Abstract
Novel sensory experiences, particularly those associated with epochal developmental events like nursing alter cortical representation, affecting memory, perception and behavior. Functional MRI was used here to test whether the sensoricortical map of the ventrum is modified during lactation. Three stimuli were used to drive cortical activation in primiparous rats: natural, artificial suckling stimulation and general mechanical rubbing of the skin of the ventrum. These stimuli significantly activated the somatosensory cortex of dams. Of the three stimuli, artificial and pup suckling robustly activated much of the cerebrum, most notably the visual, auditory and olfactory cortices. Surprisingly, activation occurred even in the absence of pups, with artificial suckling. This finding suggests that incoming information from a single modality was sufficient to drive activity of others. Enhanced sensitivity across the cortical mantle during nursing may help the dam to perceive, process, and remember stimuli critical to the care and protection of her young.
Keywords: Lactation, Breastfeeding, Nursing, Suckling, Rats, BOLD, fMRI, Maternal Behavior, Neural Circuits, Sensory, Multisensory
Introduction
Lactation plays a critical role in the survival of mammals. It is an intermittent event, in which mothers must forage for their own sustenance while simultaneously converting the food into nutrient-rich milk that can be provided to hungry young on demand. During the reproductive period defined by lactation, a bond is formed between a mother and her offspring that favors their protection and social development. Perhaps an important advantage in the evolution of nursing is the potential for a mother to significantly influence the social development of her young (Fleming et al., 2002). A prime example is the discovery that naturally occurring variations in maternal licking and grooming and arched-back nursing leads to non-genomic transmission of individual behavioral differences across generations (Francis et al., 1999).
Changes in cortical sensory processing in mothers may be important for the successful protection and care of offspring. It has been reported that suckling stimulation from pups modulates the expression of maternal behaviors in rats by promoting arched back nursing postures (Stern and Johnson, 1990; Stern et al., 2002) and slow-wave sleep (Blyton et al., 2002; Lincoln et al., 1980). Given the importance of nursing stimuli for mother-pup interactions (Stern, 1990) and the importance of breastfeeding in humans (Fergusson and Woodward, 1999), it is surprising how little is known about the areas of the cerebrum that represent the sensory maps associated with suckling. The sensory system is organized such that information coming from the landscape of peripheral receptors (i.e. body surface, cochlea and retina) is relayed to the cortex through the spinothalamic pathway and topographically represented in the cerebrum. In the case of the mammillae, primary afferent fibers terminate in the ipsilateral dorsal root ganglia between spinal segments C5 and L6 (Tasker et al., 1986), with afferent relays along the lateral cervical nucleus, the dorsal column nuclei and the sensory and spinal portions of the trigeminal complex (Dubois-Dauphin et al., 1985; Stern et al., 2002). Several in vitro studies have used immediate early gene expression as a surrogate marker for in vivo neuronal activation in order to map central neuronal populations selectively responsive to suckling in rats (Li et al., 1999; Lin et al., 1998; Lonstein and Stern, 1997b; Lonstein et al., 1998; Walsh et al., 1996). The results have shown intricate patterns of cortical and subcortical activation of gene expression, highlighting the complex nature of the stimulus and the brains’ response to it. However, despite the exquisite cellular spatial detail of cFos immunolabelling techniques, the detection of in vivo brain activity during the actual act of nursing is limited by the very wide temporal window (usually taken 60-120 min post-stimulus). Findings from electrophysiological recordings taken from neurons in the somatosensory cortex of the anesthetized rat indicate that the receptive field for the ventrum skin surrounding the nipple area is doubled in size during the lactation period (Xerri et al. 1994). However, it is important to underscore the fact that the cortical representation of afferent signals coming from the glabrous skin of the nipples and areolae of mammals is unknown (Xerri et al., 1994). Tactile or suckling stimulation of the nipple does not evoke neuronal activity in the somatosensory cortex of multiparous dams even though the same cortical area is highly responsive to deflection of the hair immediately surrounding the areolar skin (Xerri et al., 1994). Contrary to the cFos studies cited above, neuronal recordings provide a subsecond temporal resolution while dramatically narrowing spatial localization.
With the advent of functional magnetic resonance imaging (fMRI) in conscious animals, would it be possible to scan the entire cortical mantle for changes in brain activity during lactation to help identify the location of the cortical map for suckling sensation? Functional MRI is a non-invasive technology with exceptional temporal and spatial resolution making it possible to map in seconds, functionally relevant neural networks activated by a variety of environmental and chemical stimuli (Boyett-Anderson et al., 2003; Fize et al., 2003; Lahti et al., 1999; Tenney et al., 2004). For example, in our own laboratory fMRI has been used to examine the contribution of oxytocinergic neurotransmission on suckling evoked brain activity in the lactating dams (Febo et al., 2005). In a another study, we discovered that primiparous dams show a similar activation of the dopaminergic reward pathway when exposed to pup suckling, evidence that breastfeeding is a reinforcing experience promoting mother/infant bonding (Ferris et al., 2005). Interestingly, it was noted over the course of these nursing studies that dams showed a robust activation in the olfactory cortex when pups started to suckle. This seemed peculiar, since suckling activated a cortical area associated with processing sensory information related to smell not touch. These preliminary data only added to the mystery of where the sensations of suckling and lactation are represented and organized in the cerebral cortex. To resolve this issue, the present studies were undertaken to assess suckling-induced changes across multiple cortical areas using fMRI and 3D computational analysis.
Methods
Animals
Virgin Sprague-Dawley rats, 80-90 days old, were obtained from Charles River Laboratories (MA) and housed individually in 48 × 25 × 20 cm Plexiglas cages. Animals were maintained on a 12 hr light-dark cycle with lights on at 0700 hr and provided food and water ad libitum. Prior to breeding, animals were acclimated to the rodent restrainer and the imaging protocol as described below. All dams were primiparous with a minimum litter size of 10 pups. The use of animals adhered to the animal welfare act the document entitled "Principle for Use of Animals" and “Guide for the Care and Use of Laboratory Animals." The protocols used in this study were in compliance with the regulations of the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School.
