Irx3 homozygous and heterozygous knock out in mouse resulted in ventricular tachyarrhythmias in the setting of high sympathetic tone in otherwise normal hearts. Novel IRX3 mutations were found in patients with idiopathic ventricular fibrillation that occurred related to physical activities. Our finding should be useful for identification of healthy individuals at high risk of sudden death especially during exercise.
Keywords: Ventricular fibrillation, Sudden cardiac death, Cardiac conduction system
Abstract
Aim
Ventricular fibrillation (VF), the main cause of sudden cardiac death (SCD), occurs most frequently in the acute phase of myocardial infarction: a certain fraction of VF, however, develops in an apparently healthy heart, referred as idiopathic VF. The contribution of perturbation in the fast conduction system in the ventricle, the His-Purkinje system, for idiopathic VF has been implicated, but the underlying mechanism remains unknown. Irx3/IRX3 encodes a transcription factor specifically expressed in the His-Purkinje system in the heart. Genetic deletion of Irx3 provides a mouse model of ventricular fast conduction disturbance without anatomical or contraction abnormalities. The aim of this study was to examine the link between perturbed His-Purkinje system and idiopathic VF in Irx3-null mice, and to search for IRX3 genetic defects in idiopathic VF patients in human.
Methods and results
Telemetry electrocardiogram recording showed that Irx3-deleted mice developed frequent ventricular tachyarrhythmias mostly at night. Ventricular tachyarrhythmias were enhanced by exercise and sympathetic nerve activation. In human, the sequence analysis of IRX3 exons in 130 probands of idiopathic VF without SCN5A mutations revealed two novel IRX3 mutations, 1262G>C (R421P) and 1453C>A (P485T). Ventricular fibrillation associated with physical activities in both probands with IRX3 mutations. In HL-1 cells and neonatal mouse ventricular myocytes, IRX3 transfection up-regulated SCN5A and connexin-40 mRNA, which was attenuated by IRX3 mutations.
Conclusion
IRX3 genetic defects and resultant functional perturbation in the His-Purkinje system are novel genetic risk factors of idiopathic VF, and would improve risk stratification and preventive therapy for SCD in otherwise healthy hearts.
See page 1476 for the editorial comment on this article (doi:10.1093/eurheartj/ehv516)
Clinical summary.
Irx3 homozygous and heterozygous knock out in mouse resulted in ventricular tachyarrhythmias in the setting of high sympathetic tone in otherwise normal hearts. Novel IRX3 mutations were found in patients with idiopathic ventricular fibrillation that occurred related to physical activities. Our finding should be useful for identification of healthy individuals at high risk of sudden death especially during exercise.
Introduction
Sudden cardiac death (SCD) is a leading cause of mortality in Western countries, with an incidence close to one per 1000 individuals per year.1 Sudden cardiac death results most frequently from ventricular fibrillation (VF) in the setting of coronary artery disease.2 In 5–10% of cases, however, SCD and VF occur in the absence of identifiable structural heart disease, referred as idiopathic VF.2 The mechanism underlying idiopathic VF remains largely unknown, except for rare hereditary cases with genetic mutations in cardiac ion channels or their regulators.
In contrast to the atrium of the heart, where the electrical signal propagates from top to bottom, in the ventricles electrical signals propagate upward from apex to base, resulting in coordinated contraction of the heart and efficient cardiac output. To achieve reverse propagation of electrical signals, the ventricle is equipped with a specialized conduction network, the His-Purkinje system. A plethora of clinical information and recent experimental data have indicated that perturbation in the His-Purkinje system is tightly associated with cardiac arrhythmias and SCD3,4; however, the mechanistic and genetic link between the His-Purkinje system and VF remain largely unknown.
Irx3 is a member of the Iroquois homebox homeodomain transcription factors. Irx3 is expressed predominantly in the His-Purkinje system in the heart, and its genetic deletion results in perturbation of the His-Purkinje system in apparently normal hearts.5 Thus, Irx3-null (Irx3−/−) mouse should provide a good opportunity to study the relationship between the His-Purkinje system and idiopathic VF. Here we examined the arrhythmogenicity in Irx3−/− mice. Since we found that Irx3−/− mice were highly arrhythmogenic, we subsequently set up for a genetic screening of IRX3 mutations in patients with idiopathic VF. Our data showed that genetic defects in Irx3/IRX3 are linked to arrhythmias in apparently healthy hearts that mostly occur in the settings with elevated sympathetic nervous system activity.
Methods
Detailed methods are described in Supplementary material.
