Abstract
The effect of killer-cell immunoglobulin-like receptor (KIR)-ligand matching on outcomes after unrelated cord blood (CB) transplantation was studied in patients with acute myeloid leukemia (AML; n=461), categorizing KIR-ligand for HLA-C groups C1 and C2, and Bw4. Donor-recipient HLA-matching considered allele-level matching at HLA-A, -B, -C and –DRB1. Separate analyses were conducted for 6–7/8 HLA-matched and 3–5/8 HLA-matched transplants as HLA-matching confounded KIR-ligand matching (i.e., KIR-ligand mismatching was less likely with better HLA-matching). All patients received single CB unit and myeloablative conditioning. There were no significant differences in non-relapse mortality (NRM), relapse and overall mortality by KIR ligand match status. But, among recipients of 3–5/8 HLA-matched transplants, NRM (HR 2.26, p=0.008) and overall mortality (HR 1.78, p=0.008) but not relapse were higher with KIR-ligand mismatched (host vs. graft [HVG] direction) compared to KIR ligand-matched transplants. These data do not support selecting CB units based on KIR ligand match status for transplants mismatched at 1 or 2 HLA loci. Although transplants mismatched at 3 or more HLA-loci are not recommended avoiding KIR ligand mismatching in this setting lowers mortality risks.
Keywords: KIR ligand, unrelated transplant, cord blood, acute leukemia
Introduction
Natural killer (NK) cell alloreactivity may play an important role in determining the outcome of patients given allogeneic hematopoietic stem cell transplantation (HSCT). NK-cell function is controlled by an array of inhibitory and activating signals that are processed by cell-surface receptors, including the inhibitory and activating killer-cell immunoglobulin-like receptors (KIRs)(1, 2). Earlier models of NK alloreactivity in HSCT focused on the interactions between inhibitory KIRs and HLA class I ligands, in which the alloreactivity of donor NK cells is triggered by lack of self-HLA class I engagement of inhibitory KIRs. (3, 4) In retrospective studies of outcomes after HSCT, particularly for patients with acute myeloid leukemia (AML), KIR ligand incompatibilities have been associated with better survival, less relapse and lower incidence of graft-versus-host disease (GvHD).(3, 5–7). This relationship is most evident in T-cell depleted HLAhaplotype disparate HSCT, in which recipients who lack the HLA ligand present in the stem-cell donor benefit from lower relapse rates. (3, 6)
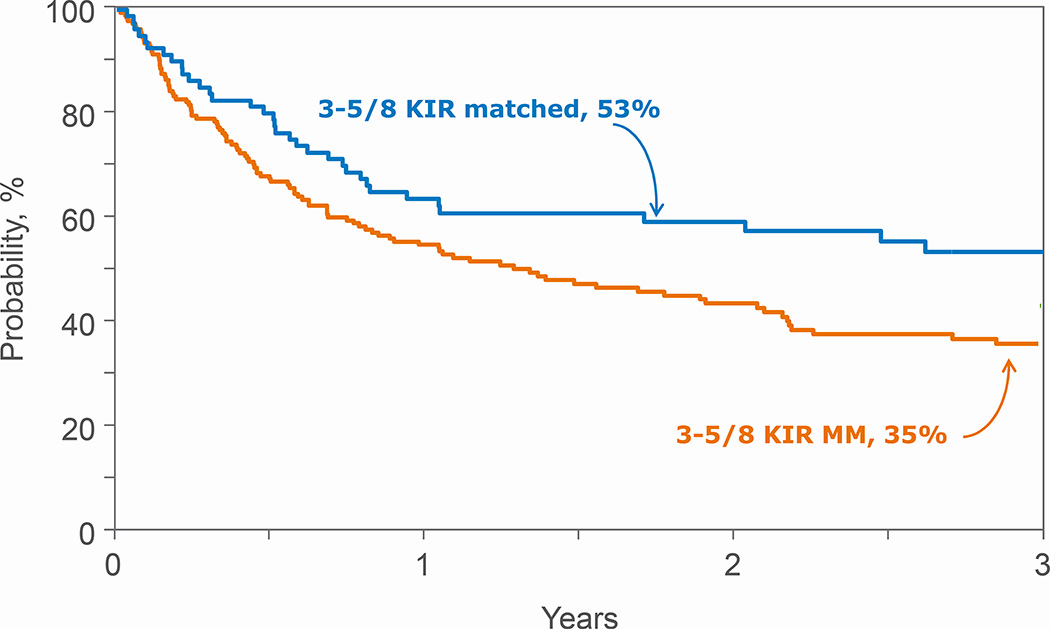
Figure 1.
