Summary
Background/objective:
The aim of this study was to investigate the effects of different concentrations of ozone (O3) therapy on bone regeneration in response to an expansion of the inter-premaxillary suture in rats.
Materials and methods:
Forty-eight Wistar rats were randomly divided into four groups (n = 12). In groups I, II, and III, 1ml of O3 at 10, 25, and 40 µg/ml was injected at the premaxillary suture, respectively. In group IV (control group), 1ml of saline solution was injected at the same point during the expansion procedure for 5 days. Bone regeneration in the suture was evaluated histomorphometrically. The area of new bone and fibrotic area, the number of osteoblasts and osteoclasts, and the amount of vascularity were measured and compared. The density of the newly formed bone in the expansion area was measured by using cone beam computed tomography. Data were analyzed using the Kruskal–Wallis one-way analysis of variance and post hoc Student-Newman–Keuls tests.
Results:
New bone area, fibrotic area, osteoblast and osteoclast numbers, and the amount of vascularity were significantly higher in experimental groups compared with the control group (P < 0.001). The density of newly formed bone (P < 0.001), new bone formation (P = 0.009), number of capillaries (P < 0.001), number of osteoclasts (P = 0.016), and number of osteoblasts (P < 0.001) in the maxillary sutures were highest in the 25 μg/ml O3 group compared with the other experimental groups and control group.
Conclusions/implications:
The application of O3 therapy can stimulate bone regeneration in an orthopedically expanded inter-premaxillary suture during both the expansion and retention periods.
Introduction
Rapid maxillary expansion (RME) is one of the most common treatment protocols for expanding a transversally narrow maxilla (1). This treatment protocol increases the posterior dental arch width in the transverse dimension, ensuring the ideal size of the maxilla (2).
Despite the fact that the reasons for a post-expansion relapse are not completely understood, the quality and rigidity in the sutural region during and after expansion may affect a post-treatment relapse (3). Potentially favourable effects of accelerating bone formation in the sutural area during and after expansion have been reported to prevent a relapse of the transversal arch width and shorten the retention period (3, 4).
Ozone (O3) is a three-atom molecule consisting of three oxygen atoms. O3 has been used in both an aqueous and gaseous form in medicine and dentistry (5). O3 therapy has antimicrobial, anti-inflammatory, and wound healing effects (6–8). Additionally, O3 therapy has immunostimulating, antihypoxic, analgesic, detoxicating, bioenergetic, and biosynthetic effects on the human body (9).
O3 provides for the synthesis of a group of cytokines, such as leukotrienes, interleukins, and prostaglandins. O3 influences both the cellular and humoral immune system and stimulates the proliferation of immunocompetent cells and the synthesis of immunoglobulins. O3 also activates the function of macrophages and increases the sensitivity of microorganisms to phagocytosis. There are different ways to administer O3 gas, including minor and major autohemotherapies, insufflations, rectally, intraarticuler, and local and topical applications (10).
The aim of this study was to investigate the effects of various concentrations of O3 therapy on bone regeneration in response to the expansion of the inter-premaxillary suture in rats. These effects were evaluated with quantitative bone histomorphometric and cone beam computed tomography (CBCT) examinations. The research hypothesis of this study states that the administration of O3 therapy has positive effects on bone formation during the inter-premaxillary suture expansion in rats.
Materials and methods
Forty-eight 11–12 week-old Wistar albino male rats with a mean weight of 203.47±9.19g were randomly divided into four groups of 12 animals each. Ethical approval for this study was obtained from the University of Erciyes Regional Animal Research Ethics Committee (13 February 2013-13/23). All rats were kept in polycarbonate cages and subjected to a 12-hour light-dark cycle at the constant temperature of 23°C and fed a normal pellet diet (Expanded pellets, Stepfield Witham, Essex, UK) with water provided ad libitum.
