Abstract
Background
Ticks serve as vectors and reservoirs for a variety of bacterial, viral and protozoan pathogens affecting humans and animals. Unusual increased tick aggressiveness was observed in 2008–2009 in northeastern Greece. The aim of the study was to check ticks removed from persons during 2009 for infection with Rickettsia species.
Methods
A total of 159 ticks were removed from 147 persons who sought medical advice in a hospital. Tick identification was performed morphologically using taxonomic keys. DNA was extracted from each individual tick and a PCR assay targeting the rickettsial outer membrane protein A gene of Rickettsia spp. was applied.
Results
Most of the adult ticks (132/153, 86.3%) were Rhipicephalus sanguineus. Rickettsiae were detected in 23 of the 153 (15.0%) adult ticks. Five Rickettsiae species were identified: R. aeschlimannii, R. africae (n=6), R. massilae (4), R. monacensis (1), and Candidatus R. barbariae (1). To our knowledge, this is the first report of R. africae, R. monacensis, and Candidatus R. barbariae in Greece.
Conclusions
Several Rickettsia species were identified in ticks removed from humans in Greece, including those that are prevalent in northern and southern latitudes.
Keywords: Greece, Rickettsia, Ticks
Introduction
Ticks serve as vectors and reservoirs for a variety of bacterial, viral, and protozoan pathogens affecting humans and animals.1 Several factors such as climatic and ecological changes, bird migration and animal movements play a role on the tick population and the rate of human exposure to ticks, as well as in the emergence of a tick-borne pathogen in a previously unaffected region. In addition, the massive application of molecular tools, including next generation sequencing, enables the detection and identification of pathogens providing better knowledge about their distribution and evolution.
Several tick-borne Rickettsia species have been identified in the Mediterranean countries, including Greece. Mediterranean spotted fever cases caused by Rickettsia conorii are observed every year, especially in summer, with the brown dog tick, Rhipicephalus sanguineus, being its main vector. Additional Rickettsia species, like R. massiliae, R. monacensis, R. aeschlimannii and R. helvetica, are considered as etiological agents of Mediterranean spotted fever-like illness in the Mediterranean basin.2 Four tick-borne Rickettsiae have been currently identified in humans in Greece: R. conorii, R. aeschlimannii, R. sibirica mongolotimonae and R. slovaca, while R. massiliae and R. rhipicephali were identified only in ticks.3–9
Unusual increased tick aggressiveness was observed in summer 2008 in northeastern Greece resulting in increased number of persons bitten by ticks, and two fatal cases, one caused by R. conorii and one by Crimean-Congo hemorrhagic fever virus (the first and only report in Greece).7,10 As a result, many residents of the region sought medical advice in the emergency departments of the regional hospitals. A study conducted in the two main hospitals (in Alexandroupolis and Komotini) during July–September 2008 showed that the majority of the 537 removed ticks were Rh. sanguineus (81.5%), while Hyalomma marginatum (the main vector of Crimean-Congo hemorrhagic fever virus) accounted for 5.2%.11 Similar aggressiveness, although to a lesser extent, was observed during 2009. The aim of the present study was to check ticks removed during 2009 from persons who referred to the University General Hospital of Alexandroupolis for infection with Rickettsia species.
Materials and methods
Collection of ticks and morphological identification
During April to October 2009, 147 persons (86 males, 58.5%) aged 0.6–88 years (median age 28 years) addressed the Emergency Department of the University General Hospital of Alexandroupolis, in northeastern Greece, because of tick bites. In total, 159 ticks were removed. The approximate time from the tick bite was 1–24 h (based on the persons’ report). Ticks were stored without any preservatives at −20oC until their transport to the laboratory in Thessaloniki where they were stored at −80oC until testing. Tick identification was performed morphologically using taxonomic keys.12
Molecular methods
Ticks were homogenized individually in a Thermo FastPrep FP120 Cell Disrupter (LabCommerce Inc., San Jose, CA, USA) and DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Ticks were tested for probable infection with Rickettsia spp. using a PCR assay targeting the rickettsial outer membrane protein (ompA) gene.13 PCR products were sequenced in an 3130 ABI Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) and nucleotide sequences were compared with those available in GenBank using the National Center for Biotechnology Information (NCBI, Bethesda, MD, USA) Basic Local Alignment Sequence Tool (BLAST) search engine (http://blast.ncbi.nlm.nih.gov/blast.cgi).