Overall Survival after 3–5/8 HLA mismatched transplantations by KIR ligand match status
The majority of donor-recipient cord blood transplant pairs are HLA-mismatched at one or two HLA-loci, considering antigen-level (low resolution) HLA matching at HLA-A and –B or at the allele-level at HLA-DRB1, and matching at the HLA-C locus is not generally considered. Previous studies investigating the effect of KIR-HLA ligand matching on HSCT outcomes have shown varied and conflicting results. While some studies showed a beneficial effect of KIR-ligand mismatching, others found deleterious effects, and some, none. (8–11) (12, 13) These differing results may be explained by the heterogeneity of the patient cohorts studied, the degree of T-cell alloreactivity secondary to HLA mismatch, and the different models used to determine KIR-HLA incompatibility. (3, 14–16)
To date, four retrospective studies investigating donor-versus-recipient alloreactivity after single- and double-unit unrelated cord blood transplantation (UCBT) using the ligand-incompatibility model have been reported, with conflicting results.. (17–20) There are a number of significant differences between these studies, including sample size, population composition and the definition of KIR-ligand matching assignment (inclusion of HLA-A associated Bw4 ligands versus no inclusion). All of these studies considered low-resolution typing and definitions of matching for HLA-A, -B, -C, and high-resolution typing for HLA-DRB1, with a relatively small number of patients, with heterogeneous diseases and conditioning regimens. We have shown that matching at the HLA-C locus lowers mortality after single-unit UCBT for acute leukemia (21, 22), but whether selecting CB units on the basis of KIR-ligand matching improves survival and other outcomes is unclear. This analysis sought to study the KIR ligand effect, using high-resolution HLA typing, and matching of HLA-A, -B, -C and -DRB1 in a large series of single-unit UCBT, using myeloablative conditioning, for AML.
Patients and Methods
Patients
Data were obtained from the Center for International Blood and Marrow Transplant Research or Eurocord-European Group for Blood and Marrow Transplantation. Patients received a single UCB unit after a myeloablative conditioning regimen for treatment of acute leukemia with cyclosporine or tacrolimus-containing GVHD prophylaxis. All transplants were performed between 2000 and 2010. Those patients (n=218) that had been previously reported in a Eurocord report (17) were excluded. The Institutional Review Board of the National Marrow Donor Program and the Eurocord-Netcord scientific committee approved this study.
HLA typing and KIR-ligand classification
Donor and recipient HLA typing at HLA-A, B, C and DRB1 was completed using molecular techniques with a minimum of antigen-split level resolution for HLA-A, -B, -C and allele-level resolution at HLA-DRB1. Details of HLA-typing and imputation (Haplogic℠ III developed by the National Marrow Donor Program) have previously been reported.(21) Patients and donors were categorized by KIR-ligand expression for HLA C group 1 or 2 and Bw4, as KIR ligand-matched or mismatched. We further classified the KIR ligand mismatches as in the GVH (graft-versus-host) direction or HVG (host-versus-graft) direction. KIR-ligand mismatch in the GVH direction was present when the donor’s KIR ligand was not shared by patients; KIR ligand mismatch in the HVG direction was present when the patient’s KIR ligand was not shared by donors and bi-directional mismatching was present when there were mismatches in both the GVH and HVG directions.
Outcomes
The primary endpoints were leukemia-free survival (LFS), defined as being alive and in remission (leukemia recurrence or death from any cause was considered an event; treatment failure, inverse of LFS) and overall survival. Other outcomes evaluated were: grade 2–4 acute GVHD(23), chronic GVHD(24), non-relapse mortality (NRM) and relapse.
Statistical methods
The probability of NRM was calculated using the cumulative incidence estimator with relapse as the competing risk.(25) The probabilities of OS and LFS were calculated using the Kaplan-Meier estimator.(26) HLA-matching and KIR ligand matching were confounded (p<0.0001); recipients of better HLA-matched transplants were less likely to be KIR ligand mismatched. Therefore, separate analyses were undertaken for recipients of 6–7/8 HLA-matched and 3–5/8 HLA-matched transplants to separate the effect of KIR ligand match status from HLA disparity. The HLA-matching groups were defined based on the results of an earlier report that examined the effects of HLA matching at the allele level in UCBT recipients.(21) Cox regression models(27) were built for acute and chronic GVHD, NRM, relapse, overall mortality and treatment failure and results reported as hazard ratio (HR) with 95% confidence interval (CI). Variables tested in the multivariate models are shown in Table2. Proportional hazards assumption was tested for each covariate individually and all covariates met this assumption. Multivariate models were built using a stepwise forward/backward model building procedure and variables that attained p-value ≤0.05 were retained in the model. Interactions between the KIR ligand and the adjusted covariates were tested in each model, and no significant interactions were detected at the significance level of 0.05. All p-values are two-sided and p-values ≤ 0.05 were considered statistically significant. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Table 2.