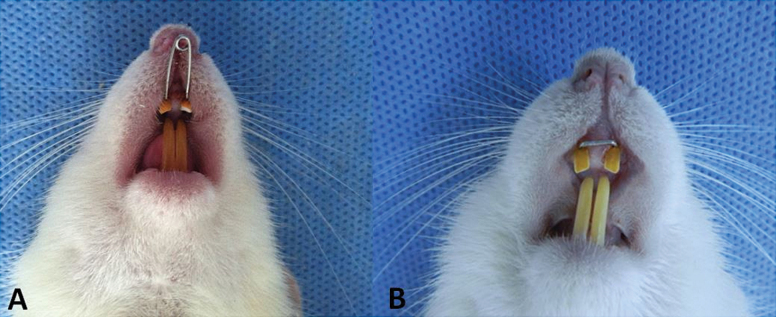
Sutural expansion was carried out in all animals using an expansion spring made of a 0.014 inch stainless steel wire inserted in holes drilled close to the gingival margins of both upper incisors. The springs were activated to deliver a force of 50g and were not reactivated during the expansion period (11, 12) (Figure 1A).
Figure 1.
(A) Expansion appliance in situ. (B) Retaining wire placed between rat incisors’ holes.
After the expansion period, the springs were removed and a 0.016×0.016 inch stainless steel rectangular wire was inserted into the holes between two incisors during a 10-day retention period (Figure 1B). The appliances were attached to the maxillary incisors of all animals under general anesthesia with 90mg/kg ketamine hydrochloride (Ketasol, Wels, Austria) and 3mg/kg xylazine (Rompun-Bayer, Leverkusen, Germany) via an intraperitoneal injection.
In this histomorphometrical and CBCT study, three experimental groups were treated with different O3 gas concentrations (group I: 10 µg/ml; group II: 25 µg/ml; and group III: 40 µg/ml). O3 therapy was performed using an O3 generator (Ozonosan® Dr. J. Hänsler GmbH, Iffezheim, Germany) and all O3 therapy volumes in the experimental groups were 1ml. The O3 therapy injections were repeated five times during the expansion period. The control group (Group IV) received a 1ml saline solution five times during the expansion procedure (5 days). One day after the expansion appliance placement, various oxygen–O3 concentrations, or saline solutions, were injected into the inter-premaxillary suture with a silicon-coated micro-syringe (Hamilton injection syringe, Hamilton Company, NV, USA).
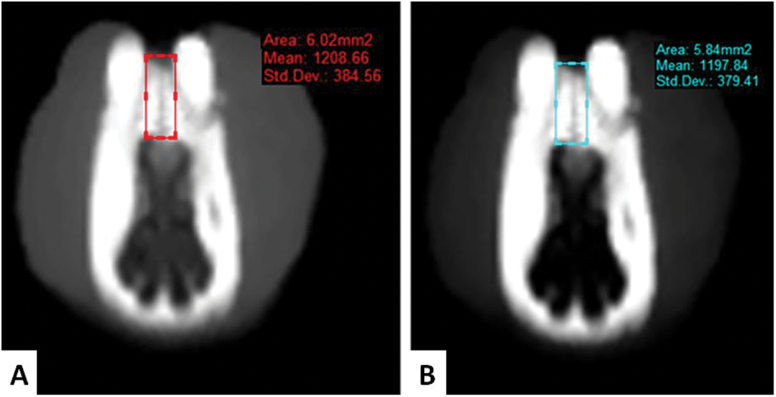
The density of the newly formed bone was measured using a CBCT. All axial images were obtained in the standard position by CBCT (NewTom 5G, QR Verona, Italy). The machine scanning time was 14–18 seconds with a limited field of view (8cm × 8cm), a voxel size of 0.3mm3, and an exposure time of 3.6 seconds. The CBCT images were transformed to the Digital Imaging and Communications in Medicine (DICOM) format, and then the SimPlant Pro 13.0 software (Materialise HQ, Leuven, Belgium) was used to perform the density measurements of the newly formed bone as a Hounsfield unit (HU) (Figure 2). CBCT measurements were taken at the end of the expansion (T1) and after the retention period (T2).
Figure 2.
(A) Cone beam computed tomography images after the expansion and (B) retention period.
At the end of the experiment, animals were sacrificed by decapitation under intraperitoneal ketamine (75mg/kg) + xylazine (10mg/kg) anesthesia. After decapitation, the pre-maxillae were removed instantly and fixed in 10% formalin for 10 days. Then, the pre-maxillae were decalcified with 5% ethylenediaminetetraacetic acid for 8 weeks. During decalcification, the solution was changed twice a week. The pre-maxillae were cut perpendicularly to the sagittal plane, which was determined by two points: one at the alveolar crest and the other 4mm apically. This plane passed through the centre of the incisor crown at its gingival portion. The decalcified pre-maxillae were rinsed under running tap water for 24 hours, followed by dehydration through a graded alcohol series. Tissues were made transparent in xylol and embedded in paraffin. Five-micrometer-thick sections were stained with hematoxylin-eosin (H&E) and Masson’s trichrome, and photographs were taken with an Olympus BX-51 photomicroscope.