Statistical methods
Differences between tick species and affected age groups were tested by χ2, while differences in the number of removed ticks per month were tested by student’s t test.
Results
All 159 ticks belonged to the Ixodidae family. Six were Rh. sanguineus larvae. Among the 153 adult ticks, 139 (90.8%) were female. Seven tick species from four genera were identified. Specifically, 132 were Rh. sanguineus (86.3%), eight Rh. bursa (5.2%), seven Rh. turanicus (4.6%), two Ixodes ricinus (1.3%), two H. marginatum (1.3%), and one each (0.65%) H. rufipes and Dermacentor marginatus (Table 1).
Table 1.
Adult ticks removed from humans in Alexandroupolis, northeastern Greece, 2008–2009
| 2008a | 2009 | |||||
|---|---|---|---|---|---|---|
| Tick | Male | Female | Total | Male | Female | Total |
| Rhipicephalus sanguineus | 1 | 218 | 219 (94.8) | 1 | 131 | 132 (86.3) |
| Rh. bursa | 1 | 3 | 4 (1.7) | 7 | 1 | 8 (5.2) |
| Rh. turanicus | 0 | 1 | 1 (0.4) | 4 | 3 | 7 (4.5) |
| Hyalomma marginatum | 4 | 2 | 6 (2.6) | 1 | 1 | 2 (1.3) |
| H. rufipes | 1 | 0 | 1 (0.4) | 0 | 1 | 1 (0.7) |
| Ixodes ricinus | 0 | 0 | 0 | 0 | 2 | 2 (1.3) |
| Dermacentor marginatus | 0 | 0 | 0 | 1 | 0 | 1 (0.7) |
| Total | 7 | 224 | 231 (100) | 14 | 139 | 153 (100) |
a Papa et al.11
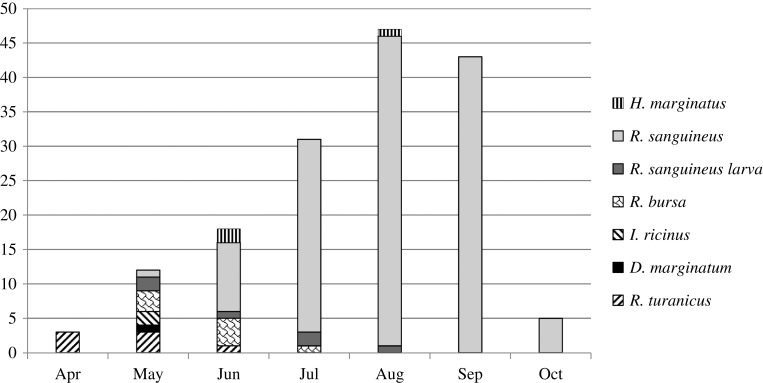
Most persons (86/147, 58.5%) were male and eight of them were bitten by more than one tick (2–3). For 123 persons the age was available; 133 ticks were removed from these persons. Most ticks (50/133, 37.6%) were removed from persons belonging to the age group of 0.1–9 years; however, the difference among groups was not significant (p>0.05) (Table 2). The number of ticks removed from humans differed significantly among months (p=0.015) (range 3–47). Most ticks were removed in August (47/159, 29.6%) and September (43/159, 27%) (Figure 1). A variety of tick species were identified during May, while the ticks removed during summer and autumn were mainly Rhipicephalus spp. (Figure 1).
Table 2.
Number and species of the ticks removed from 123 humans grouped by age, northeastern Greece, 2009
| Tick species | Age groups of humans | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | >60 | Total (%) | |
| Rhipicephalus sanguineus | 43 | 9 | 8 | 8 | 12 | 10 | 17 | 107 (80.5) |
| Rh. bursa | 0 | 0 | 1 | 2 | 1 | 1 | 3 | 8 (6.0) |
| Rh. turanicus | 2 | 2 | 0 | 1 | 2 | 0 | 0 | 7 (5.3) |
| Hyalomma marginatum | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 (1.5) |
| H. rufipes | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (0.7) |
| Ixodes ricinus | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 (0.7) |
| Dermacentor marginatus | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 (0.7) |
| Rh. sanguineus (larvae) | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 6 (4.5) |
| Total | 50 | 11 | 9 | 12 | 17 | 13 | 21 | 133 (100) |
Figure 1.