Imaging awake dams
Many of the technical and methodological problems associated with imaging of fully conscious animals in high field MR spectrometers have been resolved (Andersen et al., 2002; Febo et al., 2004; Ferris et al., 2004; King et al., 2005; Ludwig et al., 2004). Studying brain function in animals with the high temporal and spatial resolution of non-invasive MRI makes it possible to follow activation of neural pathways in a variety of behavioral and neurological models ranging from sexual arousal in monkeys (Ferris et al., 2004), and pup suckling in rat dams (Ferris et al., 2005) to generalized absence seizures in rats and monkeys (Tenney et al., 2004).
Key to imaging awake animals is controlling for motion artifact. Any minor head movement distorts the image and may also create a change in signal intensity that can be mistaken for stimulus-associated changes in brain activity (Hajnal et al., 1994). In addition to head movement, motion outside the field of view caused by respiration, swallowing and muscle contractions in the face and neck are other major sources of motion artifact (Birn et al., 1998; Yetkin et al., 1996). To minimize motion artifacts, studies were performed with a multi-concentric dual-coil, small animal restrainer developed for imaging awake rodents (Insight Neuroimaging Systems, LLC, Worcester MA). In brief, just prior to the imaging session, animals were anesthetized with 2-3% isoflurane. A topical anesthetic of 10% lidocaine gel was applied to the skin and soft tissue around the ear canals and over the bridge of the nose. A plastic semicircular headpiece with blunted ear supports that fit into the ear canals was positioned over the ears. The head was placed into a cylindrical head holder with the animal's canines secured over a bite bar and ears positioned inside the head holder with adjustable screws fitted into lateral sleeves. An adjustable surface coil built into the head holder was pressed firmly on the head and locked into place. The body of the animal was placed into a body restrainer. The body restrainer “floats” down the center of the chassis connecting at the front and rear end plates and buffered by rubber gaskets. The head piece locks into a mounting post on the front of the chassis. This design isolates all of the body movements from the head restrainer and minimizes motion artifact. Once the animal was positioned in the body holder, a volume coil was slid over the head restrainer and locked into position.
Acclimating animals to the imaging protocol
Animals were anesthetized with isoflurane as described above for securing the animal into the restrainer. When fully conscious, the restraining unit was placed into a black opaque tube “mock scanner” with a tape-recording of an MRI pulse sequence for 90 min in order to simulate the bore of the magnet and an imaging protocol. This procedure was repeated for four consecutive days. With this procedure, rats show a significant decline in respiration, heart rate, motor movements and plasma corticosterone when comparing the first to the last acclimation periods (King et al., 2005). The reduction in autonomic and somatic measures of arousal and stress improve the signal resolution and quality of the MR images.
Imaging protocol
Experiments were conducted in a Bruker Biospec 4.7-T/40-cm horizontal magnet (Oxford Instrument, Oxford, U.K.) equipped with a Biospec Bruker console (Bruker, Billerica, MA U.S.A) and a 20-G/cm magnetic field gradient insert (ID = 12 cm) capable of a 120-µs rise time (Bruker). Radiofrequency signals were sent and received with the dual coil electronics built into the animal restrainer (Ludwig et al., 2004). The volume coil for sending RF signal features an 8-element microstrip line configuration in conjunction with an outer copper shield. The arch-shaped geometry of the receiving surface coil provides excellent coverage and high signal-to-noise. To prevent mutual coil interference, the volume and surface coils were actively tuned and detuned.
Functional images were acquired using a multi-slice fast spin echo sequence. A single data acquisition acquired twelve, 1.2 mm slices in 6 sec (FOV 3.0 cm; data matrix 64 × 64; TR 1.4 sec TE 7 msec; NEX 1). This sequence was repeated 80 times in an 8 min imaging session consisting of 3 min of baseline data followed by 5 min of stimulation data. At the end of each imaging session a high resolution anatomical data set was collected using the RARE pulse sequence (12 slice; 1.2 mm; FOV 3.0 cm; 256 × 256; TR 2.1 sec; TE 12.4 msec; NEX 12; 13 min acquisition time).
Experimental Design
Suckling and General Tactile Stimulation in Awake Animals
Four stimulation conditions were studied using four groups of seven animals each. In one condition, lactating dams between postnatal days 4-8 were exposed to their pups for a five min stimulation period. In the second condition, a separate group of lactating dams between postnatal days 4-8 were artificially suckled in the absence of pups. In the third condition, a separate group of lactating dams between postnatal days 4-8 were exposed to gentle rubbing of the ventrum around the nipples with a flat edged wooden ruler. In the fourth condition, virgin females were exposed to rubbing stimulus. Data acquisition for control (3 min) and stimulation (5 min) periods was continuous. All animals were temporally anesthetized with 2-3% isoflurane, secured in the holder and allowed to awaken as described previously. For all stimulation studies, the hind limbs of the animals were loosely tethered and raised just above the floor of the body tube. This provided a visual inspection of the ventrum from outside the magnet and prevented the animals from kicking at the stimulus. A cradle containing six pups was positioned under the dam in the magnet. The body tube had a window exposing the dam’s ventrum to the pups. A thin plastic shield separated the pups from the mother. The shield was carefully positioned so it did not touch the mother when pulled. When the shield was pulled away the pups were exposed to the six hind-limb nipples and would begin suckling. We were able to visually confirm when pups came onto the most caudal teats but were not able to determine whether all teats were suckled during the 5 min stimulation period. In all seven pup stimulation studies, suckling occurred within seconds of removing the shield. To promote suckling in the magnet, dams were prevented from having physical contact with their pups for two hours prior to imaging. Dams given rubbing and artificial suction were also separate from their pups for the same amount of time. This was accomplished by inverting a shallow perforated Plexiglas box over the huddled pups. It should also be noted that (Mattson et al., 2001) showed that a 2-3 hr separation was necessary for a female to learn conditioned place preference for pups.
Artificial suction on the nipples was provided bilaterally by using a manual ‘pumping’ device constructed out of two 36-inch PVC infusion lines (Infusion Devices, Oklahoma City, OK), a plastic 3-way stopcock and a 5 cc syringe. The infusion lines had a 1 mm inner diameter, with one side having a screw-on cap for connecting with the stopcock and the other a 5-6 mm wide opening was adhered to the nipple. While under isoflurane anesthesia (2-3%), dams were placed on their backs and the hair surrounding two midsection nipples (one on each side) was removed with electrical clippers. The ends of the two lines were sealed tight with surgical glue, taking care not to affect nipple mobility during drying. At the other ends, the lines were connected via the stopcock to a 5 cc syringe. Before setting up the animal in the restrainer, negative pressure was applied to the nipples by pulling on the syringe (at approx. 1 Hz) to verify that forced pressure (push and pull) was applied correctly (the nipples extended and retracted during artificial suckling stimulation). The lines were secured to the sides of the animals within the restrainer, and they reached just outside the magnet bore for easy access. An 8-min functional scan was acquired while dams were awake, with 3 min baseline and 5 minutes of artificial suckling stimulation.
