Abstract
The convergence of science and technology in our dynamic digital era has resulted in the development of innovative digital health devices that allow easy and accurate characterization in health and disease. Technological advancements and the miniaturization of diagnostic instruments to modern smartphone-connected and mobile health (mHealth) devices such as the iECG, handheld ultrasound, and lab-on-a-chip technologies have led to increasing enthusiasm for patient care with promises to decrease healthcare costs and to improve outcomes. This ‘hype’ for mHealth has recently intersected with the ‘real world’ and is providing important insights into how patients and practitioners are utilizing digital health technologies. It is also raising important questions regarding the evidence supporting widespread device use. In this state-of-the-art review, we assess the current literature of mHealth and aim to provide a framework for the advances in mHealth by understanding the various device, patient, and clinical factors as they relate to digital health from device designs and patient engagement, to clinical workflow and device regulation. We also outline new strategies for generation and analysis of mHealth data at the individual and population-based levels.
Keywords: Digital health, mHealth, Medical technology, Sensors, Patient-generated data
The digitization of healthcare
Within these early years of the 21st century, we have witnessed remarkable technological progress with the developments of powerful and portable computing devices. Simultaneously, a global connection resulting from broadband and satellite technologies has resulted in an increasing number of ‘connected users’ for information sharing. The emergence of new mobile health (mHealth) technologies has resulted from the temporal intersection of several coincidental movements: (i) an urgent need to address the rising burden of chronic diseases; (ii) Moore's law—the exponential increase in computing power resulting in the development of smaller and cheaper mobile electronics1; and (iii) shifting healthcare model to an increasingly patient-centric designs.2 mHealth is defined by the practice of medicine supported by portable diagnostic devices. Use of these devices at the point-of-care is resulting in a change in the method of healthcare delivery from one that was health-systems generated to one that is remote and patient generated.3,4 The culmination of these factors presents unparalleled opportunities to increase patient engagement, to reduce healthcare costs, and to improve outcomes.5
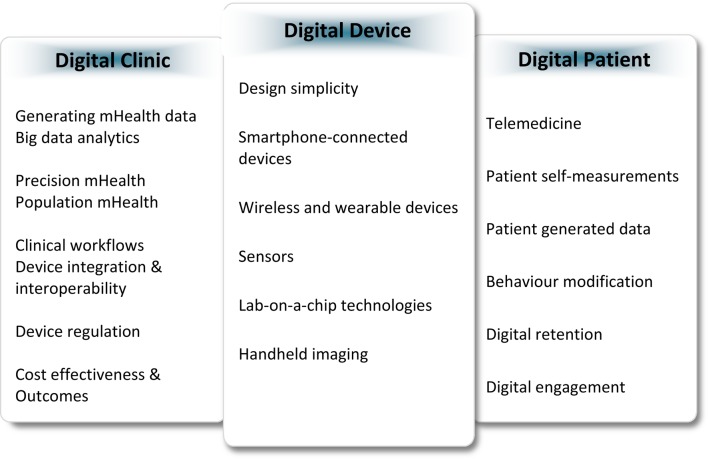
To reach the transformative potential of mHealth, a great deal of validation of the technical capabilities and accuracy, as well as the clinical impact of these technologies, is needed before we know they are effective. The real-world practice of medicine is complex and raises important questions on how we can generate clinically meaningful digital health data. Clinicians are beginning to enquire whether more devices necessarily mean more information and if some information may be redundant or even unnecessary. As mHealth devices become increasingly available, three important questions arise: who should be the first digital health adopter: the patient, the provider, or the healthcare system? What factors of mHealth are most effective? And what is the evidence supporting the clinical utilization of such devices? As we aim to determine the effectiveness of these technologies, what are the outcomes—morbidity and mortality—or are patient-generated outcomes such as quality of life equally important? Are patients prepared to understand mHealth findings particularly elderly patients or those with complex disease states? Do patients modify their behaviour? Will user-generated data lead to patients seeking out therapies for digital data rather than true disease states? We present these questions as they relate across the digital device, the digital patient, and the digital clinic (Figure 1), and discuss the literature evaluating mHealth towards their answers.
Figure 1.
Factors related to mHealth adoption across the digital device, the digital patient, and the digital clinic.
Digital devices
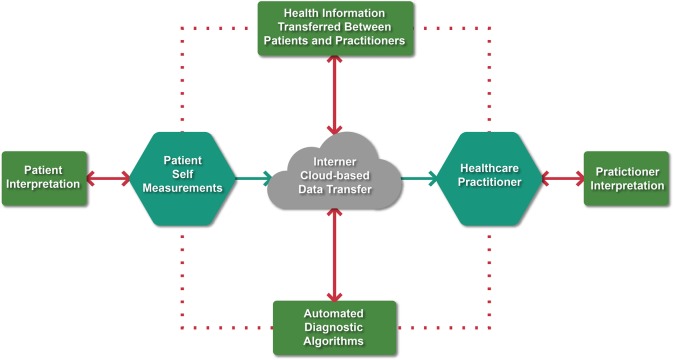
Which components of digital devices make them usable and how do these devices help to solve clinical problems? Five classifications of mHealth technologies have been developed: smartphone health ‘apps’ (>160 000 currently available),6 smartphone-connected devices; wearable and wireless devices; handheld-imaging platforms, and miniaturized sensor-based technologies.7,8 Conditions such as hypertension, diabetes, and heart failure (HF), as well as medication adherence monitoring, have seen significant advances across most technological categories (see Supplementary material online, Table S1). As new technologies are devloped, data transfer becomes increasingly important, especially when considering how data derived from mHealth devices integrates into clinical workflows. In general, a closed data loop is necessary and involves a cycle initiated by the patient or provider, followed by Internet (cloud)-based data transfer, interpretation of these findings or automated algorithms, and the data being returned to the patient and provider for clinical decisions (Figure 2). Herein, we discuss several mHealth technologies that have been approved for use by EU and US regulatory authorities and how such technologies advance our understanding of common clinical problems.
Figure 2.
The mHealth data flow for clinical care. To maximize clinical care, a closed loop is necessary that involves patient- or practitioner-derived mHealth data, Internet-based data transfer interpreted by patients, practitioners, or with automated algorithms, and returned back to patients and providers for clinical decisions.