In vivo and ex vivo studies in mice
In wild-type (WT), Irx3+/−, and Irx3−/− mice, surface electrocardiogram (ECG) was recorded in the lead II. In subgroup of animals, ambulatory ECG monitoring was performed under baseline, during swimming, after administration of isoproterenol, and after surgical creation of myocardial infarction. Ultrasound echocardiography was performed to evaluate left ventricular contractility and dimension in short-axis view at the level of the papillary muscles. In vivo electrophysiological study was performed with a custom-made 1 Fr four polar catheter. Ex vivo optical mapping was performed on excised hearts under the Langendorff perfusion with a voltage-sensitive dye, di-4-ANEPPS (Sigma-Aldrich).
Patient collection and detection of mutations
We obtained genome DNA from lymphocytes in patients with VF including idiopathic VF, Brugada syndrome, early repolarization syndrome, and short-QT syndrome. Written informed consents were obtained from the patients and were approved by the institutional review boards of each institute.
Genomic DNA was isolated from blood sample, was amplified with PCR, and direct sequencing was performed.
In vitro analysis
In vitro analysis was performed in HL-1, a cell line derived from mouse atrial myocytes, or in neonatal murine ventricular myocytes. Murine Irx3 with or without mutation was subcloned into plasmid vector, pcDNA3.1+ (Life Technologies) or adenoviral vector pAD-CMV-DEST (Life Technology) and was introduced into HL-1 or neonatal murine ventricular myocytes. Quantitative RT-PCR was performed using extracted murine mRNA.
Statistical analyses
All data are shown in terms of mean and SD values. Two-group comparison was analysed by unpaired two-tailed Student's t-test unless described otherwise, and multiple-group comparison was performed by analysis of variance, followed by the Fisher's protected least significant difference test for comparison of each group. Categorical data were compared with the Fisher's exact test. Statistical analyses were performed with Statview (version 5). P value of <.05 was considered statistically significant.
Results
Arrhythmogenicity is increased in Irx3−/− mice
The Irx3-null (Irx3−/−) mouse that we generated (Supplementary material online, Figure S1A–C) showed reduced expression of Cx40 and Scn5a (Supplementary material online, Figures S4–S7), and ventricular conduction disturbance as previously reported (Supplementary material online, Figure S2B and Table S1).5 Gross anatomical analysis found no apparent malformation, and echocardiography showed no difference in ventricular chamber size, ventricular wall thickness, or left ventricular contractile function between WT and Irx3−/− mice (Supplementary material online, Figure S3 and Table S2). Thus, Irx3−/− mice have disturbance exclusively in the conduction of the His-Purkinje system in otherwise normal hearts, providing a suitable model to evaluate the possible link between His-Purkinje system conduction disturbance and arrhythmogenicity in apparently normal hearts.
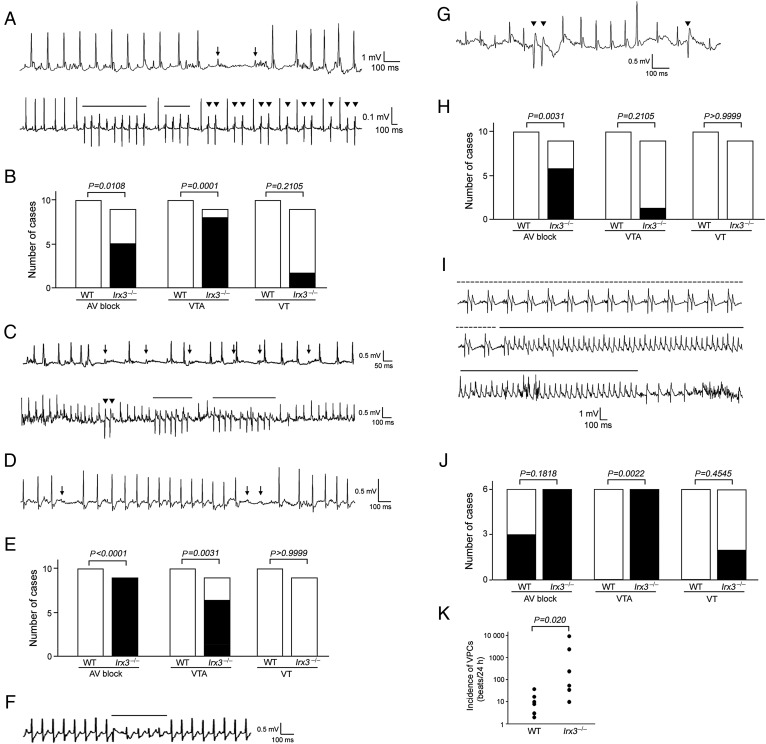
Telemetry ECG recordings in ambulatory conditions showed that both homozygous Irx3−/− and heterozygous Irx3+/− mice but not WT mice frequently exhibited complete atrio-ventricular (AV) blocks, ventricular tachyarrhytmias (VTAs) defined as consecutive ventricular premature contractions (VPCs) more than couplets, and ventricular tachycardias (VTs) defined as VPCs more than triplets (Figure 1A–C). Strikingly, these arrhythmic events occurred predominantly at night, an active phase of mice, implicating relation of these arrhythmias to elevated sympathetic nervous system tone. We tested this possibility by monitoring telemetric ECG under several pathophysiological conditions, in which sympathetic nerve activity is believed to be high. Administration of a sympathetic nerve β-receptor agonist, isoproterenol (0.05 mg/kg, i.p.), induced transient AV block and VTAs in homozygotic Irx3−/− mice (Figure 1D and E) and heterozygotic Irx3+/− (Figure 1F) mice, but never in WT mice. In Irx3−/− mice, ECGs during swimming revealed frequent AV block and occasional VTAs (Figure 1G and H). In the acute phase of myocardial infarction, activated sympathetic nervous system plays a role in development of life-threatening arrhythmias.6 Within 24 h after onset of myocardial infarction, Irx3−/− mice, but not WT mice exhibited frequent VTAs (Figure 1I and J). Ventricular premature contractions were seen in both mice: however, the frequency of VPCs was markedly higher in Irx3−/− mice than in WT mice (Figure 1K).