Characteristics of patients with AML who received 3–5/8 HLA-matched transplants
| KIR Ligand Matched |
KIR Ligand Mismatched |
P-value | |
|---|---|---|---|
| Total number | 79 | 183 | |
| Gender | 0.85 | ||
| Male | 42 (53%) | 95 (52%) | |
| Female | 37 (47%) | 88 (48%) | |
| Age, years | 0.63 | ||
| ≤ 16 | 47 (59%) | 103 (56%) | |
| > 16 | 32 (41%) | 80 (44%) | |
| Cytomegalovirus serostatus | 0.67 | ||
| Positive | 42 (53%) | 102 (56%) | |
| Negative | 36 (46%) | 76 (42%) | |
| Not reported | 1 (1%) | 5 (3%) | |
| Disease status at transplantation | 0.64 | ||
| 1st Complete remission | 32 (41%) | 69 (38%) | |
| 2nd Complete remission | 25 (32%) | 69 (38%) | |
| Relapse | 22 (28%) | 45 (25%) | |
| Unit total nucleated cell dose | 0.07 | ||
| <3 × 107/kg | 7 (9%) | 35 (19%) | |
| ≥3 × 107/kg | 72 (91%) | 146 (80%) | |
| Unknown | 2 (1%) | ||
| Donor-recipient HLA-match A/B low resolution, DRB1 |
0.20 | ||
| 6/6 HLA-match | 2 (3%) | 3 (2%) | |
| 5/6 HLA-match | 20 (25%) | 40 (22%) | |
| 4/6 HLA-match | 55 (70%) | 134 (73%) | |
| 3/6 HLA-match | 2 (3%) | 6 (3%) | |
| Donor-recipient allele-level HLA-match A/B/C/DRB1* |
0.71 | ||
| 5/8 HLA-match | 45 (57%) | 96 (52%) | |
| 4/8 HLA-match | 26 (33%) | 70 (38%) | |
| 3/8 HLA-match | 8 (10%) | 17 (10%) | |
| Conditioning regimen | 0.21 | ||
| TBI containing regimens | 39 (49%) | 75 (41%) | |
| Non-TBI regimens | 40 (51%) | 108 (59%) | |
| In vivo T-cell depletion | 0.04 | ||
| Yes | 51 (65%) | 145 (79%) | |
| No | 25 (32%) | 35 (19%) | |
| Not reported | 3 (4%) | 3 (2%) | |
| Transplant period | 0.44 | ||
| 2000 – 2004 | 18 (23%) | 50 (27%) | |
| 2005 – 2010 | 61 (77%) | 133 (73%) | |
| Follow-up, surviving patients | |||
| Median (range), months | 38 (6 – 96) | 41 (3 – 124) |
*KIR ligand matched HCT: N=3 single mismatch at HLA-C locus. Additional mismatches at HLA-C locus occurred with mismatches at HLA-A (N=9), HLA-B (N=14) and HLA-DRB1 (N=3)
*KIR ligand mismatched HCT: N=4 single mismatch at HLA-C locus and N=1 double mismatch at HLA-C loci. Additional mismatches at HLA-C locus occurred with mismatches at HLA-A (N=11), HLA-B (N=26) and HLA-DRB1 (N=11)
Results
Patient, disease and transplant characteristics
The characteristics of patients who received 6–7/8 HLA-matched transplants are shown in Table 1. Fifty-seven percent of transplantations were KIR ligand matched and 43%, mismatched. There were no differences in patient, disease and transplant characteristics by KIR ligand matching status. The characteristics of patients with AML and ALL who received 3–5/8 HLA-matched transplants are shown in Table 2. Consistent with the confounding effect of HLA disparity and KIR-ligand match status, 30% of transplantations were KIR-ligand matched and 70%, mismatched. There were no differences in patient, disease and transplant characteristics by KIR ligand match status, except that in vivo T cell depletion with anti-thymocyte globulin (ATG) was slightly more common for KIR ligand mismatched transplants.
Table 1.