Smartphone-connected rhythm monitoring devices
One such technology is the iECG, a smartphone case that incorporates electrodes for wireless cardiac telemetry monitoring (AliveCor), and was approved for use by the US Food and Drug Administration (US-FDA) and EU Medical Device Directive (EU-MDD) in 2013. A 30-s single-lead (lead I) rhythm strip is produced by a case-like attachment when held in the right and left hands. A real-time display of the cardiac rhythm is created by conversion of an electrical signal into ultrasound and is captured by the smartphone microphone. Automated algorithms were developed and approved for use, which provide the user with an immediate rhythm analysis of atrial fibrillation (http://www.accessdata.fda.gov/cdrh_docs/pdf14/K142743.pdf). To maximize the clinical effectiveness, the iECG should be used among patients at high risk for the development of an arrhythmia, and to capture the arrhythmia in real time for prompt clinical decisions. For example, the occurrence of sub-clinical atrial arrhythmias is a well-known cause of a cryptogenic stroke.9 Compared with usual care and intermittent monitoring strategies, studies investigating extended 6-month electrocardiographic monitoring with external event monitoring devices or internal devices such as pacemakers and loop recorders have identified a 9–16% incidence of sub-clinical atrial fibrillation among patients with known cerebrovascular disease and among those with hypertension, diabetes, or ischaemic heart disease.10–12 In the aggregate, ∼10 such patients need to be screened with extended monitoring to establish one new diagnosis of atrial fibrillation.9 The iECG is not designed as a continuous rhythm monitor; however, the relatively low cost (US$70–90 or £70–90) and high patient utilization make it a potentially practical alternative to monitor high-risk individuals for a prolonged duration.13 Several potential clinical applications of the iECG have recently emerged. The iTRANSMIT investigators demonstrated a 100% diagnostic accuracy of the iECG to detect the recurrence of an atrial arrhythmia after an ablation when compared with traditional transtelephonic monitoring.14 Future developments include extending the single-lead monitor to multiple iECG leads for remote monitoring of an acute coronary syndrome.15
Wireless and wearable devices
Analogous to smartphone-based devices, continuous blood pressure and glucose-monitoring technologies have also been developed. In contrast to intermittent cuff-based blood pressure devices, continuous 24-h ambulatory devices have been manufactured and form fitted to watch-like configurations (BPro, HealthStats Inc.). Approved for use in 2014, the device uses applanation tonometry by applying mild pressure to partially flatten the radial artery to acquire measurements of central aortic systolic pressure. The subsequent systolic waveform produces a digital blood pressure signal from the radial artery to the overlying watch that is transmitted at 15-min intervals and recorded for up to 24 h (Supplementary material online, Figure S3A). The findings from Ambulatory Central Aortic Pressure study demonstrated the application of this technique and compared the watch-like device with conventional cuff-based ambulatory measurements.16 Among 171 hypertensive participants, tonometric measurements correlated within a 5-mmHg margin to conventional ambulatory measurements and tracked the reduction in blood pressure with antihypertensive therapy over a follow-up duration of 3 months. Continuous monitoring of blood pressure in the ambulatory setting may be important among patients with drug resistant hypertension or those with orthostatic hypotension. Continuous glucose monitoring (CGM) with minimally invasive sensor technologies (Dexcom) involves the implantation of a small transcutaneous electrode into the subcutaneous tissue of the abdomen or arm where a glucose oxidase chemical reaction produces a current reflecting the interstitial glucose concentration.17,18 This electrical signal is converted into glucose concentrations and is transmitted to a smartphone or tablet computer at 5-min intervals for real-time continuous monitoring (Supplementary material online, Figure S3B). Several aspects of CGM are proving effective among diabetic patients including the prevention of hypoglycaemic episodes with early detection, and as a method for long-term glycaemic control resulting from positive behavioural changes such as diet, exercise, and medication compliance that are facilitated by the awareness of glucose measurements and real-time trends.19
Implantable and ingestible sensors
Unlike the average car that is equipped with sensors that gauge the vehicle's position, speed, and fluid levels alerting the driver when readings are out of range, the human body has not been designed with similar alert mechanisms to monitor internal physiological functions. Nanoparticle biosensors have been designed with some that are fully implantable. These sensors act as a ‘fuel gauge’ transmitting internal measurements of physiological function in a step towards digitizing the human body.20,21
Implantable sensors
The signs and symptoms of congestion in HF commonly precede changes in vital signs or those markers that predict a decompensation. Presently, the assessment of filling pressures in the ambulatory setting include devices that measure right ventricular and pulmonary artery pressures.22 Approved in 2014, the CardioMEMS device is a fully implantable micro-electromechanical pulmonary artery pressure monitoring system (Supplementary material online, Figure S3C). The sensing platform is designed with a combination of an inductor coil and a pressure-sensitive capacitor creating a resonant circuit that changes in response to pressures. The system is leadless and battery-free, and is implanted with passive fixation during a right heart catheterization. Pulmonary artery pressure is continuously monitored, and sensor readings are wirelessly transmitted to an external unit and to a cloud-based platform for clinical review. The CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) trial was a prospective, single-blind study that randomized 550 class III patients with a prior HF hospitalization to the CardioMEMS device with wireless implantable haemodynamic monitoring (W-IHM), or to controls with an implanted device and monitoring turned off.23 Patients were instructed to take daily measurements, and a review of pressure data by trial coordinators occurred at least once weekly. At 6 months, W-IHM was associated with a 30% reduction in HF readmissions (hazard ratio 0.70, 95% CI 0.60–0.84, P < 0.0001), a shorter HF-related hospital length of stay among those admitted for treatment (2.2 vs. 3.8 days, P = 0.02), and was associated with a greater number of changes to neurohormonal and diuretic therapies than was the control group (nine changes per patient vs. four, P < 0.0001). A post hoc analysis among 119 patients with HF and preserved ejection fraction showed that the 6- and 18-month HF readmission rates were 46 and 50% lower in the W-IHM group than in controls, respectively.24 These results underscore the significance of remotely monitoring cardiopulmonary pressures, and may be of particular significance in the preserved ejection fraction cohort given the lack of effective therapies to mitigate adverse outcomes in this population.
Ingestible sensors
Non-adherence to medications has been documented to occur in >60% of patients with cardiovascular diseases and remains one of the most common contributing factors resulting in symptoms recurrence and adverse outcomes.25 Wireless observed therapy with a novel ingestible sensor (Proteus Digital) is an approved and unique technology to monitor medication compliance.26 This system consists of two major components: an edible sensor and a wearable receiver patch. The edible sensor is an integrated circuit with 1 mm in diameter and 200 µm in thickness, and is composed of magnesium and copper (Supplementary material online, Figure S3D). Gastric fluids activate the sensor, and an electrochemical reaction produces a voltage across the circuit creating a biogalvanic battery and an electrical field. The signal remains active for 8–10 min and is transmitted to an overlying Band-Aid size abdominal patch. The digital data are subsequently transferred to a smartphone application and a cloud-based platform for review by patients and practitioners. The safety and performance of this networked sensor system has been evaluated in patients with hypertension and HF, and has demonstrated a positive detection accuracy of 97% after 3400 sensor ingestions with false signals observed in <2% of ingestions.27 These sensors may be most effective where there is the greatest need to monitor compliance, and among patients where medication non-adherence risks adverse outcomes. Such scenarios include monitoring adherence to diuretic and β-blocker therapy among HF patients at high risk for readmissions, anticoagulation therapy among atrial fibrillation patients at increased risk for bleeding or thromboembolic complications, and to reduce the risk of stent thrombosis among those receiving dual antiplatelet therapy.