Figure 1.
Arrhythmia development in Irx3−/− and Irx3+/− mice. (A) Representative ambulatory telemetric electrocardiogram recordings in homozygous Irx3−/− mice. Upper panel shows transient atrio-ventricular block. Arrows indicate P waves without following ventricular excitations. Lower panel shows spontaneous non-sustained ventricular tachycardias. Solid lines indicate the timing with non-sustained ventricular tachycardias. Reverse triangles show ventricular premature contractions. (B) Comparison of frequency of atrio-ventricular block, ventricular tachyarrhytmias, and ventricular tachycardias in ambulatory telemetric electrocardiography in WT (n = 10) and Irx3−/− (n = 9) mice. Ventricular tachyarrhytmias was defined as consecutive ventricular premature contractions more than couplets, and ventricular tachycardias as consecutive ventricular premature contractions more than triplets. Statistical analysis was done with Fisher's exact test. (C) Representative ambulatory telemetric electrocardiogram recordings in heterozygous Irx3+/−. Upper panel shows transient atrio-ventricular block. Arrows indicate P waves without following ventricular excitations. Lower panel shows spontaneous non-sustained ventricular tachycardias. Solid lines indicate the timing with non-sustained ventricular tachycardias. Reverse triangles show ventricular premature contractions. (D) Transient atrio-ventricular block induced by isoproterenol infusion in Irx3−/− mice. Arrows represent P waves without following ventricular excitation. (E) Comparison of frequency of atrio-ventricular block, ventricular tachyarrhytmias, and ventricular tachycardias after isoproterenol infusion in WT (n = 10) and Irx3−/− (n = 9) mice. Statistical analysis was done with by Fisher's exact test. (F) Non-sustained ventricular tachycardias induced by isoproterenol infusion in Irx3+/− mice. Solid lines indicate the timing with non-sustained ventricular tachycardias. (G) Representative electrocardiogram recordings during swimming in Irx3−/− mice. Reverse triangles show ventricular premature contractions. (H) Comparison of frequency of atrio-ventricular block, ventricular tachyarrhytmias, and ventricular tachycardias during swimming in wild-type (n = 10) and Irx3−/− (n = 9) mice. Statistical analysis was done with Fisher's exact test. (I) Representative electrocardiogram recordings within 24 h after surgical creation of myocardial infarction in Irx3−/− mice. Dotted lines indicate the timing with bigeminy, and solid lines indicate the timing with ventricular tachycardias. (J) Comparison of frequency of atrio-ventricular block, ventricular tachyarrhytmias, and ventricular tachycardias within 24 h after myocardial infarction in wild-type (n = 6) and Irx3−/− (n = 6) mice. Statistical analysis was done with Fisher's exact test. (K) Incidence of ventricular premature contractions within 24 h after surgical creation of myocardial infarction in wild-type (n = 6) and Irx3−/− (n = 6) mice. Statistical analysis was done with Mann–Whitney U test.