Monthly distribution of human-parasitizing ticks, northern Greece, 2009. D: Dermacentor; H: Hyalomma; I: Ixodes; R: Rhipicephalus
Rickettsia DNA was detected in 23 of the 153 (15%) adult ticks; all larvae were negative. The 23 Rickettsia spp. positive ticks were removed from 22 humans (14 males, 63.6%) with a median age of 33 years (range 1.4–88 years). Five Rickettsia species were identified: R. aeschlimannii (n=11), R. africae (6), R. massilae (4), R. monacensis (1), and Candidatus R. barbariae (1) (Table 3). The first four species are associated with human disease, while the pathogenicity of the last one remains to be elucidated.
Table 3.
Rickettsia (R.) species detected in adult ticks removed from humans, in northeastern Greece, 2009
| Tick | Female | Male | Total |
|---|---|---|---|
| n positive/n tested | n positive/n tested | n positive/n tested | |
| Rhipicephalus sanguineus | 17/131 | 0/1 | 17/132 (12.9%) |
| 11 R. aeschlimannii | |||
| 6 R. africae | |||
| Rh. bursa | 0/1 | 2/7 | 2/8 (0.25%) |
| 1 Candidatus R. barbariae | |||
| 1 R. massiliae | |||
| Rh. turanicus | 2/3 | 1/4 | 3/7 (42.8%) |
| 2 R. massiliae | 1 R. massiliae | ||
| Ixodes ricinus | 1/2 | 0 | 1/2 (50.0%) |
| 1 R. monacensis | |||
| Dermacentor marginatus | 0 | 0/1 | 0/1 |
| Hyalomma marginatum | 0/1 | 0/1 | 0/2 |
| H. rufipes | 0/1 | 0 | 0/1 |
| Total | 20/139 (14.4%) | 3/14 (21.4%) | 23/153 (15.0%) |
Specifically, R. aeschlimannii was detected in 11/131 (8.4%) Rh. sanguineus. Two groups of sequences were obtained, presenting 99.5% and 98.2% identity at nucleotide and amino acid level, respectively. One group consisted of six sequences which were identical to each other and to R. aeschlimannii strain TR/Orkun-H.aegyp85/Ankara (accession number JQ691727) obtained from an H. aegyptium in Ankara, Turkey14; the second group consisted of five sequences which were identical to each other and to R. aeschlimannii strain TR/Orkun-H.nymph88/Ankara (accession number JQ691729) obtained from an Hyalomma spp. nymph in Ankara, Turkey.14 R. africae was detected in 6/131 (4.6%) Rh. sanguineus: all sequences were identical each other, and identical to the sequence of strain Rickettsia sp. UzbekistanHA123S (AB795208) detected in H. aegyptium fed on a tortoise (Tetsudo horsfieldii) imported to Japan from Uzbekistan,15 presenting 0.4% nucleotide difference from the respective sequence of strain EgyRickHd-Qalet El-Nakhl-9 (HQ335137) detected in H. dromedarii in Egypt.16 R. massilae was detected in 3/7 (42.8%) Rh. turanicus and 1/10 (10%) Rh. bursa; sequences were identical to the respective sequence of the strain MTU5 (CP000683) isolated from Rh. turanicus collected on horses in Camargue, France.17 R. monacensis was detected in 1/3 (33%) I. ricinus, being identical to the respective sequence of the type strain IrR Munich isolated from I. ricinus collected in a city park in Munich, Germany (KT119437).18 Candidatus R. barbariae was detected in 1/10 (10%) Rh. bursa, with sequence identical to a strain detected in Rh. turanicus in Sardinia, Italy (EU272186).19
Discussion
Due to an unusual tick aggressiveness observed in northeastern Greece during 2008 and 2009, studies were conducted to identify ticks attached to persons living in this region and check them for infection with Rickettsia species. Most (132/153, 86.3%) of the adult ticks removed from humans in 2009 were Rh. sanguineus. The respective percentage in summer of 2008 in the same hospital was even higher (219/231; 94.8%)11 (Table 1). This difference is because the 2008 study started in June, following the fatal Crimean-Congo hemorrhagic fever and Mediterranean spotted fever cases, while the present study started earlier, in April, resulting in the collection of additional tick species, like I. ricinus and D. marginatus, which prefer colder weather. However, it was evident that again Rh. sanguineus was almost the only tick species removed from humans from July up to the end of the study in mid-October (Figure 1). A similar study conducted in the urban area of Istanbul showed that among 1054 removed ticks, most were H. aegyptium nymphs (50%) and I. ricinus female ticks (27%).20