The digital patient
Will patients use and engage with mHealth devices? As clinicians are well aware, changing patient behaviour and sustaining behavioural changes are exceedingly difficult. An expectation from the use of mHealth is a positive behavioural change resulting from patients actively participating in self-care and shared decision-making.28,29 Device-related factors including design simplicity and usability are important in determining which technologies may be most effective. Equally important are patient factors including patient selection and motivation towards self-monitoring.30,31 In our opinion, we can consider four categories of patients who engage with mHealth technologies: the first self-select as high-efficiency utilizers, those who are predetermined to modify their behaviours and where devices largely become a bystander in the positive behavioural change; the second are initial adopters but rapidly decline and do not retain device use; the third do not adopt; and the fourth demonstrate a change as the underlying condition, and symptoms improve resulting from modifying behaviours and treatments enabled by device use. The ultimate goal of mHealth is to transition Category 2 and 3 patients to Category 4.
Telemedicine and patient self-measurements
Several studies have demonstrated important observations of mHealth and telemedicine across various patient populations (Table 1). Low cost, and widely accessible interventions including text messaging and smartphone apps are effective strategies to promote smoking cessation32, as a method to improve medication adherence33 and are simple interventions to prevent diabetes in at-risk patients,34 and to improve outcomes among patients with coronary heart disease.35,36 Self-measurements with mHealth devices have been associated with improvements in blood pressure (mean systolic blood pressure reduction of 3-9 mmHg),37 and improved glycemic control (mean reduction in HbA1c of 0.1-0.3%)38 among hypertensive and diabetic patients, respectively, and is associated with an increased activity of 2500 steps/day among individuals using pedometers for a monitoring duration of up to 6 months.39 Numerous studies evaluating mHealth in patients with cardiac disease, particularly HF, have been conducted over the past decade.40 Device evolution during this time has permitted the design of telemedicine trials that remotely monitor multiple physiological parameters including blood pressure, weight, and heart rate. The established body of clinical trial data has largely demonstrated a beneficial impact of telemedicine in HF including improved survival and reduced HF-related hospitalization when compared with usual care and scheduled patient follow-up.40,41 In contrast, some studies have demonstrated no difference on outcomes.42,43 In the critical analysis of a rapidly evolving field, this difference requires explanation. Since devices are generally used similarly and clinical decisions for the management of HF symptoms are largely standard, this difference may result more from different patient classifications than device-related factors. These include elderly patients, those with multiple comorbidities, and patients with advanced disease states where the transmission of surrogate markers of cardiopulmonary pressures such as changes in weight and blood pressure may not reflect minor changes in already elevated filling pressures, or a rapid rise in pressure that prompts symptoms and decompensation.44,45 Determining a match between patients and digital technologies is necessary to determine the effectiveness of telemedicine and to identify which patients are suitable for device-based self-care. Such circumstances include selecting the appropriate technology that is based on the desired outcome, i.e. glycaemic46 or blood pressure control,47,48 weight loss,49 or for reducing hospital readmissions.42,43 This match is particularly important when considering remote monitoring among patients with advanced disease states and in scenarios when a healthcare visitation may be more important than telemedicine and device-based self-measurements.50
Table 1.
Select trials investigating an mHealth device, text messaging, or a smartphone health application
| Study | Study size | Study population | Digital health technology intervention group | Comparator | Outcomes | Salient findings |
|---|---|---|---|---|---|---|
| Hypertension | ||||||
| McManus et al.47 TASMIN-SR Randomized trial UK |
552 | Hypertensive patients with a history of stroke, coronary heart disease, diabetes, or chronic renal failure | Microlife Watch home-based blood pressure monitoring with medication self-titration | Usual care | 12-month difference in systolic blood pressure | Greater systolic blood pressure reduction with self-monitoring and medication titration (mean difference of −9 mmHg, 95% CI 6–13 mmHg) |
| Magid et al.48 Randomized trial USA |
348 | Adult patients with hypertension | Home-based blood pressure monitoring and Heart360 Web-based platform | Usual care | 6-month proportion of patients achieving blood pressure target of <140/90 | Greater proportion of patients achieving blood pressure reduction with home-based monitoring (54 vs. 35%, P < 0.001) and a mean reduction in systolic blood pressure of 12 mmHg (95% CI −16 to −9 mmHg) |
| Diabetes | ||||||
| Ramachandran et al.34 Randomized trial India |
537 | Adult men with impaired glucose tolerance | Text messaging to promote exercise and dietary habits | Usual care | 2-year incidence of biochemically proven type 2 diabetes | Lower incidence of type 2 diabetes in the text messaging group than in controls [(18 vs. 27%) hazard ratio 0.64 (95% CI 0.45–0.92), P = 0.015] |
| Quinn et al.68 Mobile Diabetes Intervention Study Cluster randomized trial USA |
163 | Adult patients with type II diabetes and HbA1c ≥ 7.5% | Smartphone diabetes application for medication reconciliation and self-care measures as well as clinical decision support | Usual care | Glycaemic control and HbA1c at 12 months | Greater reduction in HbA1c with smartphone application and clinical decision support (−1.9 vs. −0.7%, P < 0.001) |
| Holmen et al.46 RENEWING HEALTH Randomized trial |
151 | Adult patients with type II diabetes | Few touch smartphone application | Usual care | Glycaemic control and HbA1c at 4 months | No difference in HbA1c glycaemic control |
| Cardiac arrest | ||||||
| Ringh et al.69 Randomized trial Sweden |
9928 | Lay volunteers trained in cardiopulmonary resuscitation | Mobile-phone positioning system activated upon notification of an out-of-hospital cardiac arrest and emergency medical services. Simultaneous notification sent to nearby volunteers | Text message or phone call notification not delivered to control group volunteers | Bystander-initiated cardiopulmonary resuscitation before arrival of emergency medical services | The primary outcome measure of bystander-initiated cardiopulmonary resuscitation was significantly higher in the intervention group than in the control group (62 vs. 48%, P < 0.001) |
| Heart failure | ||||||
| Koehler et al.42 TIM-HF Randomized trial Germany |
710 | Ambulatory class II–III HF patients with ejection fraction ≤35% | Weight scale, blood pressure, and single lead ECG | Usual care | Composite outcome of hospital admission for HF and/or all-cause mortality at 24 months | No significant difference in outcomes of mortality or HF hospitalization [(15 vs. 17%) hazard ratio 0.89 (95% CI 0.67–1.19, P = 0.44)] |
| Weintraub et al.41 SPAN-CHF II Randomized trial USA |