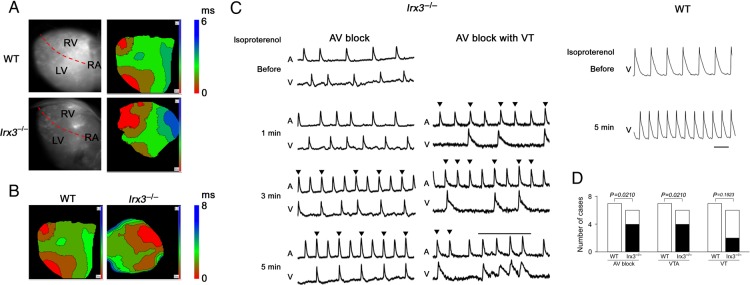
In the cardiac conduction system, the sino-atrial and AV nodes are innervated by the autonomic nervous systems, while the His-Purkinje system is relatively devoid of autonomic nerve supply. Thus, sympathetic nerve activation enhances the automaticity in the sino-atrial node and the conductivity in the AV node that can stress the His-Purkinje system.7 We hypothesized that sympathetic nerve activation-induced stress in His-Purkinje system may bring out an arrhythmogenic phenotype. To test this hypothesis, we carried out ex vivo optical mapping. In the control condition, propagation of the action potential over the epicardium was significantly slower in Irx3−/− mice than in WT mice (1.65 ± 0.50 vs. 1.07 ± 0.32 m/s, P = 0.047) (Figure 2A), consistent with disturbed conduction in Irx3−/− mice in the basal condition. Isoproterenol administration (10 nM) hastened the heart rate both in WT and Irx3−/− mice. In all of seven WT mice tested, action potentials exhibited an identical propagation pattern to that of the basal condition—propagation from apex to base—without propagation delay. In four of six Irx3−/− mice, action potentials exhibited an entirely different propagation pattern with marked slowing of propagation (Figure 2B). In Irx3−/− mice, but not in WT mice, various types of arrhythmias including AV block, VTAs, and VTs were detected (Figure 2C and D).
Figure 2.
Ex vivo optical epicardial mapping and arrhythmia development in Irx3−/− mice. (A) Representative optical epicardial mapping in wild-type and Irx3−/− mice in basal condition. (B) Representative optical epicardial mapping in wild-type and Irx3−/− mice after isoproterenol application. In Irx3−/− mice, epicardial breakthrough occurs from the base of the right ventricle, and propagates to the apex; the propagation of depolarization became markedly slow. (C) Arrhythmias observed in wild-type and Irx3−/− mice after isoproterenol application. In Irx3−/− mice, atrio-ventricular block and atrio-ventricular block with non-sustained ventricular tachycardias occurred. In wild-type mice, only sinus tachycardia occurred. Reverse triangles indicate atrial action potential without following ventricular action potential. Solid bar indicates non-sustained ventricular tachycardias. (D) Comparison of frequency of atrio-ventricular block, ventricular tachyarrhytmias, and ventricular tachycardias after isoproterenol injection in wild-type (n = 7) and Irx3−/− (n = 6) mice. Statistical analysis was done with Fisher's exact test.
IRX3 gene defects are found in patients with idiopathic ventricular fibrillation