Data Analysis
Within subjects analysis
Anatomy images for each subject were obtained at a resolution of 2562 × 12 slices and a FOV = 30mm with slice thickness of 1.2 mm. Subsequent functional imaging was performed at a resolution of 642 × 12 slices with the same FOV and slice thickness. Eighty data acquisitions were performed with a period of 6s each for a total lapse time of 480s or 8 minutes. The first 30 acquisitions were used for acclimation and control. The monitoring continued for an additional 50 acquisitions with the stimulus (suckling or ventrum rubbing) being introduced at time period 3 min. Motion artifact was assessed by qualitative analysis of time series movies looking for voxel displacement and analysis of raw data time series for course spikes. The time series movies correlated with course spike activity. The multiple data sets collected from these imaging sessions showed very little motion artifact using these criteria. There were no discernable differences in motion artifact as a function of stimulus type. On the rare occasion there would be a course spike usually caused by movement of the mouth such as in swallowing. The data for these images were excluded.
ROI-based statistical analysis was done using Medical Image Visualization and Analysis (MIVA) software (http://ccni.wpi.edu/cwbench/cwbench-tiles.jsp). Details of the alignment of scans to the rat brain atlas have been published elsewhere (Ferris et al., 2005; Wu and Sullivan, 2003). Each subject was registered or aligned to a fully segmented rat brain atlas that has the potential to delineate and analyze more than 1200 distinct anatomical volumes within the brain. The electronic rat brain atlas is based on published 2D textbook images (Paxinos and Watson, 1997; Swanson, 1999). The detailed regions are collected into 96 sub-volumes (e.g., dentate gyrus, insular cortex, anterior dorsal thalamus, accumbens) that are grouped into 12 major regions of the brain (e.g., amygdaloid complex, cerebrum, cerebellum, hypothalamus). The alignment process began by outlining the brain perimeters for each slice of the anatomy image sets. A marching cubes algorithm with automated linearization creates accurate 3D surface shells for each subject (Wu and Sullivan, 2003). This enhanced surface generation strategy eliminates the characteristic stair-stepped behavior of the marching cubes algorithms while simultaneously increasing the accuracy of the geometry representation. These anatomy shells are aligned to the atlas shell. The affined registration involved translation, rotation, and scaling in all 3 dimensions, independently. The matrices that transformed the subject’s anatomy to the atlas space were used to embed each slice within the atlas. All transformed pixel locations of the anatomy images were tagged with the segmented atlas major and minor regions creating a fully segmented representation of each subject. The inverse transformation matrix [Ti]−1for each subject (i) was also calculated. Approximately 10 min per subject were required to align and create the final segmented anatomy.
Statistical t tests were performed on each subject within their original coordinate system. The control window was the first 30 time periods. The stimulation window was the remaining 50 time periods. The baseline threshold was set at 2% following are previous data showing that BOLD signal changes above this threshold are reliably above noise levels for awake rat imaging (Brevard et al., 2003). The t test statistics used a 95% confidence level, two-tailed distributions, and heteroscedastic variance assumptions. As a result of the multiple t test analyses performed, a false-positive detection controlling mechanism was introduced (Genovese et al., 2002). This subsequent filter guaranteed that, on average, the false-positive detection rate was below our cutoff of 0.05. The formulation of the filter satisfied the following expression:
where P(i) is the p value based on the t test analysis. Each pixel (i) within the region of interest (ROI) containing (V) pixels was ranked based on its probability value. The false-positive filter value q was set to be 0.05 for our analyses, and the predetermined constant c(V) was set to unity, which is appropriate for data containing Gaussian noise such as fMRI data (Genovese et al., 2002). These analysis settings provided conservative estimates for significance. Those pixels deemed statistically significant retained their percentage change values (stimulation mean minus control mean) relative to control mean. All other pixel values were set to zero.
A statistical composite was created for each group of subjects. The individual analyses were summed within groups. The composite statistics were built using the inverse transformation matrices. Each composite pixel location (i.e., row, column, and slice), premultiplied by [Ti]−1, mapped it within a voxel of subject (i). A tri-linear interpolation of the subject’s voxel values (percentage change) determined the statistical contribution of subject (i) to the composite (row, column, and slice) location. The use of [Ti]−1 ensured that the full volume set of the composite was populated with subject contributions. The average value from all subjects within the group determined the composite value. The BOLD response maps of the composite were somewhat broader in their spatial coverage than in an individual subject; so only average number of activated pixels that has highest composite percent change values in particular ROI was displayed in composite map. Activated composite pixels are calculated as follows:
The composite percent change for the time history graphs for each region was based on the weighted average of each subject, as follows:
where N is number of subjects.
Registration of low resolution functional scans to a high resolution digital atlas of the brain results in atlas slices that are partially sampled in each individual space. To overcome this problem each subject [S] voxel is mapped to a specific location within the atlas [A]. The matrices ⌊T(Subject–to–atlas)⌋j that transformed the subject’s anatomy to the atlas space were used to embed each slice within the atlas.
This approach presented us with another challenge, namely, since the atlas resolution [512 × 512 × 300 slices] is much higher than the subject resolution [64 × 64 × 12 slices], this results in multiple atlas voxels occupying a single subject voxel. To overcome this we used nearest neighbor voxel information and verified if voxels are not a boundary voxel to improve classification accuracy. Since we do not use minor areas, this approach gives fairly accurate mapping.