188 | Symptomatic HF patients with a prior hospitalization within 2 weeks | Weight scale, blood pressure, and heart rate monitor | Usual care | HF readmission at 3 months | At 3 months, telemedicine interventions were associated with a reduction in HF readmission [(10 vs. 19%) hazard ratio 0.50 (95% CI 0.25–0.99, P = 0.05)] |
| Cardiac surgery | ||||||
| Cook et al.70 Prospective observational USA |
149 | Adult patients >50 years of age undergoing cardiac or vascular surgery | Wireless activity monitor | – | Relationship between activity and post-operative length of stay | Patients with a shorter length of stay were associated with a greater number of steps compared with patients with longer length of stay (818 vs. 223 steps/day, P < 0.001) |
| Arrhythmia | ||||||
| Barrett et al.71 Prospective observational USA |
146 | Patients referred cardiac arrhythmia management | Zio Patch wireless telemetry monitor | Simultaneous Holter monitor | Comparison of the arrhythmia detection over the total wear time | Zio Patch detected significantly more events over the total wear time compared with Holter monitoring (96 vs. 61 events, P < 0.001) |
| Lowres et al.72 SEARCH-AF Prospective observational Australia |
1000 | Patients aged 65 or greater screened for the presence of an atrial arrhythmia | AliveCor smartphone iECG | – | Prevalence of newly diagnosed atrial fibrillation | Smartphone rhythm screening by pharmacists demonstrated a 7% prevalence and a 1.5% incidence of newly diagnosed atrial fibrillation in a community cohort of elderly patients |
| Coronary heart disease | ||||||
| Chow et al.33 TEXT ME Randomized trial Australia |
710 | Adult patients with coronary heart disease established by a prior history of a myocardial infarction or angiographically proven | Text messaging to promote tobacco abstinence, healthy eating, and maintaining physical activity | Usual care | 6-month LDL-C levels, systolic blood pressure, body mass index, physical activity, and smoking status | At 6 months, text messaging was associated with a lower LDL-C (−5 mg/dL), a greater reduction in systolic blood pressure (−7.6 mmHg), a lower body mass index (−1.3), increases in physical activity (+2.93 metabolic equivalents), and a significant reduction in smoking (26 vs. 44%) compared with controls |
| Cardiac rehabilitation | ||||||
| Varnfield et al.73 Randomized trial Australia |
120 | Patients with a recent myocardial infarction | Smartphone-based home services including health and exercise monitoring | Usual care | 6-month adherence to cardiac rehabilitation programmes, 6-min walk test, and quality-of-life assessments | Smartphone-based interventions were associated with a greater adherence (94 vs. 68%) and completion (80 vs. 47%) to rehabilitation programmes and a greater improvement in quality of life than controls. No difference in 6-min walk distance |
| Chronic diseases | ||||||
| Steventon et al.52 Whole Systems Demonstrator Cluster randomized trial UK |
3230 | Adults with diabetes, HF, or chronic pulmonary diseases | Telehealth devices including weight scales, glucometers, and pulse oxymeters | Usual care | 12-month hospital admission and/or mortality | Telehealth was associated with a 18% lower risk of hospital admissions (odds ratio 0.82, 95% CI 0.7–0.97, P = 0.017) and 46% lower risk of death (odds ratio 0.54, 95% CI 0.39–0.75, P < 0.001) |
| Healthy lifestyle | ||||||
| Mattila et al.54 Randomized trial Finland |
114 | Healthy adults | Technology toolbox—weight scales, blood pressure monitors, pedometers, wellness diary, and online food log | Usual employee wellness information | Percentage of sustained use at 6 months Changes in weight, blood pressure, and fasting lipid profile |
30% sustained use at 6 months Overall, no significant difference in weight, blood pressure, or fasting lipid profile between groups. Weight, body fat, and body mass index decreased in sustained users when compared with non-sustained users |
| Laing et al.49 mFit trial Randomized trial USA |
212 | Patients with a body mass index >25 kg/m2 | MyFitness Pal Smartphone Application | Usual care | Changes in weight and blood pressure at 6 months | No significant difference in weight [(mean group difference −0.3 kg (95% CI −1.5 to 1.0 kg, P = 0.63)] or blood pressure [(mean group difference −1.7 mmHg (95% CI −7.1 to 3.8 mmHg, P = 0.55)] between groups |
| Smoking cessation | ||||||
| Free et al.32 txt2stop trial Randomized trial UK |
5800 | Adult smokers aged 16 or greater | Text messaging to promote smoking cessation using motivational messages and behavioural change support | Text messaging unrelated to smoking cessation | 6-month outcome of self-reported tobacco abstinence and biochemical verification with salivary cotinine testing | Biochemically verified continuous abstinence at 6 months was greater in the text messaging group than in controls [(10.7 vs. 4.9%) hazard ratio 2.20 (95% CI 1.80–2.68, P < 0.001)] |
Digital retention
The efficient use of mHealth devices may occur when used among patients who understand the nuances of electronic technologies including the Internet and smartphones and are able to apply the cross-functionality of one device to another.51 The Whole Systems Demonstrator randomized trial in the UK investigated the impact of telemedicine among 3000 elderly patients with chronic conditions including pulmonary diseases, HF, and diabetes on health-related outcomes. Over a 12-month monitoring period, telemedicine interventions with various mHealth and home-based monitoring devices were associated with improved survival and a lower probability of a hospitalization when compared with standard care (Table 1).52 Despite these positive findings, the investigators reported recruitment challenges with ∼40% of the 9000 eligible patients refusing enrolment and identified important patient-related reasons for non-participation and trial withdrawal, including a concern for the technical competence for operation of mHealth devices and a perception that device-based self-care will replace usual face-to-face visitations.53
The duration of remote monitoring and digital retention are important factors when considering the time required to sustain long-term behavioural changes and to achieve risk reduction in conditions such as hypertension and diabetes. In this context, Mattila et al. have provided important insights of the perceptions of mHealth and assessed the patterns of device use among a heterogeneous group of individuals seeking health improvements.54 Multiple digital tools including pedometers, weight scales, blood pressure devices, calorie counters, and Web-based programmes were assessed in a randomized trial comparing usual wellness programmes (n = 116) with mHealth interventions (n = 118) on health-related outcomes. Participants underwent testing such as body fat, aerobic fitness, and cholesterol testing at regular intervals for 1 year. Throughout the trial>75% of participants continued to state a beneficial effect of mHealth on weight loss and physical activity. Despite these positive perceptions, an early and rapid attrition in device use was observed with <50% of participants continuing to use a device at 3 months leading to a very low digital retention rate of 30% at 6 months (Figure 3). The dichotomy between high perceived utility and low sustained use presents a significant challenge to promote digital retention and may result from a lack of understanding of the requirements for self-monitoring, as well as device fatigue through repetitive use of the same technologies over time.
Figure 3.