Next, we asked if genetic defects in IRX3 are also related to lethal ventricular arrhythmias in human. We analysed the sequence of IRX3 exons in 130 probands of idiopathic VF, Brugada syndrome, early repolarization syndrome, and short-QT syndrome, in whom mutations in SCN5A had not been detected. As a control, the sequence of IRX3 exons was determined in 250 healthy volunteers.
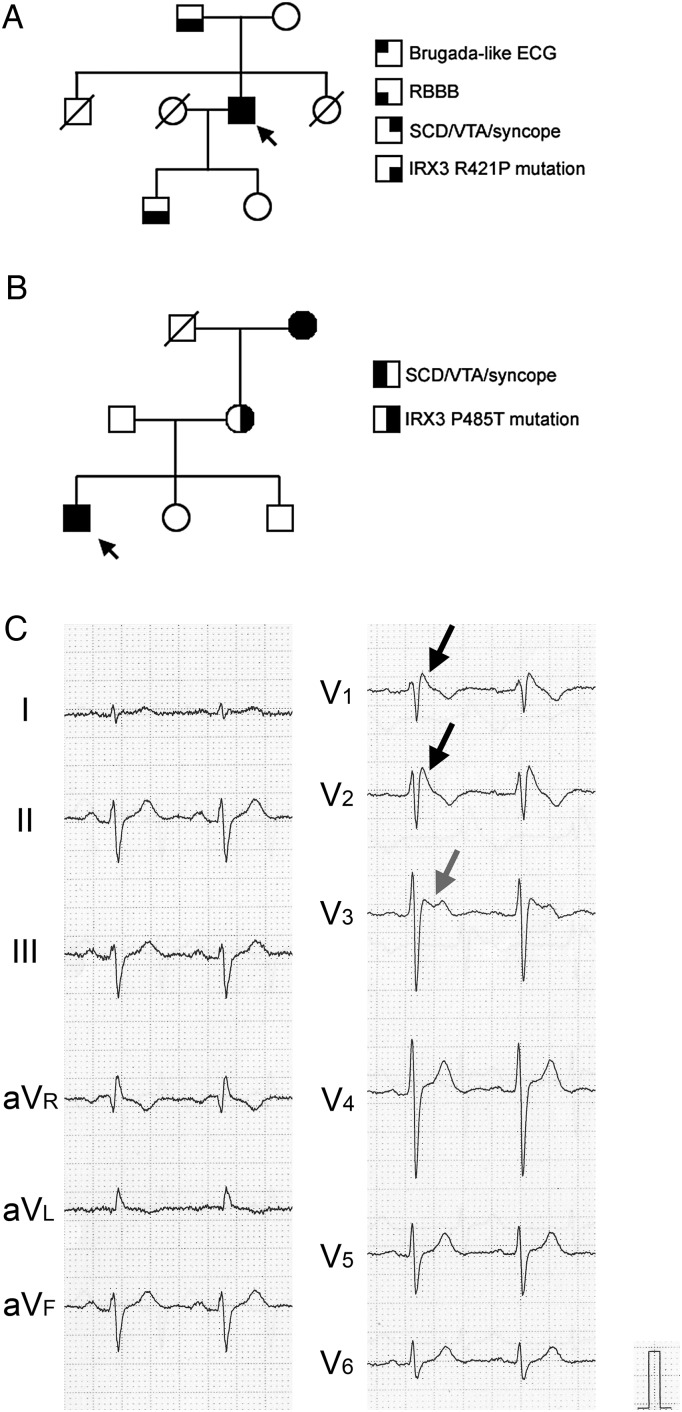
In the idiopathic VF group, we found two novel mutations of IRX3 in a family of idiopathic VF with bundle branch block (Family #1, Figure 3A) and that without bundle branch block (Family #2, Figure 3B), respectively. We examined the sequence of exons in 13 proposed Brugada syndrome-related genes in human (SCN5A, GPD1-L, CACNA1C, CACNB2, KCNE3, SCN1B, SCN3B, KCNJ8, MOG1, HCN4, KCND3, KCNE5, and SLMAP), and found no mutations in any of 130 probands with idiopathic VF or 250 healthy volunteers. The proband in the Family #1 is a 51 y.o. male who developed VF during ice skating. He showed a type 1 Brugada-type ECG with incomplete right bundle branch block (RBBB) pattern (Figure 3C). His father had complete AV block demanding pacemaker implantation (Supplementary material online, Figure S10A), and his son showed complete RBBB (Supplementary material online, Figure S10C). They had an identical point mutation, 1262G>C, resulting in replacement of arginine at residue 421 to proline (R421P) in IRX3. Mother and daughter did not have this mutation, and the ECG was normal (Supplementary material online, Figure S10B and D). The proband in the Family #2 is a 15 y.o. male and exhibited VF during commuting. His ECG did not show either Brugada-like ECG or early repolarization (Supplementary material online, Figure S11A). The proband, grandmother, and mother had identical point mutation, 1453C>A, resulting in replacement of proline at residue 485 to threonine (P485T). The grandmother had experienced syncope with unknown origin, while the mother as well as the father, the sister or the brother did not experience an episode of SCD, VTAs, or syncope. Neither R421P nor P485T mutations were found in 250 healthy volunteers, and were not reported previously including 1000 genomes database.
Figure 3.
Family pedigrees and surface electrocardiograms in patients without SCN5A mutation. (A) Pedigree of the Family #1 with R421P IRX3 mutation. An arrow indicates the proband. (B) Pedigree of the Family #2 with P485T IRX3 mutation. An arrow indicates the proband. (C) Surface electrocardiogram of the proband in the Family #1 with R421P IRX3 mutation. Electrocardiogram showed coved type ST elevation in V1 and V2 (black arrows), and saddle-back type ST elevation in V3 (grey arrow).
