Abstract
Aims
Sudden cardiac death is a major cause of mortality in adult congenital heart disease (ACHD) patients. The indications for implantable cardioverter-defibrillator (ICD) implantation in ACHD patients are still not well established. We aim to systematically review the literature on indications and outcome of ICD implantation in ACHD patients.
Methods and results
We performed a comprehensive search in EMBASE, MEDLINE, and Google Scholar to identify all studies on ICD implantation in ACHD patients. We used random effects models to calculate proportions and 95% confidence intervals. Of 1356 articles, 24 studies with 2162 patients were included, with a mean follow-up of 3.6 ± 0.9 years. Half of patients had tetralogy of Fallot. Mean age at implantation was 36.5 ± 5.5 years old and 66% was male. Implantable cardioverter-defibrillators were implanted for primary prevention in 53% (43.5–62.7). Overall, 24% (18.6–31.3) of patients received one or more appropriate ICD interventions (anti-tachycardia pacing or shocks) during 3.7 ± 0.9 years: 22% (16.9–28.8) of patients with primary prevention in 3.3 ± 0.3 years and 35% (26.6–45.2) of patients with secondary prevention in 4.3 ± 1.2 years. Inappropriate shocks occurred in 25% (20.1–31.0) in 3.7 ± 0.8 years and other, particularly lead-related complications in 26% (18.9–33.6) of patients in 3.8 ± 0.8 years. All-cause mortality was 10% during 3.7 ± 0.9 years.
Conclusions
In ACHD, remarkably high rates of appropriate ICD therapy were reported, both in primary and secondary prevention. Because of the young age and lower death rates, the cumulative beneficial effects are likely greater in ACHD patients than in acquired heart disease patients. However, considering the high rates of inappropriate shocks and complications, case-by-case weighing of costs and benefits, remains essential.
Keywords: Congenital heart disease, Implantable cardioverter-defibrillator, ICD, Indication, Appropriate ICD interventions, Appropriate shocks, Inappropriate shocks, Complications
Introduction
Sudden cardiac death (SCD) is a major cause of mortality in adult congenital heart disease (ACHD) patients, and is mostly caused by ventricular arrhythmias, i.e. ventricular tachycardia (VT) or ventricular fibrillation (VF). The overall SCD incidence in the entire population of ACHD patients is low: 0.09–0.26% annually, but still manifold higher than in the age-matched population without congenital heart disease.1,2 Although these numbers are low due to the inclusion of many patients with low-risk lesions in these analyses, e.g. those with an isolated small atrial septal defect (ASD), certain individuals may be at increased risk. Ultimately, SCD accounts for 19–26% of all deaths in ACHD patients.3–5 As more patients with congenital heart disease survive to adulthood and the population grows older as a result of improved surgical techniques, both the relative and absolute rates of SCD are expected to rise.
The implantable cardioverter-defibrillator (ICD) has been proven to effectively prevent SCD in patients with (non-)ischaemic cardiomyopathy and is recommended by extensive international guidelines.6–8 Implantable cardioverter-defibrillators have also been shown to effectively convert life-threatening arrhythmias in ACHD patients,9 but robust clinical evidence-based guidelines are lacking. The choice for ICD implantation in ACHD patients after resuscitated cardiac arrest may be evident. However, the indication for ICD implantation for primary prevention can be more challenging and is often based on the presence of multiple risk factors. Furthermore, as prospective evidence for ACHD patients is unavailable, indications for ICD implantation are often extrapolated from studies in patients with other cardiac conditions, retrospective data, or expert opinions. To our knowledge, no systematic reviews have addressed ICD implantation in the general population of ACHD patients thus far. As no randomized clinical trials are available for ICD implantation in ACHD, such data are of paramount importance to be able to weigh benefit and risk in individual patient decision-making. We therefore systematically reviewed the available literature on ICD indications, efficacy, and ICD-related harm in ACHD patients.
Methods
This systematic review was conducted in accordance with the PRISMA guidelines.10 Studies providing exclusive (i.e. not derived from the same cohort on which another study has also reported) and extractable data on ACHD patients with an ICD were included. Available data on ICD indication (primary or secondary prevention) were required for inclusion.
Participants in included studies were ACHD patients who underwent ICD implantation. Adult patients were defined as ≥16 years old. Finally, studies that also reported on non-ACHD patients were included only if the data from those patients were excludable from the analyses. Alternatively, when this was not possible, we incorporated studies with a minimum of 95% ACHD patients.
We included studies reporting on implantation of any type of ICD: transvenous, epicardial, subcutaneous (S-ICD),11 or any other configuration. Likewise, we included any type of lead configuration: single-chamber, dual-chamber, cardiac resynchronization therapy (CRT-D), or any other type of lead arrangement.
Main focus
The main focuses of this systematic review are as follows:
The indication for ICD implantation; primary or secondary prevention. Implantable cardioverter-defibrillator implantation for sustained VT or for cardiac arrest/VF defined secondary prevention. Implantable cardioverter-defibrillator implantation in the absence of a secondary prevention indication defined primary prevention.
Appropriate ICD interventions, defined as an ICD shock or anti-tachycardia pacing (ATP) in response to proven VT or VF.