Between groups statistics
The four experimental conditions (n = 7 per group) were: dam-suckling, dam-suction, dam-rubbing, virgin-rubbing. For the first stage of the fMRI analyses (described above), summary statistics per ROI for each individual functional scan was calculated independently. Activation maps were created for subjects and each map contributed to the composite maps shown in Figures 1, 2, 5. The initial analyses also provided estimated values of percent change in BOLD signal over multiple ROIs and activated voxel numbers (representative of the volume of activity), which were exported to spreadsheets and statistically analyzed on GBstat software (Silver Spring, MD). Although the number of animal per group was equal, we assumed that the data were non-normally distribution or heteroscedastic and therefore used non-parametric statistical testing. To test for differences between the groups, a Kruskall-Wallis one-way ANOVA is first employed followed by Newman-Keuls (N-K) multiple comparisons test (alpha value was set at 5%). For the ANOVA, the type of stimulus (suckling vs suction vs rubbing) was considered the independent variable and the number of voxels the dependent variable. Tests were done separately for each region of interest. Significant differences for the N-K multiple comparisons tests are summarized in the figures, with single asterisks indicating p < 0.05, N-K critical value q > 2.8, and double asterisks p < 0.01 and N-K critical value q > 3.7. BOLD signal changes over time were compared between groups using a repeated ANOVA followed by a Bonferroni test.
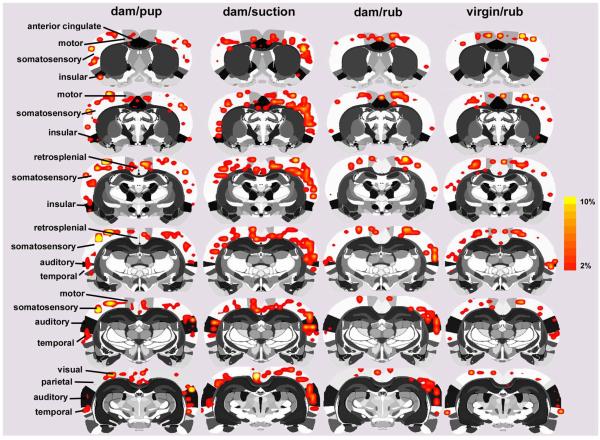
Figure 1.
Two-dimensional compositive maps of positive BOLD activation in the cortical mantle of lactating and virgin rats. Four stimulus conditions are presented: dams receiving suckling stimulation from pups, artificial suckling in the absence of pups, and general mechanical rubbing of the ventrum skin in dams and virgins (n = 7 per condition). Scale bar hue (orange-to-yellow) indicates percent increase in BOLD with a lower threshold cut-off of 2%. Various cortical regions of interest are highlighted to the left of the figure.
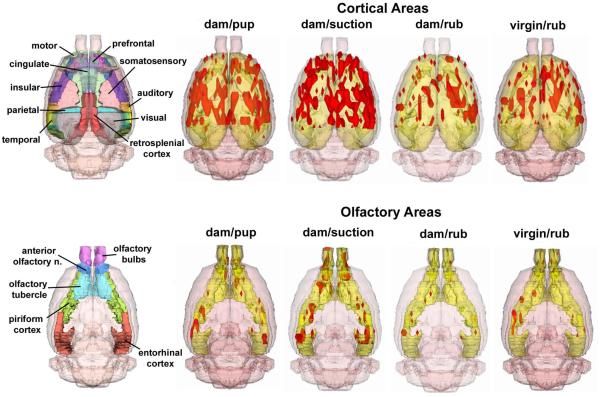
Figure 2.
Three-dimensional composite maps of positive BOLD activation in lactating and virgin rats. Data are shown for the cortical mantle (upper row) and olfactory system areas (lower row). The four stimulus conditions are the same as in Figure 1. Areas in red are volumes of positive BOLD activity. Segmented 3D atlas brains to the left show various regions of interest.
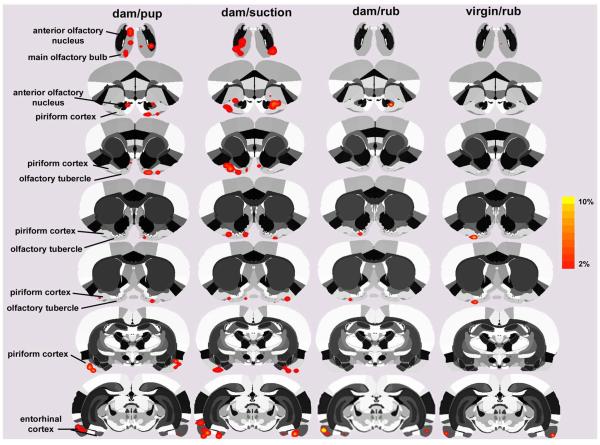
Figure 5.
Two-dimensional compositive maps of positive BOLD activation in olfactory areas of lactating and virgin rats. The four stimulus conditions are the same as in Figure 1. Scale bar hue (orange-to-yellow) indicates percent increase in BOLD with a lower threshold cut-off of 2%. Various olfactory regions of interest are highlighted to the left of the figure.
Results
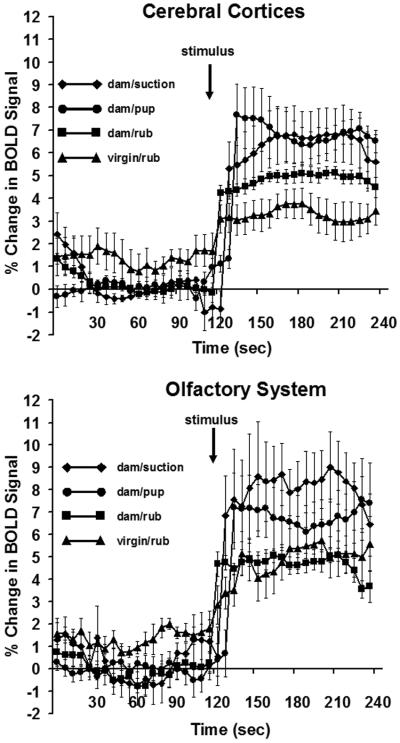
Two-dimensional activation maps of the cortical mantle for each stimulus condition are shown in Fig 1. Each 2D atlas image with its superimposed cortical functional map is a composite of 7 subjects fully registered and segmented for regions of interest (ROI) for specific areas of the cortex. Pup suckling and artificial suction in the absence of pups stimulated a bilateral pattern of enhanced BOLD signal in distributed regions of the cortex. The activation pattern was most noticeable in the somatosensory cortex, however, robust BOLD activation was observed as far rostral as the motor cortex and caudally in areas of the visual cortex. A somewhat similar bilateral pattern of BOLD signal change was observed in dams and virgin females exposed to mechanical rubbing of the ventrum. Pup suckling and artificial suction on the teats caused the most activation in the cortex, observed as both a greater volume of activated cortex (Figs 1-3) and as a greater percentage increase in BOLD (Fig 4, upper panel) than with mechanical rubbing in dams or virgin rats (repeated measures ANOVA for multiple time points, F3,23 = 3.6, p <0.05). No differences in BOLD signal changes over time were observed for the olfactory cortex (F3,23 = 2.2, p < 0.2). Although no statistically significant differences were observed when comparing pup suckling to artificial suction in the absence of pups (Figs 3 and 6), the latter group showed a more widespread BOLD activity pattern than natural pup suckling. Suction stimuli also elicited a greater percentage BOLD signal change over time than the other three conditions (Fig 4).