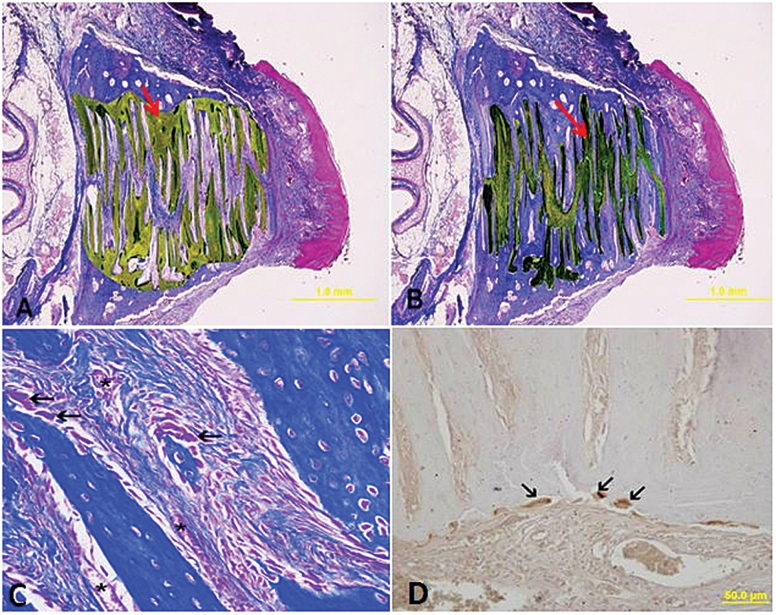
Histomorphometric evaluations were performed in a blind analysis by two experienced histologists, and the results were an average of the counts. Because bone formation on the surface area was sometimes irregular and not suitable for quantitative measurement, bone histomorphometric measurements were performed 200 μm under the surface of the osseous palate facing the oral cavity. Three non-sequential histological sections were analysed for each animal. The associated analysed parameters were the new mineralised area, the fibrosis area, the number of blood vessels, and the number of osteoblast cells within the premaxillary suture (Figures 3A–C). The differences between the groups were analysed using the Image J program (US National Institutes of Health, Bethesda, MA, USA).
Figure 3.
(A) Histomorphometric measurements of newly formed bone area (arrow) (μm2). (B) Histomorphometric measurements of fibrotic area (arrow) (μm2). (C) Osteoblast cells on the newly formed bone area (arrow) and blood vessels on the fibrotic area (asterisks) (Masson-trichrome staining). (D) Osteoclast cells on the bone surface (arrow) (immunochemical staining).
Cathepsin K expression was detected immunohistochemically using a goat polyclonal antibody (sc-6507; Santa Cruz Biotechnology, CA, USA) and the streptavidin-biotin peroxidase technique. The procedure was performed under identical conditions for all sections. Paraffin sections (5 µm) were deparaffinised in xylene. The sections were rehydrated, rinsed in deionised water, and antigen retrieval was carried out by microwave treatment in a 0.01M sodium citrate buffer (pH = 6.0) at 95°C for 5 minutes. The slides were then cooled rapidly at room temperature for 20 minutes. The sections were washed with phosphate-buffered saline (PBS), and endogenous peroxidase activity was inhibited by 3% H2O2 in methanol for 10 minutes. Five percent normal goat serum was used to block non-specific staining. The histological sections were then incubated with a cathepsin K-specific polyclonal antibody at a dilution of 2.5 µg/ml in 5% serum overnight at 4°C. After washing with PBS, sections were incubated with biotinylated secondary antibodies. The immunoreaction was then amplified with the streptavidin-avidin-peroxidase complex, and the sections were visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB) and lightly counterstained with hematoxylin. When incubation with the primary antisera was omitted, negative controls were completely unlabelled.
Three slides of each specimen were stained immunohistochemically for the osteoclast (cathepsin K positive cells) count. Osteoclasts were calculated by counting in the suture area (Figure 3D).