Inappropriate shocks, defined as an ICD shock for any other reason.
ICD-related complications. These were defined as generator infection, thrombotic events, lead failure and dislodgement, lead endocarditis, minor and major bleeding, cardiac perforation and pericardial effusion, pneumo- or haemothorax, unspecified re-intervention, and ICD-related death.
Electronic searches
A medical librarian (J.L.) performed a comprehensive search in OVID MEDLINE, OVID EMBASE, and the non-MEDLINE subset of PubMed from inception to 11 November 2014. To avoid overlooking papers that did not mention (any) congenital heart disease or ICD in the title or abstract, we also included the first 400 relevance-ranked articles found with a full-text Google Scholar search. For the MEDLINE and EMBASE search, we used both index terms and text words for ICD and congenital heart disease for both general terms and specific diagnoses. Venous and coronary anomalies were not incorporated into this search. No language or other restrictions were applied. The search included an iterative process to refine the search strategy by adding search terms as new relevant citations were identified (i.e. via reference and citation checking of relevant studies in Web of Science). The bibliographic records retrieved were imported and de-duplicated. The complete search strategy for MEDLINE is presented in Supplementary material online, Table S1.
Data collection and analysis
Two investigators (J.T.V. and T.F.B.) independently screened for eligibility of studies. In the case that studies reported serial data on the same patient cohort and overlapping data could not be excluded, only the study that provided the largest number of patients was included. Conference abstracts of unpublished studies were only included when published <1 year before the search. Case reports and review papers were excluded.
Two investigators (J.T.V. and T.F.B.) independently extracted data. In addition to data on the main focuses, we extracted data on study characteristics, patient characteristics, follow-up duration, electrophysiological studies (EPS), ICD and lead configuration, and mortality. For one study (n = 136), the patient data set was used in addition to the article to provide the most accurate and complete data.9 Differences between reviewers regarding study selection or data extraction were resolved by consensus or by consultation of a third reviewer (J.R.d.G.)
To evaluate the quality and risk of bias of included studies, we applied the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies, which was accommodated to the studies included in this systematic review.12
We performed sensitivity analyses to compare (i) studies with <50 vs. ≥50 patients, (ii) with the date of last inclusion before vs. after 1 January 2010, (iii) those with or without data on follow-up duration, and (iv) those with a higher vs. a lower study quality.
Statistical analysis
Analyses were performed using Comprehensive Meta-Analysis Version 2.2 (Biostat Inc., Englewood, NJ, USA) and Microsoft Excel 2010. For continuous data, sample size-weighted grand means and standard deviations of sample means (or medians, whichever was reported) were calculated. Categorical data were described in percentages. Meta-analysis was performed for the main focuses to calculate proportions and annual rates with corresponding 95% confidence intervals (CI). Random effects models weighted by inverse variance were used because of heterogeneity in the underlying cardiac pathology. Heterogeneity was assessed by calculating the Q-statistic and the I². A P-value of <0.05 for the Q-statistic was considered to be statistically significant. An I² of >40% was also considered to be an indication of substantial heterogeneity.
Results
Search results
The de-duplicated search yielded 1354 abstracts. After exclusion based on title and abstract, 142 full-text articles were reviewed. Twenty-four observational studies (21 published studies and 3 abstracts of unpublished studies) were included.9,13–35 Screening of reference lists of included studies and referring articles did not yield additional articles. Figure 1 shows a flowchart for selection of studies.
Figure 1.
Flowchart for study selection.
Study characteristics and risk of bias
The included studies were highly variable in numbers of patients, ranging between 2 and 1683. Similarly, congenital heart defects were diverse. Seven studies included patients with one particular congenital lesion: tetralogy of Fallot (ToF) in one23 (n = 121) and transposition of the great arteries (TGA) in six13,16,22,28,32,35 (n = 83). Appropriate and inappropriate shock rates and ICD-related complications were the most common outcomes.
All studies comprised retrospective analyses. Therefore, the study quality of most studies was low and five large studies (n = 426) were of moderate quality.9,21–24 The study quality scoring is displayed in Supplementary material online, Table S2.
A recent publication on a large US registry reported the number of procedures performed instead of number of patients.20 To minimize bias and double reporting, we incorporated the data for the 1683 initial implants only. Moreover, no follow-up data were provided. Four other studies18,21,32,34 (n = 86) overlapped with this study and were therefore only used for analyses of follow-up data.
Funnel plots did not reveal any substantial publication bias for each of the outcomes of main focus of this meta-analysis.
Outcomes
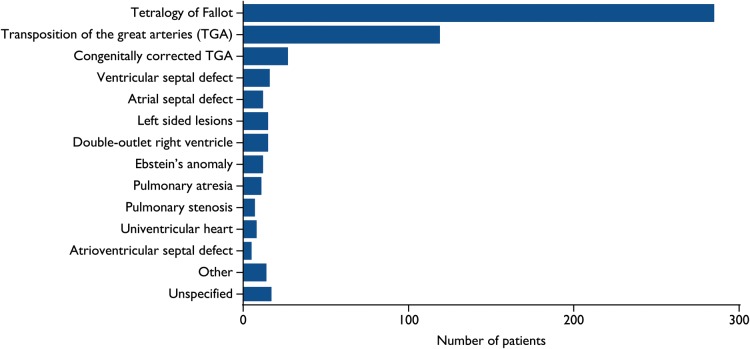
A total of 2162 exclusive ACHD patients who underwent ICD implantation were included from 24 studies. Supplementary material online, Table S3 displays the main outcomes of the studies. The congenital heart defects of patients are displayed in Figure 2 and other characteristics of included patients are displayed in Table 1.