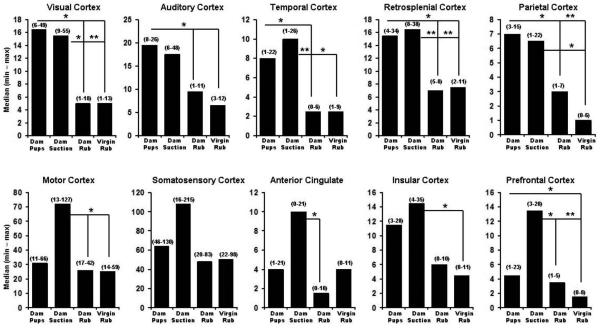
Figure 3.
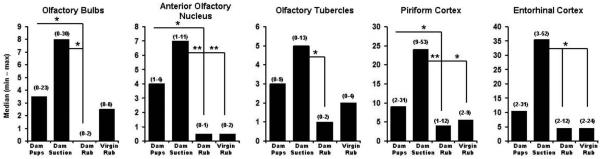
Number of positive BOLD voxels in subregions of the cortex of lactating and virgin rats. The four stimulus conditions for each panel are the same as in Figure 1. Data are expressed as median (minimum-maximum). Statistical tests were done with Newman-Keuls multiple comparisons test. * indicates q0.05, 23, 4 > 2.8, p < 0.05; ** indicates q0.05, 23, 4 > 3.7, p < 0.01.
Figure 4.
Positive BOLD signal changes over time in lactating and virgin rats. Data for the cortical mantle and olfactory system areas are shown as mean ± standard error of the mean. The four stimulus conditions are the same as in Figure 1. Arrow indicates the point of stimulus presentation during the course of the scans (suckling pups, artificial suction, or mechanical rubbing of ventrum). Statistical comparisons were done with a repeated measures analysis of variance (p < 0.05).
Figure 6.
Number of positive BOLD voxels in subregions of the olfactory system of lactating rats. The four stimulus conditions for each panel are the same as in Figure 1. Data are expressed as median (minimum-maximum). Statistical tests were done with Newman-Keuls multiple comparisons test. * indicates q0.05, 23, 4 > 2.8, p < 0.05; ** indicates q0.05, 23, 4 > 3.7, p < 0.01.
The volumes of activation comprising the cortical mantle for each stimulus condition can be viewed as 3D models (Fig 2 upper panel). This 3D perspective reveals pup suckling and artificial suction preferentially activates the most rostral midline areas, particularly the prefrontal cortex, as compared to mechanical rubbing. Again, it was observed that artificial suction on the nipple evoked robust activation of the cortex, not localized to a single region, but widespread across many cortical sites (Fig 2, upper panel). Quantitative analyses of the ROIs shown in Fig 1 and presented in Fig 3 confirm this observation. Bar graphs show the median plus minimum and maximum number of activated voxels in the different cerebral cortices for each stimulus condition. The areas of the cortex that showed greater BOLD activation with suckling and artificial suction than one or both of the two rubbing conditions were the visual, retrosplenial, auditory, temporal (compared to dam-rubbed), parietal and prefrontal cortices (compared to virgin-rubbed; specific Newman-Keuls statistical comparisons are summarized in Fig 3 legend). Additional cortical areas were significantly different only between artificial suction and one or both of the two rubbing conditions, these were: temporal cortex (compared to virgin-rubbed), motor cortex, anterior cingulate cortex (compared to dam-rubbed), prefrontal cortex (compared to dam-rubbed) and insular cortex (compared to virgin-rubbed; specific Newman-Keuls statistical comparisons are summarized in Fig 3 legend).
Two-dimensional activation maps of the olfactory system for each stimulus condition are shown in Fig 5. There is ostensibly more BOLD activation with pup suckling as compared to ventrum rubbing in either dams or virgins. The volumes of activation comprising the olfactory system for each stimulus condition can be viewed as 3D models (Fig 2 lower panel). These 3D perspectives clearly reveal a robust, bilateral pattern of activation over much of the olfactory system induced by pup suckling but not the general tactile stimulation of ventrum rubbing. As observed for the cortex, artificial suction on the teats caused the most activation of olfactory areas, observed as both a greater volume of activation (Figs 5-6) and as a greater percentage increase in BOLD (Fig 4, lower panel). It is important to indicate that suction was provided in the absence of pups. Quantitative analyses of the ROIs shown in Fig 5 and presented in Fig 6 confirm this observation (specific Newman-Keuls statistical comparisons are summarized in Fig 6 legend). There are a significantly greater number of voxels activated by pup suckling for several areas of the olfactory system (olfactory bulbs, anterior olfactory nucleus and piriform cortex) than for either ventrum rubbing in dams or virgins. It was observed that artificial suction on the nipple evoked the most robust activation of the olfactory system, most notably the olfactory bulbs (Fig 2, lower panel). The percent change in BOLD signal intensity for all four stimuli across the olfactory system is shown in Fig 4. The temporal pattern of activation is greater for artificial suction than for the other three stimulus conditions.
Given the unexpected activation of the primary olfactory system with suckling, the accessory or vomeronasal system was also analyzed under all three stimulus (data not shown). There was no significant activation in the accessory olfactory bulb, bed nucleus of the stria terminalis, nucleus of the accessory olfactory tract, or medial nucleus of the amygdala during suckling or ventrum rubbing in dams or virgin controls. Finally, it is important to make a statement on the spatial coverage over the rat brain in the present studies. Rostrally, the slices acquired extended to the caudal portions of the olfactory bulbs but did not entirely cover this area. In addition, the caudal brain slices covered the initial areas of the primary visual and the entorhinal cortex (not reported here). This ‘cut-off’ effect of slice coverage is illustrated in the composite 3D maps shown in Fig 2.