IRX3 mutations recapitulate Cx40 and Scn5a down-regulation
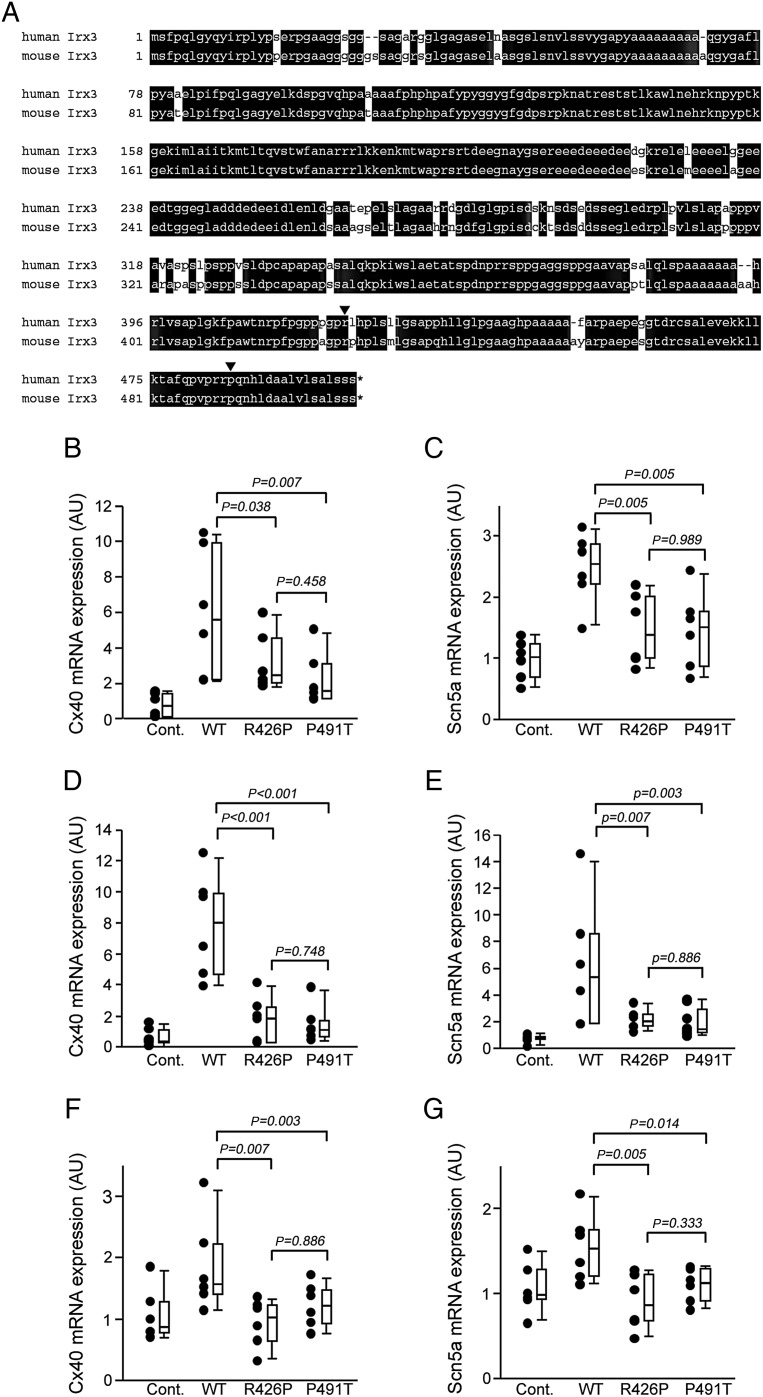
Since the disturbance of the His-Purkinje system conduction in Irx3−/− mice is attributed to decreased expression of Cx40 and Scn5a, we examined if the IRX3 mutations found in humans affected the expression of Cx40 and Scn5a. The sequence was highly conserved between human IRX3 and mouse Irx3 (85% homologous in nucleotide, and 91% in amino acid) (Figure 4A).8,9 The sites of both mutations were conserved in mouse (reverse triangles in Figure 4A). Thus, we infected adenovirus expressing mouse Irx3 without mutation or that with R426P (corresponds to human R421P) or P491T (human P485T) mutation into HL-1 cells, a cell line derived from mouse atrial myocytes or neonatal murine ventricular myocytes. We also performed transfection of Irx3 in pcDNA3 vector into HL-1 cells to exclude the non-specific effect by adenovirus. To exclude the influence of variability in Irx3 expression level, Cx40 and Scn5a mRNA expression was normalized to the expression of Irx3 mRNA using a Ct comparative method. In all three conditions, the transfection of WT Irx3 increased the expression of Cx40 and Scn5a, but not Cx43; up-regulation of Cx40 and Scn5a was significantly less with transfection of each of three mutated Irx3 than with WT Irx3 (Figure 4B–G).
Figure 4.