Figure 2.
Distribution of congenital heart diseases across 20 studies (n = 563).
Table 1.
Characteristics of included patients
| Value: years, mean ± SD or % | Number of studies providing data | Number of included patients | |
|---|---|---|---|
| Male (%) | 66% | 13 | 507 |
| Age at ICD implantation (years) | 36.5 ± 5.5 | 19 | 537 |
| Age at initial repair (years) | 9.5 ± 4.7 | 5 | 164 |
| Follow-up after ICD implant (years) | 3.6 ± 0.9 | 14 | 517 |
| Total patient years | 1935 | 14 | 517 |
| Patients with impaireda SVF (%) | 42% | 11 | 367 |
SVF, systemic ventricular function.
aAt least moderately impaired.
A transvenous ICD was implanted in 96.1% of patients (0.9% epicardial, 0.2% S-ICD, remainder not specified). Of patients with a transvenous ICD, 36% received a single-chamber ICD, 56% a dual-chamber ICD, and 7% had a CRT-D system (nine studies, n = 243).
Holter data were available in four studies (n = 299), in which Holter monitoring before ICD implantation was performed in 63% of patients, and in 33% of these patients VTs were captured.
Implantable cardioverter-defibrillator indications
Approximately half of all implantations (53.2%, 43.5–62.7) were for primary prevention indications (20 studies, n = 2162, Figure 3A). A total of 360 specific primary prevention indications were reported for 239 primary prevention patients (16 studies, n = 430). The decision for ICD for primary prevention was commonly based on multiple risk factors (Figure 3B). No information on what combination of risk factors indicated ICD implantation was reported.
Figure 3.
(A) Percentage of implantable cardioverter-defibrillator implantation for primary prevention in 20 studies (n = 2162). (B) Specified primary prevention indications in 16 studies (n = 430; 239 with primary prevention with a total of 360 non-exclusive primary prevention indications). PP, primary prevention; VT, ventricular tachycardia.
Specific indications for ICD implantation for secondary prevention in 220 patients were sustained VT in 61% and cardiac arrest in 39% (19 studies, n = 473). In the sensitivity analyses, there were no significant differences in the proportion of primary prevention indication.
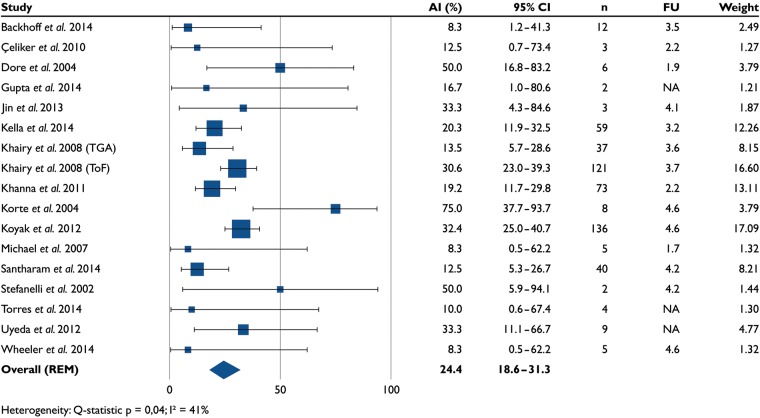
Appropriate implantable cardioverter-defibrillator interventions
Almost one in four (24.4%, 18.6–31.3) patients received one or more appropriate ICD interventions (17 studies, n = 525, Figure 4). The mean follow-up was 3.7 ± 0.9 years (14 studies, n = 514). After excluding ATP episodes, the proportion of patients who received one or more appropriate ICD shocks was 23.3% (18.3–29.3).
Figure 4.
Percentage of patients who received appropriate implantable cardioverter-defibrillator interventions in 17 studies (n = 525). AI, appropriate interventions; FU, follow-up (years).
Analysis of all appropriate ICD interventions, including patients with multiple shocks, is available in Supplementary material online, Figure S1.
Twenty-two per cent (22.3%, 16.9–28.8) of patients with primary prevention indications and 35.3% (26.6–45.2) of patients with a secondary prevention indication received appropriate ICD interventions (14 studies, n = 383; 201 with primary prevention). Follow-up was shorter for primary than for secondary prevention patients: 3.3 ± 0.3 and 4.3 ± 1.2 years, respectively (nine studies, n = 330; 180 with primary prevention).
In the sensitivity analyses, there were no significant differences between studies in the proportion of patients with appropriate interventions.