Characteristics of patients with AML who received 6–7/8 HLA-matched transplants
| KIR Ligand Matched |
KIR Ligand Mismatched |
P-value | |
|---|---|---|---|
| Total number | 114 | 85 | |
| Gender | 0.68 | ||
| Male | 53 (46%) | 37 (44%) | |
| Female | 61 (54%) | 48 (56%) | |
| Age, years | 0.87 | ||
| ≤ 16 | 82 (72%) | 62 (73%) | |
| > 16 | 32 (28%) | 23 (27%) | |
| Cytomegalovirus serostatus | 0.38 | ||
| Positive | 66 (58%) | 44 (52%) | |
| Negative | 41 (36%) | 38 (45%) | |
| Not reported | 7 (6%) | 3 (4%) | |
| Disease status at transplantation | 0.41 | ||
| 1st Complete remission | 39 (34%) | 37 (44%) | |
| 2nd Complete remission | 36 (32%) | 23 (27%) | |
| Relapse | 39 (34%) | 25 (29%) | |
| Unit total nucleated cell dose | 0.79 | ||
| <3 × 107/kg | 17 (15%) | 10 (12%) | |
| ≥3 × 107/kg | 95 (83%) | 73 (86%) | |
| Unknown | 2 (2%) | 2 (2%) | |
| Donor-recipient HLA-match A/B low resolution, DRB1 |
0.22 | ||
| 6/6 HLA-match | 22 (19%) | 12 (14%) | |
| 5/6 HLA-match | 81 (71%) | 69 (81%) | |
| 4/6 HLA-match | 11 (10%) | 4 (5%) | |
| Donor-recipient allele-level HLA-match A/B/C/DRB1* |
<0.001 | ||
| 7/8 HLA-match | 65 (57%) | 15 (18%) | |
| 6/8 HLA-match | 49 (43%) | 70 (82%) | |
| In vivo T-cell depletion | 0.66 | ||
| Yes | 78 (68%) | 62 (73%) | |
| No | 33 (29%) | 22 (26%) | |
| Not reported | 3 (3%) | 1 (1%) | |
| Conditioning regimen | 0.70 | ||
| TBI containing regimens | 46 (40%) | 32 (38%) | |
| Non-TBI regimens | 68 (60%) | 53 (62%) | |
| Transplant period | 0.44 | ||
| 2000 – 2004 | 28 (25%) | 25 (29%) | |
| 2005 – 2010 | 86 (75%) | 60 (71%) | |
| Follow-up, surviving patients | |||
| Median (range), months | 40 (9 – 120) | 48 (13 – 100) |
*KIR ligand matched HCT: N=6 single mismatch at HLA-C locus and N=1 double mismatch at HLA-C loci. Additional mismatches at HLA-C locus occurred with mismatches at HLA-A (N=6), HLA-B (N=11) and HLA-DRB1 (N=6)
*KIR ligand mismatched HCT: N=2 single mismatch at HLA-C locus and N=1 double mismatch at HLA-C loci. Additional mismatches at HLA-C locus occurred with mismatches at HLA-A (N=7), HLA-B (N=13) and HLA-DRB1 (N=5)
Transplantation Outcomes
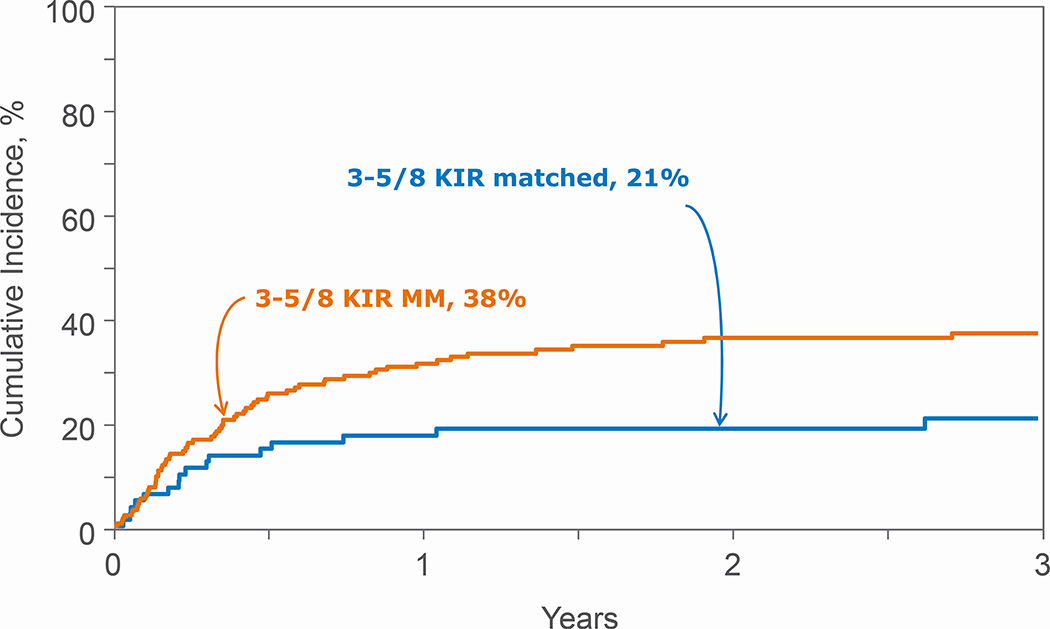
KIR ligand match status was not associated with treatment failure, overall mortality, NRM, relapse or acute and chronic GVHD after 6–7/8 HLA-matched transplants (Table 3). A similar trend was observed when examining for the effects of KIR ligand mismatched transplants in the GVH and HVG directions compared to KIR ligand matched transplants (data not shown). However, for recipients of 3–5/8 HLA-matched transplants, KIR ligand mismatching was associated with worse overall mortality and NRM but not relapse, treatment failure or acute and chronic GVHD (Table 4, Figures 1 and 2). The adverse effect of KIR ligand mismatching occurred with KIR ligand mismatching in the HVG direction; overall mortality (HR 1.78, 95% CI 1.16 – 2.74, p=0.008) and NRM (HR 2.26, 95% CI 1.23 – 4.16, p=0.008) compared to KIR ligand matched transplants. There were no significant differences in overall mortality and NRM with KIR ligand mismatched (GVH direction) compared to KIR ligand matched transplants, HR 1.33, 95% CI 0.87 – 2.02, p=0.18 and HR 1.72, 95% CI 0.95 – 3.12, p=0.07, respectively. Acute and chronic GVHD risks were not mediated by KIR ligand match status (Table 3, 4).