Statistical analysis
All data was analysed with SPSS 21 (SPSS; IBM SPSS Inc., Chicago, IL, USA). When the P-value was less than 0.05, the statistical test was determined to be significant. To evaluate and compare the density of the newly formed bone after expansion and retention (T1–T2), the Kruskal–Wallis test was used. Histologic and immunohistochemical measurements were evaluated with the Kruskal–Wallis one-way analysis of variance (ANOVA) and post hoc Student-Newman–Keuls tests.
Results
In the control and experimental groups, all animals survived to the end of the study. However, one animal in group I, one in group II, and one in group IV exhibited incisor tooth fractures; therefore, those animals were excluded from the study. Instead of these rats, three new rats were included in the study.
Inter-premaxillary suture separation was successfully achieved with the expansion spring. The body weight of the rats in the control and experimental groups decreased during the expansion period, but subsequently increased during the retention period.
The median densities of the newly formed bone at T1 were 810.64, 849.69, 802.84, and 691.70 HU in groups I, II, III, and IV, respectively. There were significant differences between the groups in the density of the newly formed bone at T1 (P < 0.001). The differences were also statistically significant between groups I and II, I and IV, and III and IV (Table 1). Moreover, the median densities of the newly formed bone at T2 were 880.28, 971.81, 885.49, and 751.23 HU in groups I, II, III, and IV, respectively. There were also significant differences between the groups in the density of the newly formed bone at T2 (P < 0.001). The highest bone density value was observed in group II (971.81 HU) (Table 2).
Table 1.
Measurement of bone density at the end of expansion period (T1) (Hounsfield Unit).
| Groups | N | Median | %25p | %75p | P |
|---|---|---|---|---|---|
| Group I | 12 | 810.64b | 778.49 | 821.33 | <0.001 |
| Group II | 12 | 849.69a | 842.40 | 901.87 | |
| Group III | 12 | 802.84b | 765.56 | 822.32 | |
| Group IV | 12 | 691.70c | 669.22 | 753.00 |
N, sample size; group I, 10 µg/ml O3; group II, 25 µg/ml O3; group III, 40 µg/ml O3; group IV, control; p, represents the differences among the groups; same letters represent the similarity, while different letters represent the differences among the groups.
Table 2.
Measurement of bone density at the end of retansion period (T2) (Hounsfield Unit).
| Groups | N | Median | %25p | %75p | P |
|---|---|---|---|---|---|
| Group I | 12 | 880.28b | 813.70 | 929.02 | <0.001 |
| Group II | 12 | 971.81a | 941.68 | 999.99 | |
| Group III | 12 | 885.49b | 826.39 | 912.27 | |
| Group IV | 12 | 751.23c | 725.09 | 835.43 |
N, sample size; group I, 10 µg/ml O3; group II, 25 µg/ml O3; group III, 40 µg/ml O3; group IV, control; p, represents the differences among the groups; same letters represent the similarity, while different letters represent the differences among the groups.
Statistical analyses showed statistically significant differences among groups for all investigated histologic and immunohistochemical parameters. New bone area, fibrotic area, blood vessels, number of osteoblasts, and osteoclasts measurements showed statistically significant differences (Tables 3 and 4; Figure 3). For all investigated histomorphometric parameters, group II showed more positive results than groups I, III, and IV with respect to new bone formation; these measurements also revealed that bone architecture in the treatment groups was improved (P < 0.001).
Table 3.
Descriptive values, Kruskal–Wallis results of histomorphometric measurements
| Parameters | Groups | N | Median | %25p | %75p | P |
|---|---|---|---|---|---|---|
| New bone area (µm2) | Group I | 12 | 651098.80b | 623055.50 | 662371.20 | 0.009 |
| Group II | 12 | 764027.00a | 675272.00 | 865265.70 | ||
| Group III | 12 | 598194.80b | 514446.00 | 730714.30 | ||
| Group IV | 12 | 484390.00b | 397921.70 | 697768.00 | ||
| Fibrotic area (µm2) | Group I | 12 | 732997.50a | 719568.20 | 766488.50 | 0.007 |
| Group II | 12 | 706363.20b | 634819.30 | 812325.80 | ||
| Group III | 12 | 590404.50c | 555543.00 | 673277.00 | ||
| Group IV | 12 | 520008.50c | 459075.00 | 709677.50 | ||
| Osteoclast | Group I | 12 | 1.00b | 0.67 | 1.50 | 0.016 |
| Group II | 12 | 2.00a | 1.33 | 2.67 | ||
| Group III | 12 | 1.00b | 0.84 | 1.50 | ||
| Group IV | 12 | 0.50b | 0.33 | 1.17 |
N, sample size; group I, 10 µg/ml O3; group II, 25 µg/ml O3; group III, 40 µg/ml O3; group IV, control; p, represents the differences among the groups; same letters represent the similarity, while different letters represent the differences among the groups.