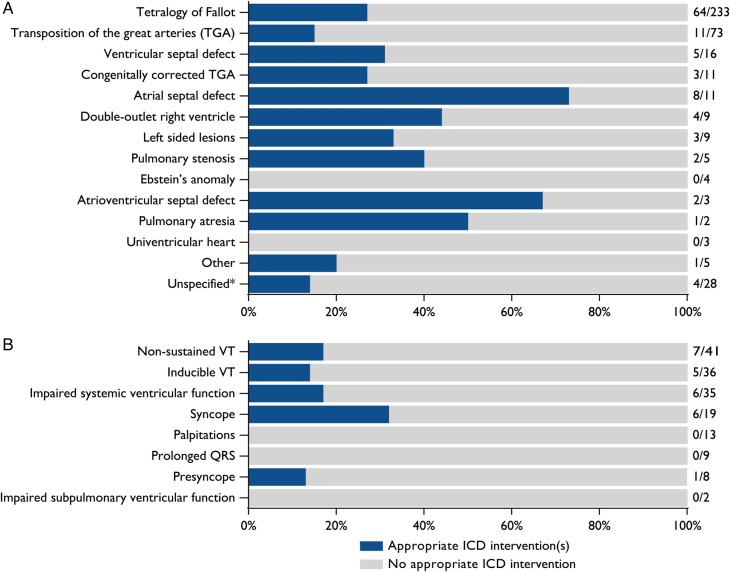
Figure 5A shows the proportions of appropriate ICD interventions in different congenital heart defects.
Figure 5.
(A) Percentage of patients with appropriate implantable cardioverter-defibrillator interventions in different congenital heart defects in 15 studies (n = 412). *One study (n = 59) displayed appropriate interventions only for tetralogy of Fallot or non-tetralogy patients.21 (B) Percentage of patients with appropriate implantable cardioverter-defibrillator interventions per non-exclusive implantable cardioverter-defibrillator indication in 10 studies (n = 213; 110 with primary prevention with a total of 163 non-exclusive indications). VT, ventricular tachycardia.
In ToF patients with vs. without surgical scars in one study (n = 121), the hazard ratio of scars for appropriate ICD interventions was 2.5 (1.1–5.4).23 Another study (n = 59) found that ToF patients, who are more likely to have ventricular scars, had a higher rate of appropriate interventions compared with non-ToF ACHD patients: 27.3% in 2.9 years vs. 11.5% in 3.7 years (P = 0.045), respectively.21 In our pooled analysis, there was no difference in appropriate intervention rates between ToF (27%) and non-ToF (25%) patients. In patients with a presumed ventricular scar [ToF, ventricular septal defect (VSD), pulmonary atresia, complete atrioventricular septal defect (AVSD), congenitally corrected TGA with VSD, double-outlet right ventricle with Rastelli surgery], 28% received appropriate ICD interventions, compared with 35% of patients without a ventricular scar (10 studies, n = 181).
Diagnostic EPS, either as part of a primary prevention indication or for other motives, was performed in 63% (206) of patients (eight studies, n = 325). Sustained monomorphic or polymorphic VT was inducible in 65% of patients (134), VF in 5% (11), and an unspecified sustained ventricular arrhythmia in 3% (7). Appropriate intervention(s) were reported for 30% (60/199) of patients who underwent EPS and 17% (19/114) of patients in whom EPS was not performed (seven studies, n = 313). Interestingly, 34% (46/134) of patients with inducible VT received appropriate interventions, whereas 9% (1/11) of patients with inducible VF and 24% (13/54) of non-inducible patients did (three studies, n = 294). The odds ratio of appropriate interventions in inducible vs. non-inducible patients was 1.2 (0.2–5.7, Q-statistic P= 0.05, I² = 66%).
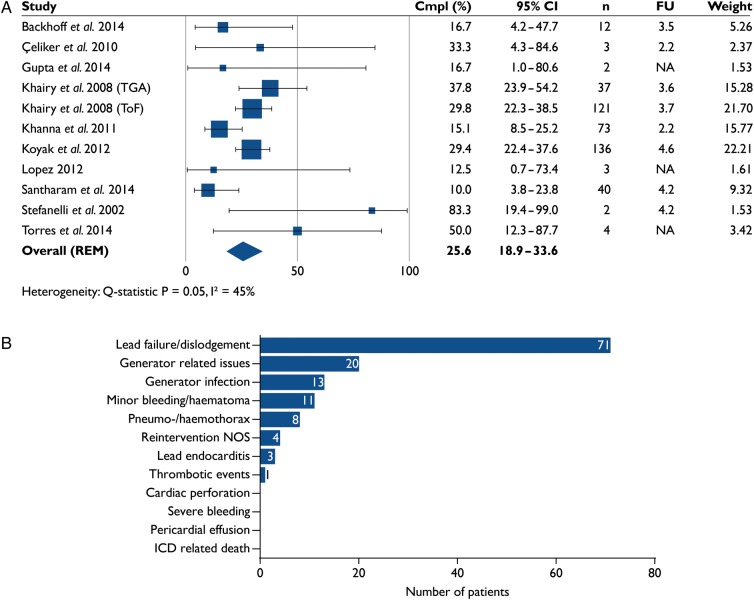
Complications and inappropriate shocks
Over one in four patients (25.6%, 18.9–33.6) experienced ICD-related complications (11 studies, n = 433, Figure 6A). Mean follow-up was 3.8 ± 0.8 years (eight studies, n = 424). Seventy-six per cent of complications involved lead- or generator-related issues (Figure 6B).