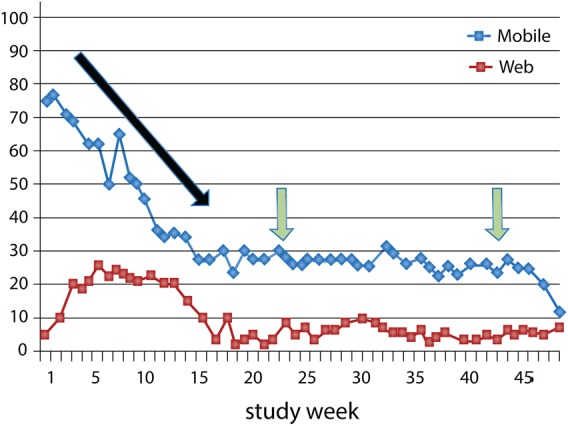
mHealth digital retention. A rapid and early attrition to device use (black arrow) and a low digital retention (green arrows) of 30% at 6 months with mHealth-based self-monitoring. Web denotes online and Internet-based platforms for health and fitness management. Reprinted and modified with permission from Mattila et al.54
The digital clinic
How can we generate mHealth data, analyse it so that it is clinically meaningful, and integrate it within clinical workflows? Each component of this question is important, and while there has been progress there are not conclusive answers. Several approaches exist to generate mHealth data. One involves precision and personalized care. The other incorporates population-based approaches and device use in new patient populations.
Precision-based mHealth and N-of-1 designs
To generate data in a field with a short duration for technology turnover, mHealth clinical trials are challenged to generate data in a time-efficient fashion and where the lengthy process from study design to execution may be surpassed by new technologies prior to the generation of trial results.3 By design, the ‘N-of-1’ trial uses the patient as his or her own control and obtains multiple repeated measurements to determine the optimal response to a particular treatment or intervention.55 Not all conditions are suitable for N-of-1 designs including monitoring individuals at risk for a myocardial infarction where acute and rapid changes are often preceded by a long periods of clinical stability. In contrast, chronic conditions such as hypertension, diabetes, and HF may be ideally suited and where physiological parameters of blood pressure, glucose, and weight are easily measurable and frequently change in a short period of time. Figure 4 illustrates a practical example of an N-of-1 design in a patient with hypertension. With the aid of frequent blood pressure measurements, we are able to visualize the blood pressure response to two different classes of antihypertensive medications, the washout and carry-over periods, and ultimately which medication may be best targeted for an individualized drug response. On one hand, the strengths of such designs include an individualized approach to treatments, and to quantify disease trends with multiple repeated measures that were previously unidentifiable.56 On the other hand, tracking minor variations may reveal sub-clinical changes that do not require interventions. The clinician must remain cognisant of noise signals and artefacts observed on mHealth devices that may result in false-positive findings, alarms, and misinterpretations when performed in real-world settings.55
Figure 4.
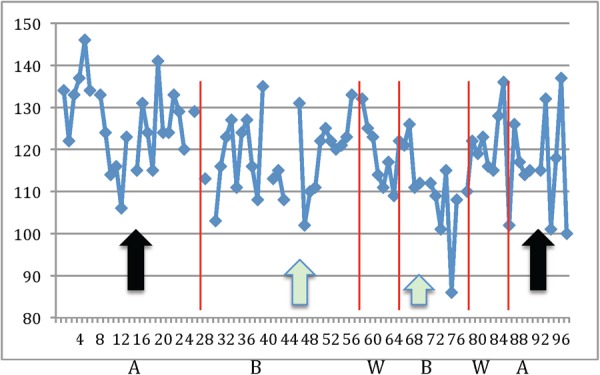
N-of-1 design and precision-based mHealth. (A and B) Denote different antihypertensive drug classes. Drug B (green arrows) lowers blood pressure more than drug A (black arrows). W denotes washout period and the x- and y-axes are the time (in days) and blood pressure (in mmHg), respectively. Figure courtesy of Nicholas Schork PhD and modified with permission.
Population-based mHealth in resource-limited areas
The mHealth advances to improve outcomes and decrease costs in the healthcare systems of industrialized nations must coincide with the efforts to improve healthcare delivery in resource-limited areas. Innovative designs are required to address the rising burden of cardiovascular diseases in developing countries that require cost-efficient and scalable solutions.57 Smartphone and app-based medication adherence and lifestyle modification intervention were recently reported in the SimCard (Simplified Multifaceted Management Program for Individuals at High Cardiovascular Risk) trial that enrolled adults with atherosclerotic cardiovascular disease in rural Tibet and India.58 Twenty-three clinical sites (n = 1095 participants) randomized to an electronic decision support system powered by Android™ devices and used by community health workers at the point-of-care demonstrated a 17 and 25% increased rate of adherence to antihypertensive therapy and aspirin, respectively, when compared with clinics randomized to usual care (n = 991 participants). Timely healthcare access for conditions such as an acute coronary syndrome remains a challenge in resource-constrained areas. The design of electronic-ICUs in such regions to remotely diagnose and monitor individuals with a myocardial infarction have been associated with marked improvements in the process of STEMI care with a 60% reduction in door-to-needle time that subsequently lead to a >70% improvement in survival.59
Ubiquitous use of cellular and Internet technologies in developing nations has permitted the design of ‘telecardiology’ programmes with cloud computing—the sharing of information on Web-based platforms—and was first investigated in the seminal ASE-REWARD (American Society of Echocardiography: Remote Echocardiography with Web-Based Assessments for Referrals at a Distance) study. Performed within a 2-day period, >1000 patients with symptoms of cardiac disease were imaged with handheld ultrasound in a remote part of India.60 The echocardiographic studies were uploaded to a cloud-based server and distributed to 75 cardiologists scattered over 60 medical centres in four countries. Scans were uploaded within 4 min and interpreted by the global consortium of readers within 12 h. Results identifying complex structural heart disease were delivered back to the local clinicians effectively creating a digital platform for providing specialty cardiology services where it may be required the most. Among the various design features of mHealth devices, the portability, ease of use, and lower cost are among the features ideally suited for use in resource-limited areas. To assess the benefit of multiple mHealth devices, we have recently initiated the ASE-VALUES (Valvular Assessments Leading to Unexplored Echocardiographic Stratagems) randomized trial in India to evaluate the effectiveness of mHealth-derived assessments including cardiac rhythm, structural abnormalities, exercise capacity, and laboratory testing with point-of-care iECG, handheld echocardiography, activity monitoring, and lab-on-a-chip devices for predicting outcomes among patients with rheumatic heart disease and aims to advance the standard-of-care in the region.
mHealth regulation and integration
Whether in a fee-for-service or a national health system, the perceptions by medical, governmental, and financial institutions have largely supported the concept that mHealth can address the growing demands of an ageing population and rising healthcare costs (https://ec.europa.eu/digital-agenda/en/news/green-paper-mobile-health-mhealth).61 Several concerns have been raised into the approval of technologies that have not included outcomes data, and we are learning that some health-related apps and devices may not work adequately in the real world.62,63 Seminal observations have emerged into the high cost of care with mHealth in a unified health system where telemedicine interventions significantly exceeded the threshold for cost-effectiveness by £60 000 per quality-adjusted-life-year among elderly patients with common chronic conditions.64 Regulatory frameworks have been developed by the US-FDA65 and the EU-MDD (http://www.mdss.com/pdf/MDD93_42EEC.pdf, https://webstore.iec.ch/preview/info_iec62304%7Bed1.0%7Den_d.pdf) to harmonize new technology approvals; however, key challenges exist between fostering new innovations that are aligned with public health objectives to improve outcomes and reduce costs.
