Abstract
The anti-fibrotic, vasodilatory, and pro-angiogenic therapeutic properties of recombinant relaxin peptide hormone have been investigated in several diseases and recent clinical trial data has shown benefit in treating acute heart failure. However, the remodeling capacity of these peptide hormones is difficult to study in chronic settings due to their short half-life and the need for intravenous administration. Here we present the first small-molecule series of human relaxin receptor 1 (RXFP1) agonists. These molecules display similar efficacy as the natural hormone in several functional assays. Mutagenesis studies indicate that the small molecules activate relaxin receptor through an allosteric site. These compounds have excellent physical and in vivo pharmacokinetic properties to support further investigation of relaxin biology and animal efficacy studies of the therapeutic benefits of RXFP1 activation.
Introduction
Despite great advances in medical science, 1 of every 2.9 deaths in the United States is due to cardiovascular disease1. Each year about 795,000 people experience a new or recurrent stroke, and 1 in 9 death certificates in the United States mention heart failure. In addition, 33.5% of US adults over 20 years of age have hypertension1. These statistics clearly illustrate both the grave need for more effective treatments and the limitations of current therapies to address cardiovascular disease in general and acute heart failure in particular.
The peptide hormone relaxin was discovered in 1926 as a hormone of pregnancy2, due to its relaxation effects on pubic ligaments (hence the name) and softening the cervix to facilitate parturition3. It has been shown that concentration of relaxin in blood rises during the first trimester of pregnancy, promoting cardiovascular and renal adjustments to meet the increased nutritional demands of the growing fetus, and the elevated requirements for renal clearance of metabolic wastes4. Relaxin induces a 20% increase in cardiac output, 30% decrease in systemic vascular resistance, 30% increase in global arterial compliance, and 45% increase in renal blood flow during pregnancy5. Numerous clinical and nonclinical studies using this hormone have now recapitulated these cardiovascular effects in both males and females, demonstrating its potential pharmacological utility in modulating cardiovascular and renal function.
The clinically observed physiological effects of relaxin are mediated though its interaction with the G protein-coupled receptor, relaxin/insulin-like family peptide receptor 1 (RXFP1), leading to the modulation of several signal transduction pathways6. Activation of RXFP1 by relaxin induces: 1) up-regulation of the endothelin system which leads to vasodilation; 2) extracellular matrix remodeling through regulation of collagen deposition, matrix metalloproteinase and tissue inhibitor of metalloproteinase expression, and overall tissue homeostasis; 3) a moderation of inflammation by reducing levels of inflammatory cytokines, such as tumor necrosis factor-alpha and transforming growth factor beta; and 4) angiogenesis by activating transcription of vascular endothelial growth factor (VEGF)6–8. The understanding of the biological effects of RXFP1 activation by relaxin has led to the evaluation of relaxin as a pharmacologic agent for the treatment of patients with acute heart failure9,10, preeclampsia11, and hypertensive diseases12,13. Given its anti-inflammatory and extracellular matrix remodeling function, several clinical trials have also evaluated the potential of relaxin as treatment for scleroderma, cervical ripening, fibromyalgia, and orthodontics8.
A similar anti-inflammatory and remodeling need also exists for cardiac rehabilitation. None of the current methodologies, such as surgery, medical devices or approved medications (angiotensin-converting-enzyme inhibitors, angiotensin II receptor blockers, digoxin, beta blockers and aldosterone antagonists), are able to prevent the development of heart tissue scar after acute heart failure, or repair heart tissue after damage is incurred. The anti-fibrotic and remodeling properties of relaxin9,14, together with its capacity to normalize blood pressure, increase blood and renal flow15 seem to be ideal for the treatment of patients with cardiovascular diseases. Clinical trial data support this theory9,16. Relaxin relieves systemic and renal vasoconstriction and increases vascular compliance, including normalization of high blood pressure, reduction of pulmonary capillary wedge pressure, increase of cardiac output, increase renal blood flow, natriuresis, and decongestion17. Recent data from the RELAX-AHF phase III clinical trial showed that the early administration of serelaxin, a recombinant analog of the natural hormone, reduced overall mortality at six months and promoted a more rapid relief of congestion with fewer signs of organ damage during the first days after admission9,10. In addition, animal pharmacology data indicate that relaxin hormone has anti-inflammatory and cardiac protection effects, including reduction of myocardial ischemia and reperfusion injury, increase of wound healing, reduction of ventricular fibrosis14, and increase of endothelial progenitor cell mobilization18.
Recombinant relaxin hormone has produced promising responses in clinical trials for the treatment of heart failure and is close to commercialization9. However, the peptide is difficult to administer as a chronic therapy. The half-life of this peptide hormone is less than 10 minutes and it has to be administrated intravenously13. The development of small-molecule agonists of RXFP1 as an alternative to recombinant hormone would have numerous benefits and will enable the investigation of additional therapeutic applications that may require chronic administration. In this article, we report the first series of small-molecule agonists of RXFP1. These small molecules are potent, highly selective, orally bioavailable and easy to synthesize.