Figure 6.
(A) Percentage of patients who experienced implantable cardioverter-defibrillator-related complications in 11 studies (n = 433). (B) Implantable cardioverter-defibrillator-related complications in 11 studies (n = 433; 112 with complications). Cmpl, complications; FU, follow-up (years); NOS, not otherwise specified.
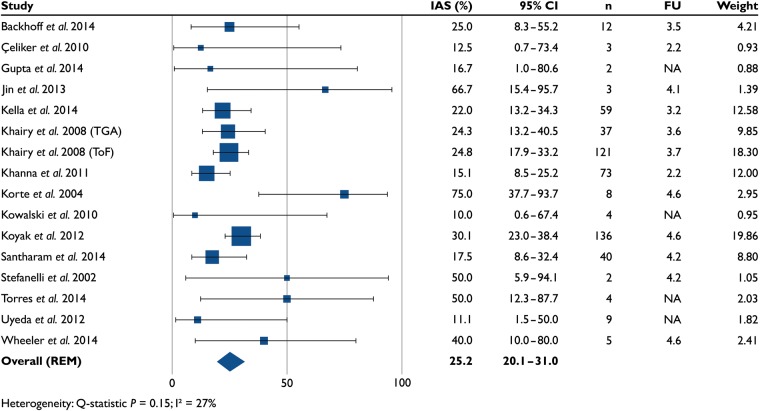
Inappropriate shocks (16 studies, n = 518) occurred in 25.2% (20.1–31.0) of patients (Figure 7A). The mean follow-up was 3.7 ± 0.8 years (12 studies, n = 499). A total of 390 inappropriate shocks were delivered to 76 patients (11 studies, n = 275). First inappropriate shocks were caused by supraventricular tachycardias in 68% of patients, sinus tachycardia in 17% of patients, and over-sensing or lead failure in 13% of patients. In the remaining 2%, the reason was not specified (14 studies, n = 506, of which 120 patients with inappropriate shocks).
Figure 7.
Percentage of patients who experienced one or more inappropriate shocks in 16 studies (n = 518). IAS, inappropriate shocks; FU, follow-up (years).
Analyses of all complications and inappropriate shocks, including patients with multiple events, are available in Supplementary material online, Figures S2 and S3.
Sensitivity analyses revealed no meaningful differences between studies in the proportion of patients with inappropriate shocks or complications.
Mortality
No ICD-related deaths were reported. Overall, 10% of patients died (15 studies, n = 440). Mean follow-up was 3.7 ± 0.9 years (11 studies, n = 404). The annual death rate was 3%. Sudden cardiac death despite ICD implantation occurred in 18% of all deaths, heart failure in 41%, other cardiac causes in 11%, and non-cardiac causes in 7%. In the remainder, the cause of death was unknown (15 studies, n = 440).
Discussion
In adults with congenital heart disease who underwent ICD implantation, a remarkably high rate of appropriate ICD interventions, both in primary prevention (22% in 3.3 years) and in secondary prevention (35% in 4.3 years), was found. Mortality in ACHD patients with an ICD was higher than in the average population of ACHD patients: 10% death during a mean follow-up of 3.7 years vs. 3% in 3.6 years, respectively,5 most likely due to more severe heart failure and general condition. However, mortality rates were much lower than in the conventional ICD population of ischaemic and non-ischaemic cardiomyopathy patients of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), in which 22% of ICD recipients died during a median of 3.8 years of follow-up.6 This may be because ACHD patients were younger at implantation (mean age 36.5 vs. 60.1 years) and likely had less co-morbidity. Thus, despite the notion that the appropriate shock rate in this study is similar to that in SCD-HeFT, the cumulative beneficial effect across decades is expected to be much greater in ACHD patients than in acquired heart disease patients, due to a more favourable appropriate intervention to mortality ratio. It is also important to note that the studies presented here included more contemporary cohorts compared with the studies in patients with acquired heart disease; those patients may currently receive less appropriate interventions as a result of improved medical therapy and advanced device programming. Therefore, it is likely that the majority of ICD implantations in ACHD patients were warranted. It may even be a sign that ICDs were only implanted in the patients at highest risk for SCD, and that some individuals who may have benefited from an ICD did not receive the device.
Appropriate intervention rates were similar for most congenital diagnoses. However, a large portion of ASD and AVSD patients received appropriate interventions (Figure 5A). This is likely an effect of low patient numbers (11 and 3, respectively) and selection bias, since only the patients with the highest perceived risk underwent ICD implantation.