Table 3.
Multivariate Analysis: 6/8 and 7/8 HLA-matched transplants
| Variables | Events/Evaluable | Hazard Ratio (95% confidence interval) |
p-value |
|---|---|---|---|
| Overall Mortality* | |||
| KIR ligand matched | 66/114 | 1.00 | |
| KIR ligand mismatched | 51/85 | 0.95 (0.66 – 1.37) | 0.79 |
| Treatment failure** | |||
| KIR ligand matched | 71/113 | 1.00 | |
| KIR ligand mismatched | 52/85 | 0.87 (0.61 – 1.25) | 0.45 |
| Relapse*** | |||
| KIR ligand matched | 45/113 | 1.00 | |
| KIR ligand mismatched | 37/85 | 0.96 (0.61 – 1.51) | 0.87 |
| NRM | |||
| KIR ligand matched | 26/113 | 1.00 | |
| KIR ligand mismatched | 15/85 | 0.69 (0.36 – 1.30) | 0.25 |
| Grade 2–4 acute GVHD# | |||
| KIR ligand matched | 40/114 | 1.00 | |
| KIR ligand mismatched | 30/85 | 0.99 (0.61 – 1.60) | 0.97 |
| Chronic GVHD## | |||
| KIR ligand matched | 15/114 | 1.00 | |
| KIR ligand mismatched | 21/85 | 1.66 (0.85 – 3.24) | 0.14 |
Model adjusted for disease status (CR2 vs. CR1 HR 1.91, 95% CI 1.15 – 3.19, p=0.01; relapse vs. CR1 HR 4.82, 95% CI 3.01 – 7.71, p<0.0001)
Model adjusted for disease status (CR2 vs. CR1 HR 1.91, 95% CI 1.15 – 3.19, p=0.01; relapse vs. CR1 HR 4.82, 95% CI 3.01 – 7.71, p<0.0001)
Model adjusted for disease status (CR2 vs. CR1 HR 2.80, 95% CI 1.40 – 5.58, p=0.004; relapse vs. CR1 HR 10.04, 95% CI 5.31 – 19.00, p<0.0001); sex (male vs. female HR 1.68, 95% CI 1.08 – 2.63, p=0.02)
Model adjusted for in vivo T-cell depletion vs. none (HR 1.79, 95% CI 1.07 – 3.01, p=0.03); patient age (>16 vs. ≤16 years HR 0.50, 95% CI 0.27 – 0.91, p=0.02)
Model adjusted for in vivo T-cell depletion vs. none (HR 2.35, 95% CI 1.20 – 4.58, p=0.01)
Table 4.
Multivariate Analysis: 3/8, 4/8 and 5/8 HLA-matched transplants
| Variables | Events/Evaluable | Hazard Ratio (95% confidence interval) |
p-value |
|---|---|---|---|
| Overall Mortality* | |||
| KIR ligand matched | 35/79 | 1.00 | |
| KIR ligand mismatched | 109/183 | 1.51 (1.03 – 2.21) | 0.035 |
| Treatment failure** | |||
| KIR ligand matched | 40/79 | 1.00 | |
| KIR ligand mismatched | 112/183 | 1.30 (0.91 – 1.88) | 0.15 |
| Relapse*** | |||
| KIR ligand matched | 24/79 | 1.00 | |
| KIR ligand mismatched | 47/183 | 0.86 (0.53 – 1.42) | 0.57 |
| NRM**** | |||
| KIR ligand matched | 16/79 | 1.00 | |
| KIR ligand mismatched | 65/183 | 1.94 (1.12 – 3.36) | 0.019 |
| Grade 2–4 acute GVHD# | |||
| KIR ligand matched | 33/79 | 1.00 | |
| KIR ligand mismatched | 50/183 | 0.71 (0.45 – 1.12) | 0.14 |
| Chronic GVHD | |||
| KIR ligand matched | 20/79 | 1.00 | |
| KIR ligand mismatched | 43/183 | 1.27 (0.73 – 2.19) | 0.39 |
Model adjusted for disease status (CR2 vs. CR1 HR 1.01, 95% CI 0.65 – 1.55, p=0.97; relapse vs. CR1 HR 3.05, 95% CI 2.05 – 4.54, p<0.0001)
Model adjusted for disease status (CR2 vs. CR1 HR 1.03, 95% CI 0.69 – 1.55, p=0.87; relapse vs. CR1 HR 2.92, 95% CI 1.99 – 4.30, p<0.0001)
Model adjusted for disease status (CR2 vs. CR1 HR 1.20, 95% CI 0.66 – 2.19, p=0.55; relapse vs. CR1 HR 3.39, 95% CI 1.90 – 6.04, p<0.0001)
Model adjusted for disease status (CR2 vs. CR1 HR 1.00, 95% CI 0.57 – 1.76, p=0.99; relapse vs. CR1 HR 2.51, 95% CI 1.49 – 4.23, p=0.0005)
Model adjusted for patient age (>16 vs. ≤16 years HR 0.46, 95% CI 0.29 – 0.75, p=0.002)
Figure 2.