Table 4.
Descriptive values, one-way analysis of vaiances and multiple comparison results of osteoblast and blood vessels number in all groups
| Parameters | Groups | N | Mean | SD | Minimum | Maximum | P |
|---|---|---|---|---|---|---|---|
| Osteoblast | Group I | 12 | 363.03a | 41.31 | 270.00 | 462.00 | <0.001 |
| Group II | 12 | 423.97b | 19.95 | 370.00 | 490.00 | ||
| Group III | 12 | 395.03b | 34.52 | 348.00 | 486.00 | ||
| Group IV | 12 | 263.33c | 52.35 | 137.00 | 340.00 | ||
| Blood vessel | Group I | 12 | 23.58a | 5.36 | 16.00 | 41.00 | <0.001 |
| Group II | 12 | 28.47a | 7.20 | 13.00 | 57.00 | ||
| Group III | 12 | 24.75a | 3.23 | 17.00 | 36.00 | ||
| Group IV | 12 | 13.31b | 5.41 | 5.00 | 27.00 |
N, sample size; group I, 10 µg/ml O3; group II, 25 µg/ml O3; group III, 40 µg/ml O3; group IV, control; p, represents the differences among the groups; same letters represent the similarity, while different letters represent the differences among the groups.
Discussion
Researchers have performed many studies involving the application of different pharmacological and biochemical agents to increase bone formation in distraction osteogenesis and sutural expansion. However, there are few studies in the orthodontic literature on stimulating regeneration in the mid-palatal suture after expansion.
In the orthodontic literature, many studies have been performed to accelerate regeneration in the inter-premaxillary suture after expansion. Sawada and Shimizu (4) applied a single dose of transforming growth factor-β1 for stimulating the expanding rat sutures. Furthermore, Chang et al. (13) evaluated the effect of an angiogenic factor on cell migration patterns and osteoblast histogenesis orthopedic expansion of the anterior maxillary suture in rats. All of these studies significantly stimulated bone regeneration in the mid-palatal/inter-premaxillary suture. Recently, Ekizer et al. (14, 15) investigated the effects of a light-emitting diode photobiomodulation and bone marrow-derived mesenchymal stem cells on bone formation in the inter-premaxillary suture in rats. This group found that these applications stimulated bone regeneration during both the expansion and retention periods. Bone marrow mesenchymal stem cells enhance bone formation in orthodontically expanded maxillae in rats. In the current study, we investigated the effects of different concentrations of O3 therapy on bone regeneration in response to the expansion of the inter-premaxillary suture in rats. We found increases in the newly formed mineralised bone area in the suture area with various O3 concentrations, especially the concentration used in group II.
Different animal models have been described for examining bone formation on expanding the inter-premaxillary suture area. Various animals, including monkeys, dogs, cats, sheep, rabbits, and rats have been used as animal models to examine the effects of biomechanical stimulation on bone (16). Monkeys and cats have similar anatomical features regarding the maxillary sutures that are, in most respects, similar to that of man; thus, these animals have been used in many maxillary expansion studies. However, the ideal animals are rabbits and rats for maxillary expansion due to the production of a clear picture of maxillary and sutural changes under stimulation and the ease of manufacturing (17). In accordance with the literature, we used rats as the experimental animal model (11, 12, 14, 15).
In this study, the positive effects of O3 therapy on the quantity of new bone formation during maxillary expansion were investigated using a histomorphometric and CBCT evaluation. Bone histomorphometry is a reliable histology technique commonly used in quantitative evaluations of bone remodelling in experimental studies (2–4, 18). Additionally, the density of the newly formed bone in the expansion area, both after the expansion and after the retention period, was measured with the CBCT device. CBCT measurements are reliable and accurate to a subvoxel size and can potentially be used as a quantitative orthodontic diagnostic tool (19, 20).