Implantable cardioverter-defibrillators were implanted for primary and secondary prevention at a roughly 50/50 ratio. Indications for primary prevention were variable and multifactorial with non-sustained VT, impaired systemic ventricular function, inducible VT, and syncope being the most prevalent. Patients in whom syncope was the indication for ICD implantation received the most appropriate interventions (30%); therefore, ICD implantation in these patients was likely appropriate. Patients with non-sustained VT, inducible VT, impaired SVF, and presyncope received fewer appropriate interventions (∼15%). Implantable cardioverter-defibrillator implantation for these indications may therefore also have been appropriate, especially in the presence of several of these risk factors. Patients with palpitations, prolonged QRS, or impaired sub-pulmonary ventricular function did not receive appropriate interventions. Hence, the latter indications may point to potentially unneeded ICD implantations. When compared with non-inducible patients, inducible VT in EP studies did not predict appropriate interventions [odds ratio 1.2 (0.2–5.7)], in contrast to what was found in another study for sustained VT or SCD in ToF patients.36 Ventricular tachycardia ablation may have been performed in patients with inducible VT, and we cannot rule out that this leads to the lower predictive value of inducible VT for appropriate ICD interventions. Although no data are available in the studies included here, VT ablation may prevent ventricular arrhythmias and shocks in ACHD patients, and requires further studying. Surgical ventricular scars are potentially an important substrate for ventricular arrhythmias. Implantable cardioverter-defibrillator implantation occurred several decades after surgical repair, which may indicate that the risk of SCD is not perceived until long after surgical repair. This emphasizes the importance of follow-up after surgery and continuing attention to the risk of SCD.
The complexity of decision-making underlines the importance of clear primary prevention indications. The 2015 European Society of Cardiology guidelines on ventricular arrhythmias and the prevention of SCD, in which the evidence for ICD implantation is derived from retrospective studies, expert opinions, or extrapolation from other patient groups, confirm this. Our findings support these guidelines; the indications listed there are indeed associated with appropriate ICD interventions (Figure 5B).
Aside from high appropriate intervention rates, numerous patients experienced inappropriate shocks and ICD-related complications. These rates were higher compared with SCD-HeFT (26% complications vs. 14%, both in 3.8 years).6 Although it is reasonable to assume that a large portion of complications appear during or shortly after implantation, cumulative numbers may still be substantial.
The vast majority of complications were due to lead failure or dislodgement. There are a number of potential reasons for this high lead-failure rate: lead placement can be more difficult in ACHD patients due to the complex anatomy, causing more unstable leads. Adult congenital heart disease patients are younger and have more active lifestyles than the much older patients with acquired heart disease. Patients may also face several generator replacements and additional cardiac surgery, which can destabilise leads. Advanced device and lead technology may substantially reduce complication rates and inappropriate shocks resulting from failed leads. The S-ICD may especially be valuable for ACHD patients, since to our knowledge no lead failures with this device have been reported thus far, with similar efficacy. Moreover, potential anatomical challenges of transvenous lead implantation in ACHD patients can be overcome with a subcutaneous approach.
Impaired ventricular sensing and high defibrillation thresholds in ACHD patients may add to inappropriate therapy, implying that involvement of an electrophysiologist with the implantation is advised. Although no data were available in these studies, advanced device programming may help reduce both appropriate and inappropriate shocks in ACHD patients.
Studies reporting solely on patients in whom an ICD was implanted deliver only limited data on the efficacy of the device. A multicentre randomized controlled trial on ICD implantation in adults with congenital heart disease is of paramount importance, although ethical and practical objections may prevent its execution. Prospective studies, in which patients with risk factors for SCD are compared with patients without risk factors, are an important first step towards a randomized trial. Such studies are urgently needed to fill this knowledge gap, and to prepare for the future where the number of ACHD patients will increase and their age will advance.
Limitations
All included studies were retrospective cohort studies. Lower levels of evidence and a higher risk of selection bias, incomplete outcome data, and reporting bias may apply to this study design. We therefore grade the overall level of evidence as low to moderate, with only five studies being of moderate quality.9,21–24 Funnel plots did not reveal any significant publication bias. There was substantial heterogeneity in patient numbers, although sensitivity analysis did not reveal significant differences in outcomes in studies with fewer or more than 50 patients. There was also heterogeneity in the types of congenital heart defects, indications, and outcomes between included studies. Regional differences (USA vs. Europe/Asia) are a likely explanation for part of the heterogeneity in implantation indications. Patients with ToF and TGA account for a large portion of ICD recipients in studies with aggregate ACHD patients, and several studies reported solely on patients with these diagnoses. Thus, these patients may be over-represented in our systematic review. Primary prevention indications were often multifactorial, but in most cases reported separately. This precluded us to report on which combination of risk factors indicated ICD implantation and most often lead to appropriate interventions.
Conclusions
A remarkably high rate of appropriate ICD interventions was reported in ACHD patients with an ICD, both in secondary and primary prevention. Thus, ICD implantation based on a multifactorial decision determined by parameters derived from studies in patients with acquired heart disease and retrospective studies in ACHD patients appears adequate. Because ACHD patients were younger and death rates were much lower compared with patients with acquired heart disease, ACHD patients will likely face many more years of ICD therapy. The cumulative beneficial, but also harmful, effects of the device may therefore be greater. Although current improvements in ICD technology may help reduce the many lead-related issues, the substantial complication and inappropriate shock rates stress careful weighing of costs and benefits per individual patient.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
J.T.V. performed statistical analysis. J.R.d.G., B.J.M.M., and B.J.B. handled funding and supervision. J.T.V., T.F.B., and J.L. acquired the data. J.T.V. and J.R.d.G. conceived and designed the research. J.T.V., T.F.B., J.L., R.E.K., B.J.B., B.J.M.M., and J.R.d.G. drafted the manuscript. J.T.V., T.F.B., J.L., R.E.K., B.J.B., B.J.M.M., and J.R.d.G. made critical revision of the manuscript for key intellectual content.