Discussion
In the current study it was found that wide areas of the postpartum rat cerebrum exhibit an increase in the fMRI BOLD signal during suckling stimulation, suggesting that neural activity is modified over wide areas of the cerebral cortex in response to a rather specific stimulus. In previous work from this laboratory, similar findings have been reported (Febo et al., 2005): the natural suckling stimulus was associated with a positive BOLD response over large areas of the cerebral cortex. The innovation of the current work was to show that an artificial suckling stimulus caused a similar degree of cortical activation. Therefore, although auditory, olfactory, and non-suckling tactile stimulation from pups may contribute to cortical activity, the isolated suckling stimulus is fully capable of causing a widespread cortical response.
What might be the possible function of such a widespread cortical response during suckling? Research indicates that ventral stimulation from nuzzling and suckling pups influences the following maternal functions in rodents: nursing behavior (Stern and Johnson, 1990); ongoing maternal motivation and the development of maternal memory (Morgan et al., 1992); the formation of a conditioned place preference where the mother learns to return to the specific site where she received suckling stimulation (Fleming and Walsh, 1994; Walsh et al., 1996); and maternal aggression (Stern and Kolunie, 1993). The role of suckling stimulation in the induction of specific nursing postures is not likely to involve cortical activation, but instead depends on suckling-induced modifications of brainstem-spinal circuits (Lonstein and Stern, 1997a; Lonstein and Stern, 1997b). However, suckling-induced cortical activation may be importantly involved in the other functions listed above.
Maternal aggression is the heightened aggressive response that the lactating mother shows toward intruders at the nest site, and this behavior serves to protect the young from threatening conspecifics and possibly from predators [see (Numan and Insel, 2003) for a review]. Importantly, if pups are separated from the mother for several hours or if the mother’s ventral surface is anesthetized so that she cannot detect suckling/nuzzling pups, then maternal aggression wanes. In mice, thelectomy (nipple removal) prevents the occurrence of maternal aggression (Svare and Gandelman, 1976). Other studies have also shown that the olfactory system is important for the display of maternal aggression (Hansen and Ferreira, 1986; Mayer and Rosenblatt, 1993; Stern and Kolunie, 1993). An interesting hypothesis, therefore, is that suckling-induced modifications of olfactory cortical representations are one of the processes involved in the maintenance of maternal aggression.
Maternal motivation and maternal memory are likely to be interrelated since maternal memory involves a modification of maternal responsiveness. When a primiparous female rat gives birth and is exposed to pups for the first time, her maternal responsiveness is highly dependent upon the endocrine changes associated with pregnancy termination, and a critical process involved in this hormonal induction of maternal behavior is a shift in the valence of pups odors from negative (or aversive) to positive (or attractive) (Numan and Insel, 2003). In the naïve nulliparous female rat, pup odors are primarily aversive, leading to avoidance behavior. Interestingly, if the primiparous female is allowed only a few hours of maternal contact with pups, her maternal responsiveness is permanently modified: the pups can be removed and when young pups are returned several weeks later the female will care for them rather than avoid them, even though she is not under the influence of the endocrine changes associated with the end of pregnancy (Bridges, 1975; Orpen et al., 1987). One interpretation of these results is that the initial maternal experience modifies the brain so that pup odors become permanently attractive and activate approach behavior instead of avoidance behavior, even in the absence of hormonal stimulation. Importantly, primiparous females who are unable to receive both ventral tactile stimulation (which includes suckling stimulation) and perioral tactile stimulation from pups show very little maternal behavior when first exposed to pups postpartum and they also do not develop maternal memory or the long-term retention of maternal reponsiveness (Morgan et al., 1992). It is, therefore, possible that suckling-induced activation of the olfactory cortex may contribute to causing a relatively permanent shift in the valence of pup odors from negative to positive. Perhaps, there are reciprocal pathways to the olfactory bulbs that are activated when a female is suckling, hence cuing her senses more into the odors of young.
During a conditioned place preference procedure an organism is exposed to a positive stimulus while in a cage compartment, which has distinct stimulus attributes which differentiate it from another cage compartment, which does not contain the positive stimulus. Postpartum rats develop such a conditioned place preference if they are allowed proximal contact with pups, which includes suckling stimulation (Fleming and Walsh, 1994). In fact, anesthetization of the ventral surface, including the nipple region, prevents conditioning from occurring (Walsh et al., 1996). One interpretation of these results is that engaging in maternal behavior, which includes suckling stimulation, is a primary reinforcer and that neutral stimuli that are associated with such positive reinforcement become attractive. This learning process then causes females to spend more time in the compartment where maternal behavior took place, even when pups are not present; it is as if the female is searching for the pups in the place where they were last found. With respect to the ecological significance of such learning, it probably allows the mother to learn the characteristics of her nest area location, so that on returning to her nest after a period of absence, her ability to find the nest is facilitated. Several characteristics related to this phenomenon are worth pointing out: (1) The mesocorticolimbic dopamine (DA) system is important for the formation of conditioned place preferences and interference with DA transmission during the pup exposure/training stage has been found to disrupt the development of a conditioned place preference based on pup stimulation (Fleming and Walsh, 1994); (2) Natural suckling stimulation has been shown to cause a positive BOLD response in various parts of the mesocorticolimbic DA system; (3) the nucleus accumbens (NA), as a component of the mesolimbic DA system, receives DA input from the ventral tegmental area (VTA) and receives cortical input from the prefrontal cortex and entorhinal cortex-hippocampal system (Pennartz et al., 1994). It is highly likely that this cortical input carries processed sensory input to NA. Therefore, during the formation of a conditioned place preference based on pup stimulation, the following scenario is possible: proximal pup stimulation, including suckling, activates both the cerebral cortex and the mesolimbic DA system. The cortical activation associated with suckling may enhance the salience of neutral stimuli that are associated with maternal behavior and may increase the neural activity of such cortical inputs to NA (Nicola et al., 2000). Concurrent DA release into NA may strengthen the ability of such cortical activity and their associated stimuli to promote approach/searching behavior at a future point in time.
How might suckling stimulation activate both the mesolimbic DA system and the cortex? One possibility is that the medial preoptic area (MPOA) is involved. The MPOA is essential for maternal behavior, it is activated by suckling stimulation, and it projects to both the VTA and to the locus coeruleus (LC) (Febo et al., 2005; Numan and Numan, 1996; Numan and Numan, 1995; Numan and Numan, 1997). Additionally, LC is activated by suckling stimulation (Li et al., 1999). It is possible, therefore, that suckling-induced activation of the MPOA activates the mesolimbic DA system via MPOA efferents to VTA. MPOA efferents to LC may cause cortical activation because the LC has widespread cortical projections and is known to be involved in cortical activation (Aston-Jones and Cohen, 2005).