Rickettsiae spp. were detected in 15.0% of the adult ticks. Apart from R. aeschlimannii and R. massilae, which have been detected previously in humans or ticks in Greece, R. africae, R. monacensis, and Candidatus R. barbariae are reported for the first time in the country. However, it has to be mentioned that R. africae has been detected in the spring of 2010 in H. marginatum larva and nymphs on migratory birds (common redstart, song thrush, and Eurasian siskin) which landed on a Greek island (Antikythira).21 Hyalomma ticks which attach as unfed larvae to migrating birds can remain up to 4 weeks on the same bird and may be transported long distances, e.g., from sub-Saharan Africa to northern Europe.21 R. africae is known to be transmitted by Amblyomma ticks in sub-Saharan Africa and the French West Indies causing African tick bite fever characterized by fever and multiple eschars.22 The detection of R. africae in 4.5% (6/132) of the Rh. sanguineus ticks attached on humans suggests that this species is already established in Greece.
None of the persons presented any symptoms of rickettsial infection. This can be explained in part by the rapid removal of the ticks (due to increased awareness), preventing infection by avoiding the inoculation of infected tick saliva. The species of the Rickettsia is also an important factor for pathogenicity. Especially for R. aeschlimannii, only one human case has been reported.5 In Spain, 35 persons bitten by R. aeschlimannii–positive ticks did not present any symptoms of spotted fever and this was attributed to the rapid removal of the ticks.23 Advanced age and immunocompromised situations are classical risk factors for severe forms of rickettsiosis.24 In the present study the median age of the persons who were bitten was 26 years (range 1.3–88 years); the oldest person was an 88-year old female bitten by Rh. bursa tick in which Candidatus R. barbariae was detected. The pathogenicity of this agent is not determined yet.19 It has been detected also in other countries, like Cyprus, Israel and Portugal (PoTiRb169).25–27 Additional Candidatus Rickettsia spp. have been described in Europe in the last 10 years, and their role for humans remains to be elucidated. A unique property of the pathogenic Rickettsiae is their affinity for vascular endothelial cells lining small and medium-sized blood vessels in humans.28 Not much information is currently available about the host and pathogen factors that enable Rickettsia to cross the species barriers and become capable of causing disease in humans. Even the known pathogenic species can cause asymptomatic infections, while agents previously considered as non human pathogens proved later to cause disease in humans.
The impact of climate on the behavior of the widely distributed Rh. sanguineus has been previously reported, when similar tick aggressiveness was observed in 2007 in southern France, where the weather in April was associated with the highest temperatures noted in the region since 1950; the authors predicted that, as a result of global warming, more pathogens transmitted by the brown dog tick may emerge in the future.29 The higher than usual summer temperatures observed in Greece in 2008 and 2009 (mainly in 2008) might affect the behavior of Rh. sanguineus.
The pathogenic potential of R. massiliae has been confirmed recently.30 In Greece, it has been isolated from R. sanguineus,8 while in Cyprus, it was identified in Rh. turanicus and Rh. sanguineus ticks collected from domestic and wild animals.26 In the present study it was detected in Rh. turanicus and Rh. bursa ticks.
H. marginatum ticks (the main vectors of Crimean-Congo hemorrhagic fever virus), accounted for only 1.2% of the ticks removed in 2009 (in 2008 the percentage was 2.6 in the same hospital, and 7.3% in the Hospital of Komotini). No Crimean-Congo hemorrhagic fever case has been reported in Greece apart from the case observed in Komotini in 2008.
Two of the 153 adult ticks, were I. ricinus, one infected by R. monacensis. This tick species was first isolated in 2002 in Germany,18 and later in other European countries.31–33 It is one of the most prevalent species in Europe, especially in relatively humid regions, and is the main vector of R. monacensis.