Funding
This was an investigator-initiated study. No specific funding was received for this study, other than from Academic Medical Center Medical Research BV.
Conflict of interest: J.T.V., T.F.B., J.L., B.J.B., and B.J.M.M. report no disclosures. R.E.K. receives unrestricted research grants and personal fees from Boston Scientific, Medtonic and St. Jude Medical. J.R.d.G. is supported by a VIDI grant from The Netherlands Organisation for Health Research and Development (ZonMw/NWO, grant 016.146.310), and receives unrestricted research grants from and is a consultant for Medtronic, St. Jude Medical and Atricure, and is a consultant for Daiichi Sankyo, Pfizer and Boehringer Ingelheim.
Acknowledgements
The authors acknowledge Dr Z. Koyak for providing the source data of her study.
References
- 1.Gallego P, Gonzalez AE, Sanchez-Recalde A, Peinado R, Polo L, Gomez-Rubin C, Lopez-Sendon JL, Oliver JM. Incidence and predictors of sudden cardiac arrest in adults with congenital heart defects repaired before adult life. Am J Cardiol 2012;110:109–117. [DOI] [PubMed] [Google Scholar]
- 2.Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol 1998;32:245–251. [DOI] [PubMed] [Google Scholar]
- 3.Koyak Z, Harris L, de Groot JR, Silversides CK, Oechslin EN, Bouma BJ, Budts W, Zwinderman AH, Van Gelder IC, Mulder BJ. Sudden cardiac death in adult congenital heart disease. Circulation 2012;126:1944–1954. [DOI] [PubMed] [Google Scholar]
- 4.Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am J Cardiol 2000;86:1111–1116. [DOI] [PubMed] [Google Scholar]
- 5.Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, van Dijk AP, Vliegen HW, Grobbee DE, Mulder BJ. Mortality in adult congenital heart disease. Eur Heart J 2010;31:1220–1229. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 7.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 8.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;17:1601–1687. [Google Scholar]
- 9.Koyak Z, de Groot JR, Van Gelder IC, Bouma BJ, van Dessel PF, Budts W, van Erven L, van Dijk AP, Wilde AA, Pieper PG, Sieswerda GT, Mulder BJ. Implantable cardioverter defibrillator therapy in adults with congenital heart disease: who is at risk of shocks? Circ Arrhythm Electrophysiol 2012;5:101–110. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV, Maass AH, de Groot JR, van Oostrom AJ, Theuns DA, Jordaens LJ, Wilde AA, Knops RE. The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol 2012;60:1933–1939. [DOI] [PubMed] [Google Scholar]
- 12.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (31 January 2016).
- 13.Backhoff D, Muller M, Ruschewski W, Paul T, Krause U. ICD therapy for primary prevention of sudden cardiac death after Mustard repair for d-transposition of the great arteries. Clin Res Cardiol 2014;103:894–901. [DOI] [PubMed] [Google Scholar]
- 14.Cannon BC, Friedman RA, Fenrich AL, Fraser CD, McKenzie ED, Kertesz NJ. Innovative techniques for placement of implantable cardioverter-defibrillator leads in patients with limited venous access to the heart. PACE 2006;29:181–187. [DOI] [PubMed] [Google Scholar]
- 15.Çeliker A, Olgun H, Karagoz T, Özer S, Özkutlu S, Alehan D. Midterm experience with implantable cardioverter-defibrillators in children and young adults. Europace 2010;12:1732–1738. [DOI] [PubMed] [Google Scholar]
- 16.Cuypers JA, Eindhoven JA, Slager MA, Opic P, Utens EM, Helbing WA, Witsenburg M, van den Bosch AE, Ouhlous M, van Domburg RT, Rizopoulos D, Meijboom FJ, Bogers AJ, Roos-Hesselink JW. The natural and unnatural history of the Mustard procedure: long-term outcome up to 40 years. Eur Heart J 2014;35:1666–1674. [DOI] [PubMed] [Google Scholar]
- 17.Dore A, Santagata P, Dubuc M, Mercier LA. Implantable cardioverter defibrillators in adults with congenital heart disease: a single center experience. PACE 2004;27:47–51. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Moore JP, Shannon K. A novel approach to eliminate intraventricular lead placement in patients with congenital heart disease. J Interv Card Electrophysiol 2012;35:115–118. [DOI] [PubMed] [Google Scholar]
- 19.Jin BK, Bang JS, Choi EY, Kim GB, Kwon BS, Bae EJ, Noh CI, Choi JY, Kim WH. Implantable cardioverter defibrillator therapy in pediatric and congenital heart disease patients: a single tertiary center experience in Korea. Korean J Pediatr 2013;56:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan CP, Freedenberg V, Wang Y, Curtis JP, Gleva MJ, Berul CI. Implant and clinical characteristics for pediatric and congenital heart patients in the national cardiovascular data registry implantable cardioverter defibrillator registry. Circ Arrhythm Electrophysiol 2014;7:1092–1100. [DOI] [PubMed] [Google Scholar]