The eHealth Action Plan 2012–20 commissioned by the EU aims to determine the present challenges for mHealth across several domains including research and development, promoting international cooperation, achieving wider interoperability, and harnessing these findings to develop new health technology regulation and future legislation (http://ec.europa.eu/health/ehealth/docs/com_2012_736_en.pdf). One specific mandate is to address the unknown mechanisms necessary to develop data integration and the interoperability of mHealth within large volumes of existing patient data in national electronic health records (EHRs). Many health-related apps and mHealth devices are programmed to integrate within existing EHRs; however, few if any have achieved this.66 It is largely unclear how we should develop the resources necessary for administrating digital health services, and the requirement for healthcare personnel to monitor the wave of incoming patient-generated data. To address the integration challenges, Redfern et al. recently initiated the CONNECT (Consumer Navigation of Electronic Cardiovascular Tools) randomized study that is designed to investigate whether a digital health strategy of a smartphone-based app that provides patients with clinical decision support and counselling tools reduces risk in 2000 individuals with cardiovascular disease. Executed in single health system in Australia, the study aims to determine the acceptability and cost-effectiveness of this connected-care strategy.67 As digital health technologies evolve and become increasingly more available, we must remain vigilant towards monitoring the effectiveness of mHealth and its integration within day-to-day practices.
Healthcare's digital future
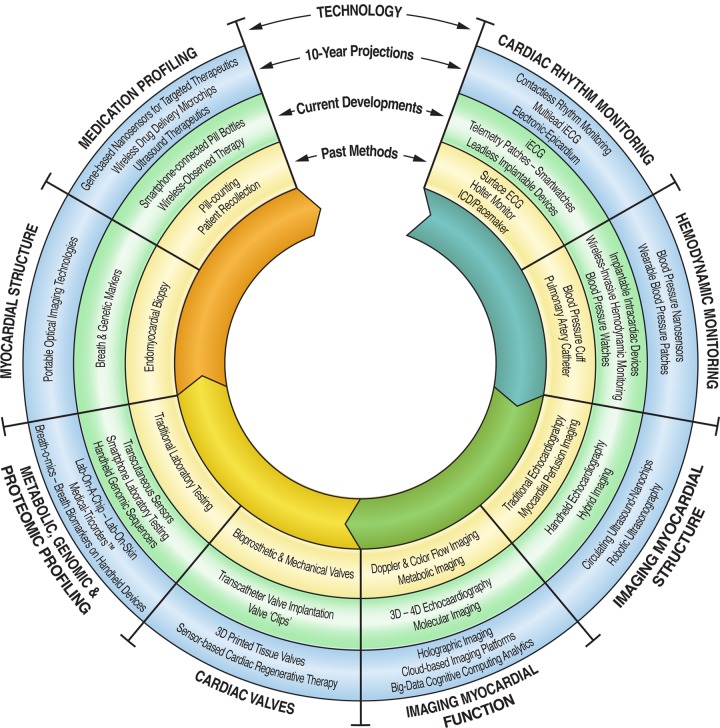
Within the next decade, we predict the development of new technologies across several areas in diagnostics, imaging, and therapeutics (Figure 5). Similar to clinical practice, the reality of mHealth is becoming increasingly complex. Our analysis of the current state of the field provides three main paths for translating mHealth to the real world: to identify new methods for patient engagement that results in beneficial and measurable behavioural changes, to develop the necessary tools to streamline clinical integration and data analytics, and to outline the regulatory factors that promote the most effective and robust technologies for clinical use. To achieve all three, we are collectively required to create an evidence base that assesses the impact of mHealth on healthcare quality, cost, and outcomes. In doing so, this interplay of digital devices, digital patients, and digital doctors holds exceptional promise for the future developments in medicine.
Figure 5.
Future mobile and digital health technologies.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
S.P.B., J.N., and P.P.S. conceived and designed the research, drafted the manuscript, and made critical revision of the manuscript for key intellectual content.
Acknowledgements
We are greatly indebted to Nicholas Schork PhD at the J. Craig Venture Institute, Elina Mattila PhD at the VTT Technical Research Institute, the Massachusetts Institute of Technology CSAIL and QUANTA laboratories for their generous contributions of the images and videos in this article, and to Akshay Bagai MD, and Nirtal Shah MSc from the University of Toronto for their comments and editorial assistance.
Conflict of interest: S.P.B. reports receiving an educational and research grant from the Qualcomm Foundation and Scripps Health. P.P.S. is adviser to Saffron Technology, TeleHealthRobotics, and Heart Test Laboratories, is a consultant with Edward Lifesciences, and has received grants from Forest Labs. J.N. reports no disclosures.
References
- 1.Moore GE. Cramming more components onto integrated circuits. Electronics 1965;38:114–117. [Google Scholar]
- 2.Topol EJ. Transforming medicine via digital innovation. Sci Transl Med 2010;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topol EJ, Steinhubl SR, Torkamani A. Digital medical tools and sensors. JAMA 2015;313:353–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, Riley WT, Shar A, Spring B, Spruijt-Metz D, Hedeker D, Honavar V, Kravitz R, Lefebvre RC, Mohr DC, Murphy SA, Quinn C, Shusterman V, Swendeman D. Mobile health technology evaluation: the mHealth evidence workshop. Am J Prev Med 2013;45:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva BM, Rodrigues JJ, de la Torre Díez I, López-Coronado M, Saleem K. Mobile-health: a review of current state in 2015. J Biomed Inform 2015;56:265–272. [DOI] [PubMed] [Google Scholar]
- 6.IMS Institute for Healthcare Informatics. Patient apps for improved healthcare: from novelty to mainstream http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS%20Health%20Institute/Reports/Patient_Apps/IIHI_Patient_Apps_Report.pdf (2013).
- 7.http://www.imedicalapps.com/2013/07/apple-android-medical-app/ (March 2015).