An implicit assumption concerning the cortical mechanisms we have just described is that the suckling-induced positive BOLD response in the cortex causes activation of cortical outputs, which promote maternal aggression, maternal responsiveness, and learning. However, a significant complication to such an assumption is that when suckling stimulation induces what is called the crouch nursing posture, the mother appears less rather than more responsive to external stimulation (Stern and Johnson, 1990). In fact, there is research that indicates that the milk-ejection reflex is associated with slow wave sleep (Lincoln et al., 1980; Voloschin and Tramezzani, 1979; Voloschin and Tramezzani, 1984), which would suggest synchronized cortical activity and decreased sensory responsiveness. Therefore, we can’t rule out the possibility that the suckling-induced positive cortical BOLD response represents the activation of local inhibitory cortical interneurons, which may depress cortical responsiveness to external stimulation.
Another interpretation for the finding that artificial suction activates multiple sensoricortical sites is that the nursing event itself has been somehow conditioned across several sensory modalities. Nursing rats not only experience the sensory stimuli at the ventrum and nipples, but also experience a wealth of sensory cues associated with this act. Pups deliver odors, vocalizations, and perhaps even gustatory stimuli (through licking), that can enhance the sensory experience of suckling, and possibly facilitate perception and behavior in mothers. Thus, the absence of one or more of these sensory stimuli (as seen in the present study design) may still result in activity in multi-sensory regions. Interestingly, suckling might also enhance the cortical responses to pup calls, pup odors, and there is ample evidence in the past behavioral neuroscience literature for this multisensory integration (Stern, 1990). Liu et al. (2006) have recently shown that the maternal mouse auditory cortex represents pup calls differently than pup-naïve females, neuronal populations responding to faster call rates in mothers. This type of neural representation might be enhanced by other co-occurring sensory events, perhaps involving associative processes. Neural plasticity necessarily occurs during the first few days of feeding and attending to pups (Xerri et al., 1994), which may favor multisensorial interactions (Stern, 1990). Indeed, there is additional evidence for multisensory interactions coming from different fields of neuroscience employing fMRI, however, this has mostly stemmed from research in humans and non-human primates. Neuronal recordings in the primate cortex have revealed that somatosensory inputs modulate oscillatory responses of auditory neuronal populations receiving tone stimuli and can actually have an additive effect on auditory neuronal responses to sounds of moderate intensity (Lakatos et al., 2007). Sensory integration between visual and auditory cortex of the primate, and facilitatory interactions between these areas, has also been demonstrated using fMRI (Kayser et al., 2007).
To move beyond the rodent data and to make some general statements, the results of the current experiment suggest that suckling stimulation during nursing may have dramatic influences on mammalian maternal behavior; it appears to influence maternal motivation and the mother-infant bond, maternal aggressiveness, and learning and memory mechanisms which probably enable the mother to recognize envirornments which are safe places to care for infants. Activation of wide areas of the cortex by suckling appears to be involved in these effects, and some of these processes may be influenced by suckling-induced release of oxytocin in selected neural sites (Febo et al., 2005).
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse to Craig F. Ferris (R01DA13517) and Marcelo Febo (R01DA019946).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen AH, et al. Functional MRI studies in awake rhesus monkeys: methodological and analytical strategies. J Neurosci Methods. 2002;118:141–52. doi: 10.1016/s0165-0270(02)00123-1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Birn RM, et al. Magnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med. 1998;40:55–60. doi: 10.1002/mrm.1910400108. [DOI] [PubMed] [Google Scholar]
- Blyton DM, et al. Lactation is associated with an increase in slow-wave sleep in women. J Sleep Res. 2002;11:297–303. doi: 10.1046/j.1365-2869.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- Boyett-Anderson JM, et al. Functional brain imaging of olfactory processing in monkeys. Neuroimage. 2003;20:257–64. doi: 10.1016/s1053-8119(03)00288-x. [DOI] [PubMed] [Google Scholar]
- Brevard ME, et al. Changes in MRI signal intensity during hypercapnic challenge under conscious and anesthetized conditions. Magn Reson Imaging. 2003;21:995–1001. doi: 10.1016/s0730-725x(03)00204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiol Behav. 1975;14:245–249. doi: 10.1016/0031-9384(75)90028-1. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, et al. Somatosensory systems and the milk-ejection reflex in the rat. II. The effects of lesions in the ventroposterior thalamic complex, dorsal columns and lateral cervical nucleus-dorsolateral funiculus. Neuroscience. 1985;15:1131–40. doi: 10.1016/0306-4522(85)90257-x. [DOI] [PubMed] [Google Scholar]
- Febo M, et al. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–44. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, et al. Imaging cocaine-induced changes in the mesocorticolimbic dopaminergic system of conscious rats. J Neurosci Methods. 2004;139:167–76. doi: 10.1016/j.jneumeth.2004.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ. Breast feeding and later psychosocial adjustment. Paediatr Perinat Epidemiol. 1999;13:144–57. doi: 10.1046/j.1365-3016.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, et al. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–56. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, et al. Activation of neural pathways associated with sexual arousal in non-human primates. J Magn Reson Imaging. 2004;19:168–75. doi: 10.1002/jmri.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fize D, et al. The retinotopic organization of primate dorsal V4 and surrounding areas: A functional magnetic resonance imaging study in awake monkeys. J Neurosci. 2003;23:7395–406. doi: 10.1523/JNEUROSCI.23-19-07395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, et al. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacol Biochem Behav. 2002;73:61–75. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Walsh C. Neuropsychology of maternal behavior in the rat: c-fos expression during mother-litter interactions. Psychoneuroendocrinology. 1994;19:429–43. doi: 10.1016/0306-4530(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Francis D, et al. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Genovese CR, et al. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hajnal JV, et al. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med. 1994;31:283–91. doi: 10.1002/mrm.1910310307. [DOI] [PubMed] [Google Scholar]