Irx3 mutations were less effective in up-regulation of Cx40 and Scn5a. (A) Homology of human IRX3 and murine Irx3. Amino acids conserved between human IRX3 and mouse Irx3 are shown by white letters in black box. Two missense mutation sites found in ventricular fibrillation patients in this study are shown by reverse triangles. (B and C) Effects of adenoviral infection with Irx3 into HL-1 cells on the expression of Cx40 (B) and Scn5a (C). The expression of Cx40 and Scn5a was normalized to that of Irx3. Adenoviral infection with wild-type Irx3 increased the expression of Cx40 and Scn5a. Up-regulation of Cx40 and Scn5a was significantly less in R426P (n = 6) and P491T (n = 6) infection than in wild-type Irx3 infection (n = 6). The data are presented actual plots beside the box whisker plot in these and following figures. (D and E) Effects of adenoviral infection with Irx3 into neonatal murine ventricular myocytes on the expression of Cx40 (D) and Scn5a (E). The expression of Cx40 and Scn5a was normalized to that of Irx3. Adenoviral infection with wild-type Irx3 into neonatal murine ventricular myocytes increased the expression of Cx40 and Scn5a. Up-regulation of Cx40 and Scn5a was significantly less in R426P (n = 6) and P491T (n = 6) infection than in wild-type Irx3 infection (n = 6). (F and G) Effects of transfection of HL-1 cells with Irx3 in pcDNA3 vector on the expression of Cx40 (F) and Scn5a (G). The expression of Cx40 and Scn5a was normalized to that of Irx3. Transfection of HL-1 cells with wild-type Irx3 increased the expression of Cx40 and Scn5a. Up-regulation of Cx40 and Scn5a was significantly less in R426P (n = 6) and P491T (n = 6) infection than in wild-type Irx3 transfection (n = 6).
Discussion
A certain fraction of SCD occurs in apparently normal hearts, referred as idiopathic VF.2 We found genetic defects in a transcription factor, IRX3, in patients with idiopathic VF. IRX3 is a transcription factor specifically expressed in the His-Purkinje system in the heart5; Irx3−/− mouse had disturbance of ventricular fast conduction without anatomical or contraction abnormalities, and exhibited frequent VTAs. Up to the present, genetic defects in at least 13 genes have been linked to idiopathic VF, including Brugada syndrome and early repolarization syndrome. IRX3/Irx3 genetic defects appear to be unique because they predominantly cause disturbance of the His-Purkinje system conduction. The link between genetic defects in cardiac transcription factors and cardiac arrhythmias has previously been reported for Nkx2.5 and Tbx5 in the context of cardiac malformation.10,11 Among the genetic defects in cardiac transcription factors, the IRX3/Irx3 mutation is also unique because it exclusively affects the electrophysiological properties without morphological insults. Thus, the IRX3 mutation could be a genetic risk for VF in healthy hearts, or idiopathic VF.
Irx3−/− mice had previously shown to exhibit reduced expression of Scn5a and Cx40, and disturbed ventricular conduction represented by prolonged QRS duration.5 In this report, arrhythmogenicity in Irx3−/− mice had not been reported.5 We believe that their finding does not contradict with our finding, because in Irx3−/− mice in our study arrhythmias were also hardly detected during daytime, and they were detected mostly at night, during application of a sympathetic nerve β-receptor agonist, isoproterenol, during exercise, or in the acute phase of myocardial infarction. Thus, Irx3−/− mice are arrhythmogenic only when the sympathetic nervous system is activated.
The finding that the proband in the Family #1 exhibited type 1 Brugada-type ECG should be discussed with care. In the Family #1, R421P mutation was segregated with conduction disturbance (AV block, complete RBBB), but not with Brugada-type ECG. In general, arrhythmic events in Brugada syndrome occur when para-sympathetic nerve activity is elevated,12 whereas in our study the proband with Brugada-type ECG exhibited VF related to physical activities. We found no genetic defects in 13 proposed Brugada syndrome-related genes in human (SCN5A, GPD1-L, CACNA1C, CACNB2, KCNE3, SCN1B, SCN3B, KCNJ8, MOG1, HCN4, KCND3, KCNE5, and SLMAP). Augmented transient outward currents or diminished voltage-dependent Na+ or Ca2+ currents are implicated in the ionic mechanism of Brugada-type ECG.13,14 Irx3-null mouse exhibited no alterations in the expression of mRNAs encoding transient outward potassium channels (Kv4.2, Kv4.3 and KChIP2), or voltage-dependent Na+ or Ca2+ channels (Sna5a and Cacna1c), in the ventricle (Supplementary material online, Figures S8 and S9). Thus, the relation of VF with Brugada-type ECG is not currently identified.
Irx3 up-regulates the expression of Cx40 and Scn5a. The Irx family commonly acts as a negative regulator. Thus, it is reasonable to assume that the direct target of Irx3 could be some un-identified negative regulator, and that down-regulation of an un-known negative regulator by Irx3 could result in up-regulation of Cx40 and Scn5a. Overexpression of Irx3 with each of two mutations resulted in less up-regulation of Cx40 and Scn5a mRNA compared with overexpression of WT Irx3, suggesting that two IRX3/Irx3 mutations act in a loss-of-function manner.