Non-relapse mortality after 3–5/8 HLA mismatched transplantations by KIR ligand match status
Discussion
This retrospective registry-based study has analyzed a large series of patients with AML transplanted with a single cord blood unit who received a myeloablative-conditioning regimen and identified several factors that are relevant when selecting CB units. First, KIR-ligand match status was confounded with HLA disparity in that KIR-ligand mismatching was more common with HLA disparity. Therefore, analyses were conducted separately for 6–7/8 and 3–5/8 HLA-matched transplants so we could distinguish the effects of HLA disparity from KIR-ligand match status. Second, KIRligand match status was not associated with outcomes after 6–7/8 HLA-matched transplants. Although donor-recipient HLA-mismatching at 3 or more loci are not generally recommended, KIR-ligand mismatching was associated with higher overall and NRM. The adverse effect of KIR-ligand mismatching was shown to occur with mismatching in the HVG direction and not the GVH direction or bi-directional. Further, the adverse effect on survival AML relapse was not mediated by relapse or GVHD. These findings do not support selecting cord blood units based on KIR-ligand match status when HLA disparity is limited to 1 or 2-loci considering allele-level HLA matching. When contemplating HLA-mismatched transplants at 3 or more loci, avoiding KIR ligand CB units mismatched in HVG direction lowers mortality risks. The results of the current analyses contradict some of the findings of an earlier Eurocord study in which KIR ligand mismatch was associated with better leukemia-free and overall survival for ALL and AML, and the effects were more pronounced for AML.(17) However, there are substantial differences between the current analyses and that report. The current analyses considers HLA-matching at the allele-level at HLA-A, -B, -C and –DRB1 and the confounding between HLA disparity and KIR ligand match status required the current analyses be conducted separately for transplants mismatched at 1- 2 HLA-loci and ≥3 HLA-loci. Further, in the current analyses KIR ligand match status did not consider Bw4 epitopes at HLA-A3 and A11. Our findings also differ from a recent report from the Japanese registry that did not find an association between KIR ligand matching and leukemia-free or overall survival in 643 recipients with acute leukemia transplanted with single cord blood units.(18) As with the Eurocord report, the Japanese registry also considered lower resolution HLA-matching between cord blood units and their recipients. Conflicting results have also been reported in the setting of double UCBT. (19) Brunstein and colleagues found that KIR ligand incompatibility was associated with higher rates of acute GVHD and lower survival after reduced-intensity conditioning regimen. However, this effect was not seen in a similar study with 80 patients with various hematological malignancies, including 31 patients with AML that received double UCBT.(20)
Interestingly, in the setting of allogeneic HSCT using other stem cell sources, the results of KIR ligand status are also somewhat contradictory among different studies. (4, 5, 10, 11, 16, 28–30) In order to reconcile these discrepant results, it has to be underlined that, from a biological point of view, patients with KIR ligand incompatibility are, by definition, at risk for donor T-cell alloreactivity in unmanipulated transplantation; thus, in patients given a minimally T-cell-depleted transplant, T-cell alloreactivity often dominates and outweighs the effect of NK cells. This observation emphasizes the concept that proper studies have to be conducted and analyzed to dissect and unveil the role played by the different components of the immune system in terms of protection against malignant recurrence. Methods that directly detect the donor KIR repertoire at the DNA, RNA, and surface protein expression levels are a more accurate measure of NK cell alloreactivity than that obtained using solely HLA-based KIR ligand defining methods.(15, 31) DNA-based methods for KIR analysis have the obvious advantage that a single DNA sample used for HLA genotyping can also be used for KIR typing. Another DNA-based approach is the KIR haplotype model, founded on the concept that the greater the number of activating KIRs the donor has, the larger the effect of NK-cell alloreactivity. (7, 32–34). Further support to the role of activating KIR genes in HSCT, was reported by recent publications that have shown positive associations of individual activating KIR genes, namely 2DS1 with reduced relapse and 3DS1 with reduced acute GvHD, and improved survival. (35, 36) Yet others have shown that the number of activating KIR genes present in the donor graft or patients KIR genotype determined using the KIR haplotype model or assessing specific aKIRs, led to poor outcomes.(28, 29, 37, 38)
Thus the effects of KIR ligand match status in the setting of UCBT remains controversial and further studies using other models of NK alloreactivity including genotypes of donor and recipients are needed to elucidate the impact of KIR on outcomes after UCBT. Nevertheless, the current analyses suggest that KIR ligand matching should not be considered in the setting of 6–7/8 HLA-matched transplants for AML. Generally 3–5/8 HLA-matched transplants are not recommended but in the event of such a transplant, avoiding KIR ligand mismatch in the HVG direction may improve survival.