Currently, O3 therapy is widely used in medicine, dentistry, and maxillofacial surgery (7, 21–23); however, its application in orthodontics is limited. Ozdemir et al. (24) aimed to evaluate the effect of O3 therapy on autogenous bone graft healing in calvarial defects. They concluded that O3 therapy enhances new bone formation using an autogenous bone graft in the rat calvarial defect model. Another study also reported that O3 therapy has a positive effect on bone formation in rat calvarial defects compared with controls (10).
O3 activates a series of biological mechanisms that lead to normalising the delivery of oxygen for several days with consequent therapeutic effects (5). Additionally, in the present study, the histomorphometric results showed faster bone formation in the 25 µg/ml dose of the O3 therapy administrated group. We think that this might be due to the fact that a low concentration of O3 therapy stimulates the immune system, while a high concentration of O3 therapy inhibits the immune system.
Agrillo et al. (25) carried out 20 tooth extractions from 15 patients with avascular bisphosphonate-related jaw osteonecrosis. They reported that O3 was effective when used 7 days before and 7 days after the tooth extraction. In our study, O3 therapy enhanced new bone formation in the inter-premaxillary sutural area. These healing properties are due to the reaction of O3 with erythrocytes, leukocytes, and platelets. As a result, O3 therapy provides greater microcirculation and improves wound healing (26).
Conclusions
The application of O3 therapy can stimulate bone regeneration in an orthopedically expanded inter-premaxillary suture during both the expansion and retention periods. Furthermore, a 25 µg/ml concentration of O3 therapy was the most effective dose for bone regeneration in rats. Further animal and clinical studies are required to determine any pharmaceutical benefits of O3 therapy.
Funding
The Scientific and Technological Research Council of Turkey (TUBITAK 113S118).
Acknowledgements
The authors would also like to thank Dr Mustafa Koseahmetoglu for many helpful suggestions concerning O3 applications.
References
- 1. Nicholson P. and Plint D (1989) A long-term study of rapid maxillary expansion and bone grafting in cleft lip and palate patients. European Journal of Orthodontics, 11, 186–192. [DOI] [PubMed] [Google Scholar]
- 2. Uysal T. Ustdal A. Sonmez M.F. and Ozturk F (2009) Stimulation of bone formation by dietary boron in an orthopedically expanded suture in rabbits. The Angle Orthodontist, 79, 984–990. [DOI] [PubMed] [Google Scholar]
- 3. Saito S. and Shimizu N (1997) Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. American Journal of Orthodontics and Dentofacial Orthopedics, 111, 525–532. [DOI] [PubMed] [Google Scholar]
- 4. Sawada M. and Shimizu N (1996) Stimulation of bone formation in the expanding mid-palatal suture by transforming growth factor-β1 in the rat. European Journal of Orthodontics, 18, 169–179. [DOI] [PubMed] [Google Scholar]
- 5. Bocci V.A. (2006) Scientific and medical aspects of ozone therapy. State of the art. Archives of Medical Research, 37, 425–435. [DOI] [PubMed] [Google Scholar]
- 6. Ripamonti C.I. Cislaghi E. Mariani L. and Maniezzo M (2011) Efficacy and safety of medical ozone (O(3)) delivered in oil suspension applications for the treatment of osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: preliminary results of a phase I–II study. Oral Oncology, 47, 185–190. [DOI] [PubMed] [Google Scholar]
- 7. Bocci V. (2004) Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediators of Inflammation, 13, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grigor’ian A. Grigor’iants L. and Guchetl M (2007) Experimental-morphological study of the anti-inflammatory action of ozone-perfluorane complex application [Article in Russian]. Stomatologiia (Mosk), 87, 4–9. [PubMed] [Google Scholar]
- 9. Seidler V. Linetskiy I. Hubálková H. Stankova H. Smucler R. and Mazanek J (2008) Ozone and its usage in general medicine and dentistry. A review article. Prague Medical Report, 109, 5–13. [PubMed] [Google Scholar]