Limitations of the study
The ticks were collected only during April–October. This was because persons started visiting the hospital because of tick bites only in late April; the number of visits peaked in August, and declined sharply in mid October (Figure 1). This can be explained by the high activity of Rh. sanguineus ticks observed in the summer months of 2008–2009. Another limitation of the study was the testing for Rickettsia using only one target gene for PCR amplification. This was selected for better identification of the spotted fever group Rickettsia. In a previous study using two target genes (17-kDa antigen gene and ompA gene) in a clinical sample both PCRs were positive (R. conorii).7 The fact that R. conorii was not detected in the present study is likely due to its pathogenicity for ticks, since it was shown that exposure of Rh. sanguineus to R. conorii strain Malish produces lasting harmful effects on their survival.34
Conclusions
The present study shows that ticks removed from humans in northern Greece harbored several Rickettsia species, including those that are prevalent in northern and southern latitudes, like R. monacensis and R. africae, respectively. Billions of birds, many of them tick-infested, migrate annually between Europe and Africa, with the potential to disseminate several tick-borne pathogens, including rickettsiae. This fact, combined with climatic and ecological changes, plays a role in the geographic distribution of the Rickettsia species. Further studies in ticks and in patients with symptoms resembling rickettsiosis will enable the better understanding of the epidemiology and pathogenicity of rickettsiae in Greece and worldwide.
Acknowledgments
Authors’ contributions: AP designed the study, evaluated the results and wrote the paper; KX performed the molecular testing; TK analysed the data and contributed in the writing; MP and EM undertook the work in the hospital; SS and IC performed the identification of the ticks; all authors read and approved the manuscript. AP is the guarantor of the paper.
Funding: This work was partially supported by the EU FP7 Programme ANTIGONE (project number 278976).
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Heyman P, Cochez C, Hofhuis A et al. A clear and present danger:tick-borne diseases in Europe. Expert Rev Anti Infect Ther 2010;8:33–50. [DOI] [PubMed] [Google Scholar]
- 2.Portillo A, Santibanez S, Garcia-Alvarez L et al. Rickettsioses in Europe. Microbe Infect 2015;17:834–8. [DOI] [PubMed] [Google Scholar]
- 3.Psaroulaki A, Germanakis A, Gikas A et al. First isolation and genotypic identification of Rickettsia conorii Malish 7 from a patient in Greece. Eur J Clin Microbiol Infect Dis 2005;24:297–8. [DOI] [PubMed] [Google Scholar]
- 4.Psaroulaki A, Germanakis A, Gikas A et al. Simultaneous detection of “Rickettsia mongolotimonae” in a patient and in a tick in Greece. J Clin Microbiol 2005;43:3558–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Germanakis A, Chochlakis D, Angelakis E et al. Rickettsia aeschlimannii infection in a man, Greece. Emerg Infect Dis 2013;19:1176–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostopoulou V, Chochlakis D, Kanta C et al. A case of human infection by Rickettsia slovaca in Greece. Jpn J Infect Dis 2015; DOI:10.7883/yoken.JJID.2015.194. [DOI] [PubMed] [Google Scholar]
- 7.Papa A, Dalla V, Petala A et al. Fatal Mediterranean spotted fever in Greece. Clin Microbiol Infect 2010;16:589–92. [DOI] [PubMed] [Google Scholar]
- 8.Babalis T, Tselentis Y, Roux V et al. Isolation and identification of a rickettsial strain related to Rickettsia massiliae in Greek ticks. Am J Trop Med Hyg 1994;50:365–72. [DOI] [PubMed] [Google Scholar]
- 9.Psaroulaki A, Ragiadakou D, Kouris G et al. Ticks, tick-borne rickettsiae, and Coxiella burnetii in the Greek Island of Cephalonia. Ann N Y Acad Sci 2006;1078:389–99. [DOI] [PubMed] [Google Scholar]
- 10.Papa A, Dalla V, Papadimitriou E et al. Emergence of Crimean–Congo haemorrhagic fever in Greece. Clin Microbiol Infect 2009;16:843–7. [DOI] [PubMed] [Google Scholar]
- 11.Papa A, Chaligiannis I, Xanthopoulou K et al. Ticks parasitizing humans in Greece. Vector Borne Zoonotic Dis 2011;11:539–42. [DOI] [PubMed] [Google Scholar]