- 21.Kella DK, Merchant FM, Veledar E, Book W, Lloyd MS. Lesion-specific differences for implantable cardioverter defibrillator therapies in adults with congenital heart disease. PACE 2014;37:1492–1498. [DOI] [PubMed] [Google Scholar]
- 22.Khairy P, Harris L, Landzberg MJ, Fernandes SM, Barlow A, Mercier LA, Viswanathan S, Chetaille P, Gordon E, Dore A, Cecchin F. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: a multicenter study. Circ Arrhythm Electrophysiol 2008;1:250–257. [DOI] [PubMed] [Google Scholar]
- 23.Khairy P, Harris L, Landzberg MJ, Viswanathan S, Barlow A, Gatzoulis MA, Fernandes SM, Beauchesne L, Therrien J, Chetaille P, Gordon E, Vonder MI, Cecchin F. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation 2008;117:363–370. [DOI] [PubMed] [Google Scholar]
- 24.Khanna AD, Warnes CA, Phillips SD, Lin G, Brady PA. Single-center experience with implantable cardioverter-defibrillators in adults with complex congenital heart disease. Am J Cardiol 2011;108:729–734. [DOI] [PubMed] [Google Scholar]
- 25.Korte T, Koditz H, Niehaus M, Paul T, Tebbenjohanns J. High incidence of appropriate and inappropriate ICD therapies in children and adolescents with implantable cardioverter defibrillator. PACE 2004;27:924–932. [DOI] [PubMed] [Google Scholar]
- 26.Kowalski M, Nicolato P, Kalahasty G, Kasirajan V, Wood MA, Ellenbogen KA, Shepard RK. An alternative technique of implanting a nontransvenous implantable cardioverter-defibrillator system in adults with no or limited venous access to the heart. Heart Rhythm 2010;7:1572–1577. [DOI] [PubMed] [Google Scholar]
- 27.Lopez JA. Implantable cardioverter defibrillator lead placement in the middle cardiac vein after tricuspid valve surgery. Europace 2012;14:853–858. [DOI] [PubMed] [Google Scholar]
- 28.Michael KA, Veldtman GR, Paisey JR, Yue AM, Robinson S, Allen S, Sunni NS, Kiesewetter C, Salmon T, Roberts PR, Morgan JM. Cardiac defibrillation therapy for at risk patients with systemic right ventricular dysfunction secondary to atrial redirection surgery for dextro-transposition of the great arteries. Europace 2007;9:281–284. [DOI] [PubMed] [Google Scholar]
- 29.Stefanelli CB, Bradley DJ, Leroy S, Dick M, Serwer GA, Fischbach PS. Implantable cardioverter defibrillator therapy for life-threatening arrhythmias in young patients. J Interv Card Electrophysiol 2002;6:235–244. [DOI] [PubMed] [Google Scholar]
- 30.Stephenson EA, Batra AS, Knilans TK, Gow RM, Gradaus R, Balaji S, Dubin AM, Rhee EK, Ro PS, Thogersen AM, Cecchin F, Triedman JK, Walsh EP, Berul CI. A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol 2006;17:41–46. [DOI] [PubMed] [Google Scholar]
- 31.Uyeda T, Inoue K, Sato J, Mizukami A, Yoshikawa T, Wada N, Ando M, Takahashi Y, Umemura J, Park IS. Outcome of implantable cardioverter defibrillator therapy for congenital heart disease. Pediatr Int 2012;54:379–382. [DOI] [PubMed] [Google Scholar]
- 32.Ludmir J, Khan A, Shah M, Kim Y. Arrhythmia burden and sudden cardiac death in adults with systemic right ventricles. Heart Rhythm 2014;11(Suppl. 5):S241. [Google Scholar]
- 33.Santharam S, Theodosiou M, Thorne S, Clift P, Hudsmith L, De BJ. Long-term follow-up of ICD in adult congenital heart disease patients: a large single centre experience. Heart 2014;100(Suppl. 3):A3–A4. [Google Scholar]
- 34.Torres JL, Kumar S, Kamalov G, Amin A, Hummel J, Daoud E, Augostini R, Houmsse M, Kalbfleisch S, Love C, Rhodes T, Tyler J, Weiss R. Subcutaneous cardiac defibrillator in patients with congenital heart disease: a single center experience. Heart Rhythm 2014;11(Suppl. 5):S488. [Google Scholar]
- 35.Wheeler M, Grigg L, Zentner D. Can we predict sudden cardiac death in long-term survivors of atrial switch surgery for transposition of the great arteries? Congenit Heart Dis 2014;9:326–332. [DOI] [PubMed] [Google Scholar]
- 36.Khairy P, Landzberg MJ, Gatzoulis MA, Lucron H, Lambert J, Marcon F, Alexander ME, Walsh EP. Value of programmed ventricular stimulation after tetralogy of Fallot repair: a multicenter study. Circulation 2004;109:1994–2000. [DOI] [PubMed] [Google Scholar]