- Hansen S, Ferreira A. Food intake, aggression, and fear behavior in the mother rat: control by neural systems concerned with milk ejection and maternal behavior. Behav Neurosci. 1986;100:64–70. doi: 10.1037//0735-7044.100.1.64. [DOI] [PubMed] [Google Scholar]
- Kayser C, et al. Functional imaging reveals visual modulation of specific fields in auditory cortex. J Neurosci. 2007;27:1824–35. doi: 10.1523/JNEUROSCI.4737-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, et al. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–60. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti KM, et al. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med. 1999;41:412–6. doi: 10.1002/(sici)1522-2594(199902)41:2<412::aid-mrm28>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lakatos P, et al. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–92. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, et al. Neural populations in the rat forebrain and brainstem activated by the suckling stimulus as demonstrated by cFos expression. Neuroscience. 1999;94:117–29. doi: 10.1016/s0306-4522(99)00236-5. [DOI] [PubMed] [Google Scholar]
- Lin SH, et al. Metabolic mapping of the brain in pregnant, parturient and lactating rats using fos immunohistochemistry. Brain Res. 1998;787:226–36. doi: 10.1016/s0006-8993(97)01484-4. [DOI] [PubMed] [Google Scholar]
- Lincoln DW, et al. Sleep: a prerequisite for reflex milk ejection in the rat. Exp Brain Res. 1980;38:151–62. doi: 10.1007/BF00236736. [DOI] [PubMed] [Google Scholar]
- Liu RC, et al. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–97. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. J Neurosci. 1997a;17:3364–78. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Somatosensory contributions to c-fos activation within the caudal periaqueductal gray of lactating rats: effects of perioral, rooting, and suckling stimuli from pups. Horm Behav. 1997b;32:155–66. doi: 10.1006/hbeh.1997.1416. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, et al. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–81. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Ludwig R, et al. A dual RF resonator system for high-field functional magnetic resonance imaging of small animals. J Neurosci Methods. 2004;132:125–35. doi: 10.1016/j.jneumeth.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, et al. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–94. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Contributions of olfaction to maternal aggression in laboratory rats (Rattus norvegicus): effects of peripheral deafferentation of the primary olfactory system. J Comp Psychol. 1993;107:12–24. doi: 10.1037/0735-7036.107.1.12. [DOI] [PubMed] [Google Scholar]
- Morgan HD, et al. Somatosensory control of the onset and retention of maternal responsiveness in primiparous Sprague-Dawley rats. Physiol Behav. 1992;51:549–55. doi: 10.1016/0031-9384(92)90178-5. [DOI] [PubMed] [Google Scholar]
- Nicola SM, et al. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior (hormones, brain, and behavior series) Springer-Verlag; New York: 2003. [Google Scholar]
- Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Importance of pup-related sensory inputs and maternal performance for the expression of Fos-like immunoreactivity in the preoptic area and ventral bed nucleus of the stria terminalis of postpartum rats. Behav Neurosci. 1995;109:135–49. doi: 10.1037//0735-7044.109.1.135. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J Neuroendocrinol. 1997;9:369–84. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- Orpen BG, et al. Hormonal influences on the duration of postpartum maternal responsiveness in the rat. Physiol Behav. 1987;40:307–15. doi: 10.1016/0031-9384(87)90052-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. Academic Press; Boston: 1997. [Google Scholar]
- Pennartz CM, et al. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–61. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Stern JM. Multisensory regulation of maternal behavior and masculine sexual behavior: a revised view. Neurosci Biobehav Rev. 1990;14:183–200. doi: 10.1016/s0149-7634(05)80219-2. [DOI] [PubMed] [Google Scholar]
- Stern JM, Johnson SK. Ventral somatosensory determinants of nursing behavior in Norway rats. I. Effects of variations in the quality and quantity of pup stimuli. Physiol Behav. 1990;47:993–1011. doi: 10.1016/0031-9384(90)90026-z. [DOI] [PubMed] [Google Scholar]
- Stern JM, Kolunie JM. Maternal aggression of rats is impaired by cutaneous anesthesia of the ventral trunk, but not by nipple removal. Physiol Behav. 1993;54:861–8. doi: 10.1016/0031-9384(93)90293-o. [DOI] [PubMed] [Google Scholar]
- Stern JM, et al. Dorsolateral columns of the spinal cord are necessary for both suckling-induced neuroendocrine reflexes and the kyphotic nursing posture in lactating rats. Brain Res. 2002;947:110–21. doi: 10.1016/s0006-8993(02)02916-5. [DOI] [PubMed] [Google Scholar]
- Svare B, Gandelman R. Postpartum aggression in mice: the influence of suckling stimulation. Horm Behav. 1976;7:407–16. doi: 10.1016/0018-506x(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Elsevier Science; Boston: 1999. [Google Scholar]
- Tasker JG, et al. Afferent projections from the mammary glands to the spinal cord in the lactating rat--I. A neuroanatomical study using the transganglionic transport of horseradish peroxidase-wheatgerm agglutinin. Neuroscience. 1986;19:495–509. doi: 10.1016/0306-4522(86)90276-9. [DOI] [PubMed] [Google Scholar]
- Tenney JR, et al. FMRI of brain activation in a genetic rat model of absence seizures. Epilepsia. 2004;45:576–82. doi: 10.1111/j.0013-9580.2004.39303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloschin LM, Tramezzani JH. Milk ejection reflex linked to slow wave sleep in nursing rats. Endocrinology. 1979;105:1202–7. doi: 10.1210/endo-105-5-1202. [DOI] [PubMed] [Google Scholar]
- Voloschin LM, Tramezzani JH. Relationship of prolactin release in lactating rats to milk ejection, sleep state, and ultrasonic vocalization by the pups. Endocrinology. 1984;114:618–23. doi: 10.1210/endo-114-2-618. [DOI] [PubMed] [Google Scholar]
- Walsh CJ, et al. The effects of olfactory and somatosensory desensitization on Fos-like immunoreactivity in the brains of pup-exposed postpartum rats. Behav Neurosci. 1996;110:134–53. doi: 10.1037//0735-7044.110.1.134. [DOI] [PubMed] [Google Scholar]
- Wu Z, Sullivan JMJ. Multiple material marching cubes algorithm. IJNME. 2003;58:189–207. [Google Scholar]
- Xerri C, et al. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci. 1994;14:1710–1721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin FZ, et al. Effect of motion outside the field of view on functional MR. AJNR Am J Neuroradiol. 1996;17:1005–9. [PMC free article] [PubMed] [Google Scholar]