- 12.Estrada-Pena A, Bouattour A, Camica J, Walzer AR.. Ticks of domestic animals in the Mediterranean region: a guide to identification of species. Spain; Atalanta, Houten, The Netherlands: University of Zaragosa; 2004. [Google Scholar]
- 13.Roux V, Fournier P, Raoult D.. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol 1996;34:2058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orkun O, Karaer Z, Cakmak A, Nalbantoglu S.. Spotted fever group rickettsiae in ticks in Turkey. Ticks Tick Borne Dis 2014;5:213–8. [DOI] [PubMed] [Google Scholar]
- 15.Andoh M, Sakata A, Takano A et al. Detection of Rickettsia and Ehrlichia spp. in ticks associated with exotic reptiles and amphibians imported into Japan. PloS One 2015;10:e0133700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel-Shafy S, Allam NA, Mediannikov O et al. Molecular detection of spotted fever group rickettsiae associated with ixodid ticks in Egypt. Vector Borne Zoonotic Dis 2012;12:346–59. [DOI] [PubMed] [Google Scholar]
- 17.Blanc G, Ogata H, Robert C et al. Lateral gene transfer between obligate intracellular bacteria:evidence from the Rickettsia massiliae genome. Genome Res 2007;17:1657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simser JA, Palmer AT, Fingerle V et al. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl Environ Microbiol 2002;68:4559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mura A, Masala G, Tola S et al. First direct detection of rickettsial pathogens and a new rickettsia, ‘Candidatus Rickettsia barbariae’, in ticks from Sardinia, Italy. Clin Microbiol Infect 2008;14:1028–33. [DOI] [PubMed] [Google Scholar]
- 20.Vatansever Z, Gargili A, Aysul NS et al. Ticks biting humans in the urban area of Istanbul. Parasitol Res 2008;102:551–3. [DOI] [PubMed] [Google Scholar]
- 21.Wallmenius K, Barboutis C, Fransson T et al. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasite Vectors 2014;7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensenius M, Fournier P, Kelly P et al. African tick bite fever. Lancet Infect Dis 2003;3:557–64. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Soto P, Encinas-Grandes A, Perez-Sanchez R.. Rickettsia aeschlimannii in Spain: molecular evidence in Hyalomma marginatum and five other tick species that feed on humans. Emerg Infect Dis 2003;9:889–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parola P, Paddock CD, Raoult D.. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev 2005;18:719–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Sousa R, Barata C, Vitorino L et al. Rickettsia sibirica isolation from a patient and detection in ticks, Portugal. Emerg Infect Dis 2006;12:1103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chochlakis D, Ioannou I, Sandalakis V et al. Spotted fever group Rickettsiae in ticks in Cyprus. Microb Ecol 2012;63:314–23. [DOI] [PubMed] [Google Scholar]
- 27.Waner T, Keysary A, Eremeeva ME et al. Rickettsia africae and Candidatus Rickettsia barbariae in ticks in Israel. Am J Trop Med Hyg 2014;90:920–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker DH, Ismail N. Emerging and re-emerging rickettsioses:endothelial cell infection and early disease events. Nature Rev Microbiol 2008;6:375–86. [DOI] [PubMed] [Google Scholar]
- 29.Parola P, Socolovschi C, Jeanjean L et al. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Neglect Trop Dis 2008;2:e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitale G, Mansuelo S, Rolain JM, Raoult D.. Rickettsia massiliae human isolation. Emerg Infect Dis 2006;12:174–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katargina O, Geller J, Ivanova A et al. Detection and identification of Rickettsia species in Ixodes tick populations from Estonia. Tick Tick Borne Dis 2015;6:689–94. [DOI] [PubMed] [Google Scholar]
- 32.Jado I, Oteo JA, Aldamiz M et al. Rickettsia monacensis and human disease, Spain. Emerg Infect Dis 2007;13:1405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madeddu G, Mancini F, Caddeo A et al. Rickettsia monacensis as cause of Mediterranean spotted fever-like illness, Italy. Emerg Infect Dis 2012;18:702–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin ML, Killmaster L, Eremeeva ME, Dasch GA.. Effects of Rickettsia conorii infection on the survival of Rhipicephalus sanguineus ticks. Clin Microbiol Infect 2009;15(Suppl 2):277–8. [DOI] [PubMed] [Google Scholar]