Results
Identification of hits as small-molecule agonists of RXFP1
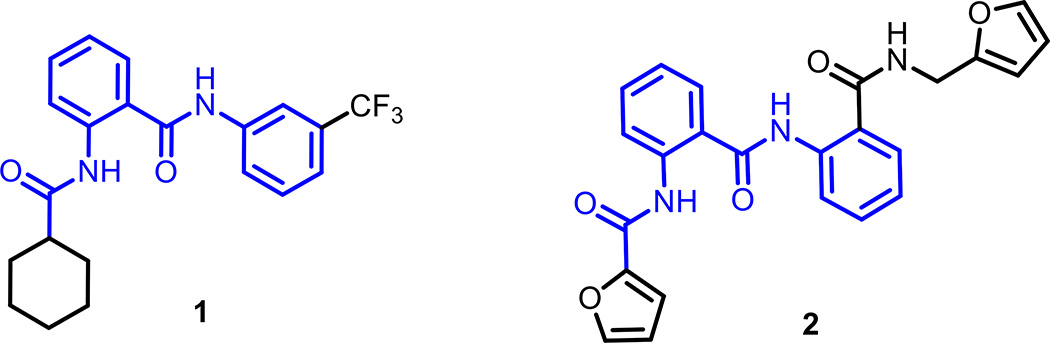
More than 350,000 compounds of the Molecular Libraries Probe Center Network library19 were tested in a quantitative high-throughput screening (qHTS)20 campaign searching for small-molecule agonists of RXFP1, by measuring the ability of compounds to elevate cyclic adenosine monophosphate (cAMP) levels in a HEK293 cell line stably transfected with human RXFP121. Maximal cAMP signal was established by treatment with porcine relaxin (1.66 nM) or the adenylyl cyclase activator forskolin (57 µM). Confirmed active compounds were further interrogated using the naive parental HEK293 cell line and cells transfected with the related receptors insulin-like 3 peptide receptor, RXFP2, or arginine vasopressin receptor 1B, AVPR1B, using the same cAMP detection kit as screens for selectivity and to eliminate compounds which non-specifically increase the cAMP signal through an RXFP1-independent mechanism. Two molecules 1 and 2 with modest activity in the primary screen were validated using these counter-screen assays21 and then resynthesized in-house. Both confirmed hits displayed similarities in their chemical scaffold containing a 2-acetamido-N-phenylbenzamide core in their structures (Fig. 1, in blue).
Figure 1. Chemical structures of hit molecules 1 and 2 identified in a RXFP1 cAMP primary screening assay.
Both molecules share a common chemical scaffold containing a core substructure of 2-acetamido-N-phenylbenzamide (in blue).
Hit-to-lead medicinal chemistry optimization
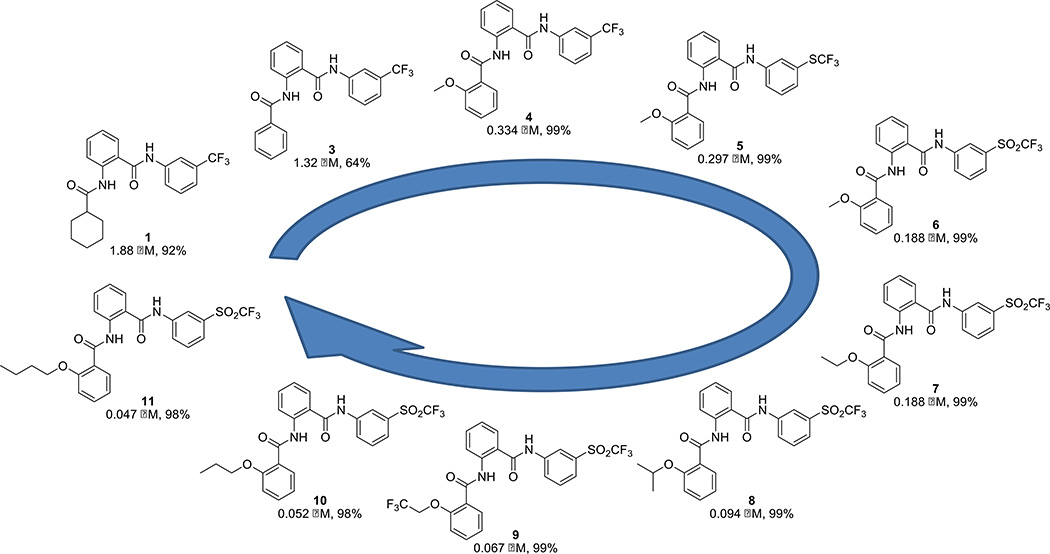
The initial screening was followed up by an extensive structure activity relationship campaign to improve the potency, efficacy and physical properties of the compounds (Fig. 2, Supplementary Table S1). Compound activity is reported through two measurements: EC50 (concentration necessary to reach 50% of the maximum cAMP signal produced by the molecule) and maximum response (efficacy, corresponding with the level of cAMP elevation normalized to relaxin control). Both EC50 and maximum response were evaluated and optimized through these efforts. Our studies on the aniline ring indicated the importance of a hydrophobic functional group having the proper field effect22 at the meta-position of this aromatic ring, with a trifluoromethylsulfonyl group providing the best activity. Meta-substituents with incremental field-effect values at this position (SO2CF3, F = 0.73; SCF3, F = 0.35; and CF3, F = 0.38)22 provide increasingly potent activity. We also investigated the impact of modifying the cyclohexane ring moiety and found that aromatic replacements, especially those with an alkoxy aliphatic chain in ortho-position, increased compound potency. Finally, most substituents and replacement modifications of the middle phenyl ring core and amide functional groups produced analogs with the same or lower activity than the corresponding parent compound.