- 8.Walsh JA III, Topol EJ, Steinhubl SR. Novel wireless devices for cardiac monitoring. Circulation 2014;130:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamel H. Heart-rhythm monitoring for evaluation of cryptogenic stroke. N Engl J Med 2014;370:2532–2533. [DOI] [PubMed] [Google Scholar]
- 10.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J, CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 11.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O'Donnell M, Laupacis A, Côté R, Sharma M, Blakely JA, Shuaib A, Hachinski V, Coutts SB, Sahlas DJ, Teal P, Yip S, Spence JD, Buck B, Verreault S, Casaubon LK, Penn A, Selchen D, Jin A, Howse D, Mehdiratta M, Boyle K, Aviv R, Kapral MK, Mamdani M, EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–2477. [DOI] [PubMed] [Google Scholar]
- 12.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH, ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 13.Saxon LA, Smith A, Doshi S. iPhone rhythm strip—the implications of wireless and ubiquitous heart rate monitoring. J Am Coll Cardiol 2012;59:E726. [Google Scholar]
- 14.Tarakji KG, Wazni OM, Callahan T, Kanj M, Hakim AH, Wolski K, Wilkoff BL, Saliba W, Lindsay BD. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015;3:554–559. [DOI] [PubMed] [Google Scholar]
- 15.Muhlestein JB, Le V, Albert D, Moreno FL, Anderson JL, Yanowitz F, Vranian RB, Barsness GW, Bethea CF, Severance HW, Ramo B, Pierce J, Barbagelata A, Muhlestein JB. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS Pilot Study. J Electrocardiol 2015;48:249–259. [DOI] [PubMed] [Google Scholar]
- 16.Williams B, Lacy PS, Baschiera F, Brunel P, Düsing R. Novel description of the 24-hour circadian rhythms of brachial versus central aortic blood pressure and the impact of blood pressure treatment in a randomized controlled clinical trial: the Ambulatory Central Aortic Pressure (AmCAP) Study. Hypertension 2013;61:1168–1176. [DOI] [PubMed] [Google Scholar]
- 17.Facchinetti A, Del Favero S, Sparacino G, Castle JR, Ward WK, Cobelli C. Modeling the glucose sensor error. IEEE Trans Biomed Eng 2014;61:620–629. [DOI] [PubMed] [Google Scholar]
- 18.Del Favero S, Facchinetti A, Sparacino G, Cobelli C, AP@home consortium. Retrofitting of continuous glucose monitoring traces allows more accurate assessment of glucose control in outpatient studies. Diabetes Technol Ther 2015;17:355–363. [DOI] [PubMed] [Google Scholar]
- 19.Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, Brown SA, Chernavvsky DR, Breton MD, Mize LB, Farret A, Place J, Bruttomesso D, Del Favero S, Boscari F, Galasso S, Avogaro A, Magni L, Di Palma F, Toffanin C, Messori M, Dassau E, Doyle FJ III. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care 2014;37:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen LY, Tee BC, Chortos AL, Schwartz G, Tse V, Lipomi DJ, Wong HS, McConnell MV, Bao Z. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat Commun 2014;5:5028. [DOI] [PubMed] [Google Scholar]
- 21.Gurun G, Tekes C, Zahorian J, Xu T, Satir S, Karaman M, Hasler J, Degertekin FL. Single-chip CMUT-on-CMOS front-end system for real-time volumetric IVUS and ICE imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2014;61:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau CP, Siu CW, Tse HF. Future of implantable devices for cardiac rhythm management. Circulation 2014;129:811–822. [DOI] [PubMed] [Google Scholar]
- 23.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 24.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935–944. [DOI] [PubMed] [Google Scholar]
- 25.Baroletti S, Dell'Orfano H. Medication adherence in cardiovascular disease. Circulation 2010;121:1455–1458. [DOI] [PubMed] [Google Scholar]
- 26.DiCarlo L, Moon G, Intondi A, Duck R, Frank J, Hafazi H, Behzadi Y, Robertson T, Costello B, Savage G, Zdeblick M. Digital health solution for using and managing medications: wirelessly observed therapy. IEEE Pulse 2012;3:23–26. [DOI] [PubMed] [Google Scholar]
- 27.Au-Yeung KY, Moon GD, Robertson TL, Dicarlo LA, Epstein MS, Weis SE, Reves RR, Engel G. Early clinical experience with networked system for promoting patient self-management. Am J Manag Care 2011;17:e277–e287. [PubMed] [Google Scholar]
- 28.Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. JAMA 2015;313:459–460. [DOI] [PubMed] [Google Scholar]
- 29.Jain SH, Powers BW, Hawkins JB, Brownstein JS. The digital phenotype. Nat Biotechnol 2015;33:462–463. [DOI] [PubMed] [Google Scholar]
- 30.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev 2012;12:CD007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castelnuovo G, Zoppis I, Santoro E, Ceccarini M, Pietrabissa G, Manzoni GM, Corti S, Borrello M, Giusti EM, Cattivelli R, Melesi A, Mauri G, Molinari E, Sicurello F. Managing chronic pathologies with a stepped mHealth-based approach in clinical psychology and medicine. Front Psychol 2015;6:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, Rodgers A, Cairns J, Kenward MG, Roberts I. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet 2011;378:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, Jan S, Graves N, de Keizer L, Barry T, Bompoint S, Stepien S, Whittaker R, Rodgers A, Thiagalingam A. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 2015;314:1255–1263. [DOI] [PubMed] [Google Scholar]
- 34.Ramachandran A, Snehalatha C, Ram J, Selvam S, Simon M, Nanditha A, Shetty AS, Godsland IF, Chaturvedi N, Majeed A, Oliver N, Toumazou C, Alberti KG, Johnston DG. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2013;1:191–198. [DOI] [PubMed] [Google Scholar]
- 35.Neubeck L, Lowres N, Benjamin EJ, Freedman SB, Coorey G, Redfern J. The mobile revolution-using smartphone apps to prevent cardiovascular disease. Nat Rev Cardiol 2015;12:350–360. [DOI] [PubMed] [Google Scholar]
- 36.Piette JD, List J, Rana GK, Townsend W, Striplin D, Heisler M. Mobile health devices as tools for worldwide cardiovascular risk reduction and disease management. Circulation 2015;132:2012–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med 2013;159:185–194. [DOI] [PubMed] [Google Scholar]
- 38.Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr 2013;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007;298:2296–2304. [DOI] [PubMed] [Google Scholar]
- 40.Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JG. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail 2011;13:1028–1040. [DOI] [PubMed] [Google Scholar]
- 41.Weintraub A, Gregory D, Patel AR, Levine D, Venesy D, Perry K, Delano C, Konstam MA. A multicenter randomized controlled evaluation of automated home monitoring and telephonic disease management in patients recently hospitalized for congestive heart failure: the SPAN-CHF II trial. J Card Fail 2010;16:285–292. [DOI] [PubMed] [Google Scholar]
- 42.Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD, Telemedical Interventional Monitoring in Heart Failure Investigators. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011;123:1873–1880. [DOI] [PubMed] [Google Scholar]