Highlights.
KIR ligand match assigned using HLA-C1, -C2 and –Bw4
KIR ligand matching is not associated with outcomes after 1 or 2 HLA-loci mismatched transplants
Higher mortality with KIR ligand mismatch in HvG direction with ≥ 3 HLA-loci mismatched transplants
Acknowledgments
This work was funded in part by grants from the National Institute of Health (Public Health Service grant U24-CA76518); a Scholar in Clinical Research Award, Leukemia and Lymphoma Society (M Eapen); Office of Naval Research, Department of Navy to the National Marrow Donor Program (N00014-11-01-0339). V Rocha and R Danby are funded of National Institute Health Research (NIHR)-Biomedical Research Centre (BRC) funding scheme and NHS-BT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship: VR, AR, SS, EG and ME designed the study, SS classified the KIR groups, TW performed the statistical analysis, VR and ME wrote the paper, RS, MA, DP, RD EG, FL, GM, WA, API, GFS provided cases and or edited and approved the manuscript.
Disclosures: The authors have no conflict of interest to disclose.
References
- 1.Locatelli F, Moretta F, Brescia L, Merli P. Natural killer cells in the treatment of high-risk acute leukaemia. Seminars in immunology. 2014;26(2):173–179. doi: 10.1016/j.smim.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. NK cell recognition. Annual review of immunology. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 4.Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102(3):814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 5.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105(12):4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, et al. Donors with group KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishara A, De Santis D, Witt CC, Brautbar C, Christiansen FT, Or R, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue antigens. 2004;63(3):204–211. doi: 10.1111/j.0001-2815.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 9.Cook MA, Milligan DW, Fegan CD, Darbyshire PJ, Mahendra P, Craddock CF, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103(4):1521–1526. doi: 10.1182/blood-2003-02-0438. [DOI] [PubMed] [Google Scholar]
- 10.Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12(8):876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Huang XJ, Zhao XY, Liu DH, Liu KY, Xu LP. Deleterious effects of KIR ligand incompatibility on clinical outcomes in haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion. Leukemia. 2007;21(4):848–851. doi: 10.1038/sj.leu.2404566. [DOI] [PubMed] [Google Scholar]
- 12.Gagne K, Brizard G, Gueglio B, Milpied N, Herry P, Bonneville F, et al. Relevance of KIR gene polymorphisms in bone marrow transplantation outcome. Human immunology. 2002;63(4):271–280. doi: 10.1016/s0198-8859(02)00373-7. [DOI] [PubMed] [Google Scholar]
- 13.Bornhauser M, Schwerdtfeger R, Martin H, Frank KH, Theuser C, Ehninger G. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood. 2004;103(7):2860–2861. doi: 10.1182/blood-2003-11-3893. author reply 2. [DOI] [PubMed] [Google Scholar]
- 14.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, et al. Determinants of antileukemia effects of allogeneic NK cells. Journal of immunology. 2004;172(1):644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 16.McQueen KL, Dorighi KM, Guethlein LA, Wong R, Sanjanwala B, Parham P. Donor-recipient combinations of group A and B KIR haplotypes and HLA class I ligand affect the outcome of HLA-matched, sibling donor hematopoietic cell transplantation. Human immunology. 2007;68(5):309–323. doi: 10.1016/j.humimm.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socie G, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23(3):492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka J, Morishima Y, Takahashi Y, Yabe T, Oba K, Takahashi S, et al. Effects of KIR ligand incompatibility on clinical outcomes of umbilical cord blood transplantation without ATG for acute leukemia in complete remission. Blood cancer journal. 2013;3:e164. doi: 10.1038/bcj.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunstein CG, Wagner JE, Weisdorf DJ, Cooley S, Noreen H, Barker JN, et al. Negative effect KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009;113(22):5628–5634. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garfall A, Kim HT, Sun L, Ho VT, Armand P, Koreth J, et al. KIR ligand incompatibility is not associated with relapse reduction after double umbilical cord blood transplantation. Bone marrow transplantation. 2013;48(7):1000–1002. doi: 10.1038/bmt.2012.272. [DOI] [PubMed] [Google Scholar]