In conclusion, IRX3 genetic defects and resultant functional perturbation in the His-Purkinje system are novel genetic risk factors of idiopathic VF, and would improve risk stratification and preventive therapy for SCD in otherwise healthy hearts, especially under the condition with elevated sympathetic nerve activity.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
The authors thank an initial contribution of M. Machii for making the Irx3 KO mouse. This work was supported by Grant-in-Aid for Scientific Research and Program for Improvement of Research Environment for Young Researchers from Special Coordination Funds for Promoting Science and Technology from MEXT of Japan, by the Research Grant for the Cardiovascular Diseases (H24-033) and Grant-in-Aid for Scientific Research on Innovative Areas (22136011) from the Ministry of Health, Labour and Welfare, Japan, and by the Joint Usage/Research Program of Medical Research Institute, Tokyo Medical and Dental University.
Conflict of interest: none declared.
References
- 1.Straus SM, Sturkenboom MC, Bleumink GS, DIeleman JP, van der Lei J, de Graeff PA, Kingma JH, Stricker BH. The incidence of sudden cardiac death in the general population. J Clin Epidemiol 2004;57:98–102. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Aliot E, Blømstrom-Lundqvist C, Bossaert L, Breidhardt G, Brugada P, Camm JA, Cappato R, Cobbe SM, Di MC, Maron BJ, McKenna WJ, Pedersen AK, Ravens U, Schwartz PJ, Trusz-Gluza M, Vardas P, Wellens HJ, Zipes DP. Task force on sudden cardiac death, European Society of Cardiology. Europace 2002;4:3–18. [DOI] [PubMed] [Google Scholar]
- 3.Nademanee K, Veerakul G, Nimmannit S, Chaowakul V, Bhuripanyo K, Likittanasombat K, Tunsanga K, Kuasirikul S, Malasit P, Tansupasawadikul S, Tatsanavivat P. Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men. Circulation 1997;96:2595–2600. [DOI] [PubMed] [Google Scholar]
- 4.Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009;6:1050–1058. [DOI] [PubMed] [Google Scholar]
- 5.Zhang SS, Kim KH, Rosen A, Smyth JW, Sakumra R, Delgado-Olguin P, Davis M, Chi NC, Puviindran V, Gaborit N, Sukonnik T, Wylie JN, Brand-Arzamendi K, Farman GP, Kim J, Rose RA, Marsden PA, Zhu Y, Zhou YQ, Miquerol L, Henkelman RM, Stainier DY, Shaw RM, Hui CC, Bruneau BG, Backx PH. Iroquois homeobox gene 3 establishes fast conduction in the cardiac His-Purkinje network. Proc Natl Acad Sci USA 2011;108:13574–13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation 1992;85:177–191. [PubMed] [Google Scholar]
- 7.Woelfel AK, Simpson RJ Jr, Gettes LS, Foster JR. Exercise-induced distal atrioventricular block. J Am Coll Cardiol 1983;2:578–581. [DOI] [PubMed] [Google Scholar]
- 8.Christoffels VM, Keijser AG, Houweling AC, Clout DE, Moorman AF. Patterning the embryonic heart: identification of five mouse Iroquois homeobox genes in the developing heart. Dev Biol 2000;224:263–274. [DOI] [PubMed] [Google Scholar]
- 9.Ragvin A, Moro E, Fredman D, Navratilova P, Drivenes Ø, Enqström PG, Alonso ME, de la Calle Mustienes E, Gómez Skarmeta JL, Tavares MJ, Casares F, Manzanares M, van Heyningen V, Molven A, Njøstad PR, Argenton F, Lenhard B, Becker TS. Long-range regulation links genomic type 2 diabetes and obesity risk regions to HHEX, SOX4, and IRX3. Proc Natl Acad Sci USA 2010;107:775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CD, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 1998;281:108–111. [DOI] [PubMed] [Google Scholar]
- 11.Postma AV, van de Meerakker JB, Mathijssen IB, Barnett P, Christoffels VM, Ilgun A, Lam J, Wilde AA, Lekanne Deprez RH, Moorman AF. A-gain-of-function TBX5 mutation is associated with atypical Holt-Oram syndrome and paroxysmal atrial fibrillation. Circ Res 2008;102:1433–1442. [DOI] [PubMed] [Google Scholar]
- 12.Wichter T, Matheja P, Eckardt L, Kies P, Schäfers K, Schulze-Bahr E, Haverkamp W, Borggrefe M, Schober O, Breithardt G, Schäfers M. Cardiac arrhythmic dysfunction in Brugada syndrome. Circulation 2002;105:702–706. [DOI] [PubMed] [Google Scholar]
- 13.Yan G-X, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation 1999;100:1660–1666. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu W, Aiba T, Kamakura S. Mechanisms of disease: current understanding and future challenges in Brugada syndrome. Nat Clin Pract Cardiovasc Med 2005;2:408–414. [DOI] [PubMed] [Google Scholar]