1):63–87. [PubMed] [Google Scholar]
- McKee MD, Farach-Carson MC, Butler WT, Hauschka PV, Nanci A. 1993. Ultrastructural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2hs-glycoprotein) proteins in rat bone. J Bone Miner Res. 8(4):485–496. [DOI] [PubMed] [Google Scholar]
- McKee MD, Hoac B, Addison WN, Barros NM, Millán JL, Chaussain C. 2013. Extracellular matrix mineralization in periodontal tissues: noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia. Periodontol 2000. 63(1):102–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto E, Darveau RP, Foster BL, Nogueira-Filho GR, Somerman MJ. 2006. Regulation of cementoblast function by P. gingivalis lipopolysaccharide via TLR2. J Dent Res. 85(8):733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus SJ, Byers MR. 2007. Endothelin receptors and endothelin-1 in developing rat teeth. Arch Oral Biol. 52(7):655–662. [DOI] [PubMed] [Google Scholar]
- Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jähn K, Kato S, Wysolmerski J, Bonewald LF. 2012. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 27(5):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P. 1977. Histologic and microradiographic observations on teeth during calcium deprivation in rats. Scand J Dent Res. 85(7):549–556. [DOI] [PubMed] [Google Scholar]
- Salmon CR, Tomazela DM, Ruiz KG, Foster BL, Paes Leme AF, Sallum EA, Somerman MJ, Nociti FH., Jr. 2013. Proteomic analysis of human dental cementum and alveolar bone. J Proteomics. 91:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada T, Ishikawa T, Shintani S, Yanagisawa T. 2012. Ultrastructural immunolocalization of dentin matrix protein 1 on Sharpey’s fibers in monkey tooth cementum. Biotech Histochem. 87(5):360–365. [DOI] [PubMed] [Google Scholar]
- Schulze E, Witt M, Kasper M, Löwik CW, Funk RH. 1999. Immunohistochemical investigations on the differentiation marker protein E11 in rat calvaria, calvaria cell culture and the osteoblastic cell line ROS 17/2.8. Histochem Cell Biol. 111(1):61–69. [DOI] [PubMed] [Google Scholar]
- Scivetti M, Pilolli GP, Corsalini M, Lucchese A, Favia G. 2007. Confocal laser scanning microscopy of human cementocytes: analysis of three-dimensional image reconstruction. Ann Anat. 189(2):169–174. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Singhrao SK, Newman GR, Waddington RJ, Embery G. 1997. A biochemical and immuno-electron microscopical analysis of chondroitin sulphate-rich proteoglycans in human alveolar bone. Histochem J. 29(1):1–9. [DOI] [PubMed] [Google Scholar]
- Stern AR, Nicolella DP. 2013. Measurement and estimation of osteocyte mechanical strain. Bone. 54(2):191–195. [DOI] [PubMed] [Google Scholar]
- Suda R, Motegi Y, Kokatsu H, Miyashita H, Hasegawa K, Tachikawa T, Yoshiki S. 1989. Study of the penetration of extrinsic tracers into exposed cementum in vitro [in Japanese]. Nippon Shishubyo Gakkai Kaishi. 31(3):849–859. [DOI] [PubMed] [Google Scholar]
- Tenorio D, Cruchley A, Hughes FJ. 1993. Immunocytochemical investigation of the rat cementoblast phenotype. J Periodontal Res. 28(6 Pt 1):411–419. [PubMed] [Google Scholar]
- van Bezooijen RL, Bronckers AL, Gortzak RA, Hogendoorn PC, van der, Wee-Pals L, Balemans W, Oostenbroek HJ, Van Hul W, Hamersma H, Dikkers FG, et al. 2009. Sclerostin in mineralized matrices and van Buchem disease. J Dent Res. 88(6):569–574. [DOI] [PubMed] [Google Scholar]
- Vandevska-Radunovic V, Fristad I, Wimalawansa SJ, Kvinnsland I. 2003. CGRP1 and NK1 receptors in postnatal, developing rat dental tissues. Eur J Oral Sci. 111(6):497–502. [DOI] [PubMed] [Google Scholar]
- Walker CG, Dangaria S, Ito Y, Luan X, Diekwisch TG. 2010. Osteopontin is required for unloading-induced osteoclast recruitment and modulation of RANKL expression during tooth drift–associated bone remodeling, but not for super-eruption. Bone. 47(6):1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CG, Ito Y, Dangaria S, Luan X, Diekwisch TG. 2008. Rankl, osteopontin, and osteoclast homeostasis in a hyperocclusion mouse model. Eur J Oral Sci. 116(4):312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterwald A, Hoffstetter W, Cecchini MG, Lanske B, Wagner C, Fleisch H, Atkinson M. 1996. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 18(2):125–132. [DOI] [PubMed] [Google Scholar]
- Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. 2011. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 26(11):2634–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, Bonewald L, Manolagas SC, O’Brien CA. 2015. Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One. 10(9):e0138189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Snider TN, Wimer HF, Yamada SS, Yang T, Holmbeck K, Foster BL. 2016. Multiple essential MT1-MMP functions in tooth root formation, dentinogenesis, and tooth eruption. Matrix Biol. 52–54:266–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Domon T, Takahashi S, Wakita M. 1996. Cellular cementogenesis in rat molars: the role of cementoblasts in the deposition of intrinsic matrix fibers of cementum proper. Anat Embryol (Berl). 193(5):495–500. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hinrichsen KV. 1993. The development of cellular cementum in rat molars, with special reference to the fiber arrangement. Anat Embryol (Berl). 188(6):537–549. [DOI] [PubMed] [Google Scholar]
- Ye L, Zhang S, Ke H, Bonewald LF, Feng JQ. 2008. Periodontal breakdown in the DMP1 null mouse model of hypophosphatemic rickets. J Dent Res. 87(7):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N. 2007. Mineralized tissue cells are a principal source of FGF23. Bone. 40(6):1565–1573. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Jiang Y, Zhao X, Sato T, Densmore M, Schüler C, Erben RG, McKee MD, Lanske B. 2014. Increased osteopontin contributes to inhibition of bone mineralization in FGF23-deficient mice. J Bone Miner Res. 29(3):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Nociti FH, Jr, Duan P, Prideaux M, Zhao H, Foster BL, Somerman MJ, Bonewald LF. 2016. Isolation and functional analysis of an immortalized murine cementocyte cell line, IDG-CM6. J Bone Mineral Res. 31(2):430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]