- 21.Eapen M, Klein JP, Ruggeri A, Spellman S, Lee SJ, Anasetti C, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eapen M, Klein JP, Sanz GF, Spellman S, Ruggeri A, Anasetti C, et al. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. The Lancet Oncology. 2011;12(13):1214–1221. doi: 10.1016/S1470-2045(11)70260-1. Epub 2011/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15(6):825–828. Epub 1995/06/01. [PubMed] [Google Scholar]
- 24.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematology/oncology clinics of North America. 1999;13(5):1091–1112. viii–ix. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 25.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Klein JP, Moeschberger ML. Survival Analysis: Statistical Methods for Censored and Truncated Data. 2nd. New York: Springer-Verlag; 2003. [Google Scholar]
- 27.Cox DR. Regression model and life tables. J R Stat Soc B. 1972;32(2):187–200. [Google Scholar]
- 28.Giebel S, Nowak I, Wojnar J, Markiewicz M, Dziaczkowska J, Wylezol I, et al. Impact of activating killer immunoglobulin-like receptor genotype on outcome of unrelated donor hematopoietic cell transplantation. Transplantation proceedings. 2006;38(1):287–291. doi: 10.1016/j.transproceed.2005.11.091. [DOI] [PubMed] [Google Scholar]
- 29.Kroger N, Binder T, Zabelina T, Wolschke C, Schieder H, Renges H, et al. Low number of donor activating killer immunoglobulin-like receptors (KIR) genes but not KIR-ligand mismatch prevents relapse and improves disease-free survival in leukemia patients after in vivo T-cell depleted unrelated stem cell transplantation. Transplantation. 2006;82(8):1024–1030. doi: 10.1097/01.tp.0000235859.24513.43. [DOI] [PubMed] [Google Scholar]
- 30.Sobecks RM, Wang T, Askar M, Gallagher MM, Haagenson M, Spellman S, et al. Impact of KIR and HLA Genotypes on Outcomes after Reduced-Intensity Conditioning Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(9):1589–1596. doi: 10.1016/j.bbmt.2015.05.002. Epub 2015/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung W, Iyengar R, Triplett B, Turner V, Behm FG, Holladay MS, et al. Comparison of killer like receptor genotyping and phenotyping for selection of allogeneic blood stem cell donors. Journal of immunology. 2005;174(10):6540–6545. doi: 10.4049/jimmunol.174.10.6540. [DOI] [PubMed] [Google Scholar]
- 32.Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(4):533–542. doi: 10.1016/j.bbmt.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroger N, Zabelina T, Berger J, Duske H, Klyuchnikov E, Binder T, et al. Donor KIR haplotype B improves progression-free and overall survival after allogeneic hematopoietic stem cell transplantation for multiple myeloma. Leukemia. 2011;25(10):1657–1661. doi: 10.1038/leu.2011.138. [DOI] [PubMed] [Google Scholar]
- 34.Chen C, Busson M, Rocha V, Appert ML, Lepage V, Dulphy N, et al. Activating KIR genes are associated with CMV reactivation and survival after non-T-cell depleted HLA-identical sibling bone marrow transplantation for malignant disorders. Bone marrow transplantation. 2006;38(6):437–444. doi: 10.1038/sj.bmt.1705468. [DOI] [PubMed] [Google Scholar]
- 35.Venstrom JM, Gooley TA, Spellman S, Pring J, Malkki M, Dupont B, et al. Donor activating KIR3DS1 is associated with decreased acute GVHD in unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2010;115(15):3162–3165. doi: 10.1182/blood-2009-08-236943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. The New England journal of medicine. 2012;367(9):805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabriel IH, Sergeant R, Szydlo R, Apperley JF, DeLavallade H, Alsuliman A, et al. Interaction between KIR3DS1 and HLA-Bw4 predicts for progression-free survival after autologous stem cell transplantation in patients with multiple myeloma. Blood. 2010;116(12):2033–2039. doi: 10.1182/blood-2010-03-273706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marin D, Gabriel IH, Ahmad S, Foroni L, de Lavallade H, Clark R, et al. KIR2DS1 genotype predicts for complete cytogenetic response and survival in newly diagnosed chronic myeloid leukemia patients treated with imatinib. Leukemia. 2012;26(2):296–302. doi: 10.1038/leu.2011.180. [DOI] [PubMed] [Google Scholar]