- 43.Lyngå P, Persson H, Hägg-Martinell A, Hägglund E, Hagerman I, Langius-Eklöf A, Rosenqvist M. Weight monitoring in patients with severe heart failure (WISH): a randomized controlled trial. Eur J Heart Fail 2012;14:438–444. [DOI] [PubMed] [Google Scholar]
- 44.Bhavnani S, Waalen J, Srivastava A, Heywood JT. Which patients? Which devices? mHealth monitoring with wearable and implantable devices in heart failure: meta analysis of randomized trials. J Am Coll Cardiol 2015;65 doi:10.1016/S0735-1097(15)61030-0. [Google Scholar]
- 45.Stevenson LW, Zile M, Bennett TD, Kueffer FJ, Jessup ML, Adamson P, Abraham WT, Manda V, Bourge RC. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail 2010;3:580–587. [DOI] [PubMed] [Google Scholar]
- 46.Holmen H, Torbjørnsen A, Wahl AK, Jenum AK, Småstuen MC, Arsand E, Ribu L. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, Part 2: one-year results from the Norwegian randomized controlled trial RENEWING HEALTH. JMIR Mhealth Uhealth 2014;2:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, Jones MI, Jowett S, Little P, Penaloza C, Schwartz C, Shackleford H, Shovelton C, Varghese J, Williams B, Hobbs FD, Gooding T, Morrey I, Fisher C, Buckley D. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA 2014;312:799–808. [DOI] [PubMed] [Google Scholar]
- 48.Magid DJ, Olson KL, Billups SJ, Wagner NM, Lyons EE, Kroner BA. A pharmacist-led, American Heart Association Heart360 Web-enabled home blood pressure monitoring program. Circ Cardiovasc Qual Outcomes 2013;6:157–163. [DOI] [PubMed] [Google Scholar]
- 49.Laing BY, Mangione CM, Tseng CH, Leng M, Vaisberg E, Mahida M, Bholat M, Glazier E, Morisky DE, Bell DS. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients: a randomized, controlled trial. Ann Intern Med 2014;161:S5–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi PY, Pecina JL, Upatising B, Chaudhry R, Shah ND, Van Houten H, Cha S, Croghan I, Naessens JM, Hanson GJ. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med 2012;172:773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bujnowska-Fedak MM, Mastalerz-Migas A. Usage of medical Internet and e-health services by the elderly. Adv Exp Med Biol 2015;834:75–80. [DOI] [PubMed] [Google Scholar]
- 52.Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Hirani S, Cartwright M, Rixon L, Knapp M, Henderson C, Rogers A, Fitzpatrick R, Hendy J, Newman S. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012;344:e3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanders C, Rogers A, Bowen R, Bower P, Hirani S, Cartwright M, Fitzpatrick R, Knapp M, Barlow J, Hendy J, Chrysanthaki T, Bardsley M, Newman SP. Exploring barriers to participation and adoption of telehealth and telecare within the Whole System Demonstrator trial: a qualitative study. BMC Health Serv Res 2012;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattila E, Orsama AL, Ahtinen A, Hopsu L, Leino T, Korhonen I. Personal health technologies in employee health promotion: usage activity, usefulness, and health-related outcomes in a 1-year randomized controlled trial. JMIR Mhealth Uhealth 2013;1:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med 2011;8:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G, PURE Investigators. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014;371:818–827. [DOI] [PubMed] [Google Scholar]
- 58.Tian M, Ajay VS, Dunzhu D, Hameed SS, Li X, Liu Z, Li C, Chen H, Cho K, Li R, Zhao X, Jindal D, Rawal I, Ali MK, Peterson ED, Ji J, Amarchand R, Krishnan A, Tandon N, Xu LQ, Wu Y, Prabhakaran D, Yan LL. A cluster-randomized, controlled trial of a Simplified Multifaceted Management Program for Individuals at High Cardiovascular Risk (SimCard Trial) in rural Tibet, China, and Haryana, India. Circulation 2015;132:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta S, Dewan S, Kaushal A, Seth A, Narula J, Varma A. eICU reduces mortality in STEMI patients in resource-limited areas. Glob Heart 2014;9:425–427. [DOI] [PubMed] [Google Scholar]
- 60.Singh S, Bansal M, Maheshwari P, Adams D, Sengupta SP, Price R, Dantin L, Smith M, Kasliwal RR, Pellikka PA, Thomas JD, Narula J, Sengupta PP, ASE-REWARD Study Investigators. American Society of Echocardiography: Remote Echocardiography with Web-Based Assessments for Referrals at a Distance (ASE-REWARD) Study. J Am Soc Echocardiogr 2013;26:221–233. [DOI] [PubMed] [Google Scholar]
- 61.Cortez NG, Cohen IG, Kesselheim AS. FDA regulation of mobile health technologies. N Engl J Med 2014;371:372–379. [DOI] [PubMed] [Google Scholar]
- 62.Semigran HL, Linder JA, Gidengil C, Mehrotra A. Evaluation of symptom checkers for self diagnosis and triage: audit study. BMJ 2015;351:h3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ioannidis JP. Stealth research: is biomedical innovation happening outside the peer-reviewed literature? JAMA 2015;313:663–664. [DOI] [PubMed] [Google Scholar]
- 64.Henderson C, Knapp M, Fernández JL, Beecham J, Hirani SP, Cartwright M, Rixon L, Beynon M, Rogers A, Bower P, Doll H, Fitzpatrick R, Steventon A, Bardsley M, Hendy J, Newman SP, Whole System Demonstrator Evaluation Team. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 2013;346:f1035. [DOI] [PubMed] [Google Scholar]
- 65.http://docs.house.gov/meetings/IF/IF00/20150519/103516/BILLS-1146ih.pdf. (July 2015).
- 66.Gagnon MP, Ngangue P, Payne-Gagnon J, Desmartis M. m-Health adoption by healthcare professionals: a systematic review. J Am Med Inform Assoc 2015; doi:10.1093/jamia/ocv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Redfern J, Usherwood T, Harris MF, Rodgers A, Hayman N, Panaretto K, Chow C, Lau AY, Neubeck L, Coorey G, Hersch F, Heeley E, Patel A, Jan S, Zwar N, Peiris D. A randomised controlled trial of a consumer-focused e-health strategy for cardiovascular risk management in primary care: the Consumer Navigation of Electronic Cardiovascular Tools (CONNECT) study protocol. BMJ Open 2014;4:e004523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther 2008;10:160–168. [DOI] [PubMed] [Google Scholar]
- 69.Ringh M, Rosenqvist M, Hollenberg J, Jonsson M, Fredman D, Nordberg P, Järnbert-Pettersson H, Hasselqvist-Ax I, Riva G, Svensson L. Mobile-phone dispatch of laypersons for CPR in out-of-hospital cardiac arrest. N Engl J Med 2015;372:2316–2325. [DOI] [PubMed] [Google Scholar]
- 70.Cook DJ, Thompson JE, Prinsen SK, Dearani JA, Deschamps C. Functional recovery in the elderly after major surgery: assessment of mobility recovery using wireless technology. Ann Thorac Surg 2013;96:1057–1061. [DOI] [PubMed] [Google Scholar]
- 71.Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, Fought AJ, Topol EJ. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014;127:95.e11–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallenhorst C, Lau JK, Brieger DB, Sy RW, Freedman SB. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111:1167–1176. [DOI] [PubMed] [Google Scholar]
- 73.Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, Smith C, Walters DL. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014;100:1770–1779. [DOI] [PubMed] [Google Scholar]