- 10. Kazancioglu H.O. Ezirganli S. and Aydin M.S (2013) Effects of laser and ozone therapies on bone healing in the calvarial defects. Journal of Craniofacial Surgery, 24, 2141–2146. [DOI] [PubMed] [Google Scholar]
- 11. Uysal T. Amasyali M. Olmez H. Enhos S. Karslioglu Y. and Gunhan O (2011) Effect of vitamin C on bone formation in the expanded inter-premaxillary suture. Early bone changes. Journal of Orofacial Orthopedics, 72, 290–300. [DOI] [PubMed] [Google Scholar]
- 12. Uysal T. Gorgulu S. Yagci A. Karslioglu Y. Gunhan O. and Sagdic D (2011) Effect of resveratrol on bone formation in the expanded inter-premaxillary suture: early bone changes. Orthodontics & Craniofacial Research, 14, 80–87. [DOI] [PubMed] [Google Scholar]
- 13. Chang H.N. Garetto L.P. Katona T.R. Potter R.H. and Eugene Roberts W (1996) Angiogenic induction and cell migration in an orthopaedically expanded maxillary suture in the rat. Archives of Oral Biology, 41, 985–994. [DOI] [PubMed] [Google Scholar]
- 14. Ekizer A. Uysal T. Güray E. and Yüksel Y (2013) Light-emitting diode photobiomodulation: effect on bone formation in orthopedically expanded suture in rats—early bone changes. Lasers in Medical Science, 28, 1263–1270. [DOI] [PubMed] [Google Scholar]
- 15. Ekizer A. Yalvac M.E. Uysal T. Sonmez M.F. and Sahin F (2015) Bone marrow mesenchymal stem cells enhance bone formation in orthodontically expanded maxillae in rats. The Angle Orthodontist, 85, 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swennen G. Dempf R. and Schliephake H (2002) Cranio-facial distraction osteogenesis: a review of the literature. Part II: experimental studies. International Journal of Oral and Maxillofacial Surgery, 31, 123–135. [DOI] [PubMed] [Google Scholar]
- 17. Storey E. (1973) Tissue response to the movement of bones. American Journal of Orthodontics, 64, 229–247. [DOI] [PubMed] [Google Scholar]
- 18. Uysal T. Amasyali M. Enhos S. Sonmez M.F. and Sagdic D (2009) Effect of ED-71, a new active vitamin D analog, on bone formation in an orthopedically expanded suture in rats. A histomorphometric study. European Journal of Dentistry, 3, 165–172. [PMC free article] [PubMed] [Google Scholar]
- 19. Gribel B.F. Gribel M.N. Frazão D.C. McNamara J.A. Jr and Manzi F.R (2011) Accuracy and reliability of craniometric measurements on lateral cephalometry and 3D measurements on CBCT scans. The Angle Orthodontist, 81, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Celikoglu M. Halicioglu K. Buyuk S.K. Sekerci A.E. and Ucar F.I (2013) Condylar and ramal vertical asymmetry in adolescent patients with cleft lip and palate evaluated with cone-beam computed tomography. American Journal of Orthodontics and Dentofacial Orthopedics, 144, 691–697. [DOI] [PubMed] [Google Scholar]
- 21. Martínez-Sánchez G. Al-Dalain S.M. Menéndez S. Re L. Giuliani A. Candelario-Jalil E. Alvarez H. Fernández-Montequín J.I. and León O.S (2005) Therapeutic efficacy of ozone in patients with diabetic foot. European Journal of Pharmacology, 523, 151–161. [DOI] [PubMed] [Google Scholar]
- 22. Magni E. Ferrari M. Papacchini F. Hickel R. and Ilie N (2010) Influence of ozone application on the repair strength of silorane-based and ormocer-based composites. American Journal of Dentistry, 23, 260–264. [PubMed] [Google Scholar]
- 23. Stübinger S. Sader R. and Filippi A (2006) The use of ozone in dentistry and maxillofacial surgery: a review. Quintessence International, 37, 353–359. [PubMed] [Google Scholar]
- 24. Ozdemir H. Toker H. Balcı H. and Ozer H (2013) Effect of ozone therapy on autogenous bone graft healing in calvarial defects: a histologic and histometric study in rats. Journal of Periodontal Research, 48, 722–726. [DOI] [PubMed] [Google Scholar]
- 25. Agrillo A. Ungari C. Filiaci F. Priore P. and Iannetti G (2007) Ozone therapy in the treatment of avascular bisphosphonate-related jaw osteonecrosis. Journal of Craniofacial Surgery, 18, 1071–1075. [DOI] [PubMed] [Google Scholar]
- 26. Azarpazhooh A. and Limeback H (2008) The application of ozone in dentistry: a systematic review of literature. Journal of Dentistry, 36, 104–116. [DOI] [PubMed] [Google Scholar]