Figure 2. Structure activity relationship optimization campaign.
Ten representative compounds, 1 and 3–11, highlight the key steps in the hit-to-lead evolution. The EC50 and relative activity for each compound are shown using the RXFP1 primary screening assay. 100% relative activity was normalized to 57.7 µM forskolin stimulation, and 0% relative activity was normalized to compound vehicle control (0.58% DMSO). Complete concentration-response data are also provided (Supplementary Fig. 1).
Profiling of representative lead compounds
Table 1 displays the profile of compounds 5–11 with an EC50 below 300 nM in the RXFP1 transfected cAMP assay, and also includes their activities in a human monocytic leukemia cell line (THP1) which endogenously expresses the RXFP1 receptor, their selectivity against HEK293 cells transfected with the receptors RXFP2 and AVPR1B, ATP cytotoxicity, aqueous phosphate buffered saline solubility, and mouse liver microsomal stability (Supplementary Table S2). As expected, due to the lipophilic character of our molecules, these compounds have poor water solubility, although our most potent compounds (8–11, EC50 < 100 nM) display a solubility of 3.3–17.0 µM which is 49–327 times higher than their corresponding EC50 for RXFP1 in HEK293 cells.
Table 1. Profiles of potent analogs 5–11 with EC50 less than 300 nM in the RXFP1 transfected HEK293 cAMP assay.
EC50s of compounds are given in micromolar units for the HEK293 cells stably transfected with RXFP1 (HEK-RXFP1), for THP1 cells which endogenously express RXFP1, for HEK293 cells stably transfected with RXFP2 (HEK-RXFP2), as well as the vasopressin receptor (HEK-V1b), and for cytotoxicity at 72 h using the HEK293 cells stably transfected with RXFP1 (HEK-RXFP1). Replicate compound concentration-response data is also provided (Supplementary Fig. S1). The series has good selectivity against RXFP2 and AVPR1B receptors stably transfected into HEK293 cells. Phosphate buffered saline (PBS) solubility analysis was performed by Analiza Inc. and based upon quantitative nitrogen detection as described (www.analiza.com). Mouse liver microsomal (MLM) stability analyses were performed by Pharmaron and are based upon duplicate incubations of test reagent in pooled male mouse liver microsomes (www.pharmaron.com). Analysis in the absence and presence of glutamate synthase (NADPH) was performed to assess NADPH free degradation. Half-lifes (T1/2) were calcuated only in NADPH (+) degradation (Supplementary Table S2)
| ID | HEK-RXFP1 EC50 (µM) |
THP1 EC50 (µM) |
HEK-RXFP2 EC50 (µM) |
HEK-V1b EC50 (µM) |
ATP Tox. EC50 (µM) |
PBS Solubility (µM) |
MLM Stability (T1/2 in min.) |
|---|---|---|---|---|---|---|---|
| 5 | 0.297 | 0.523 | 3.34 | inactive | 3.74 | 2.9 | N/A |
| 6 | 0.188 | 0.358 | inactive | inactive | 29.7 | 6.3 | N/A |
| 7 | 0.188 | 0.362 | 7.47 | inactive | 18.8 | < 1.1 | 1732 |
| 8 | 0.094 | 0.200 | inactive | inactive | 9.4 | 7.0 | 122 |
| 9 | 0.067 | 0.107 | inactive | inactive | 29.7 | 3.3 | 100 |
| 10 | 0.052 | 0.105 | inactive | inactive | 9.4 | 17.0 | 133 |
| 11 | 0.047 | 0.124 | inactive | inactive | 59.3 | 5.3 | 178 |
Lead compounds increase VEGF expression in THP1 cells
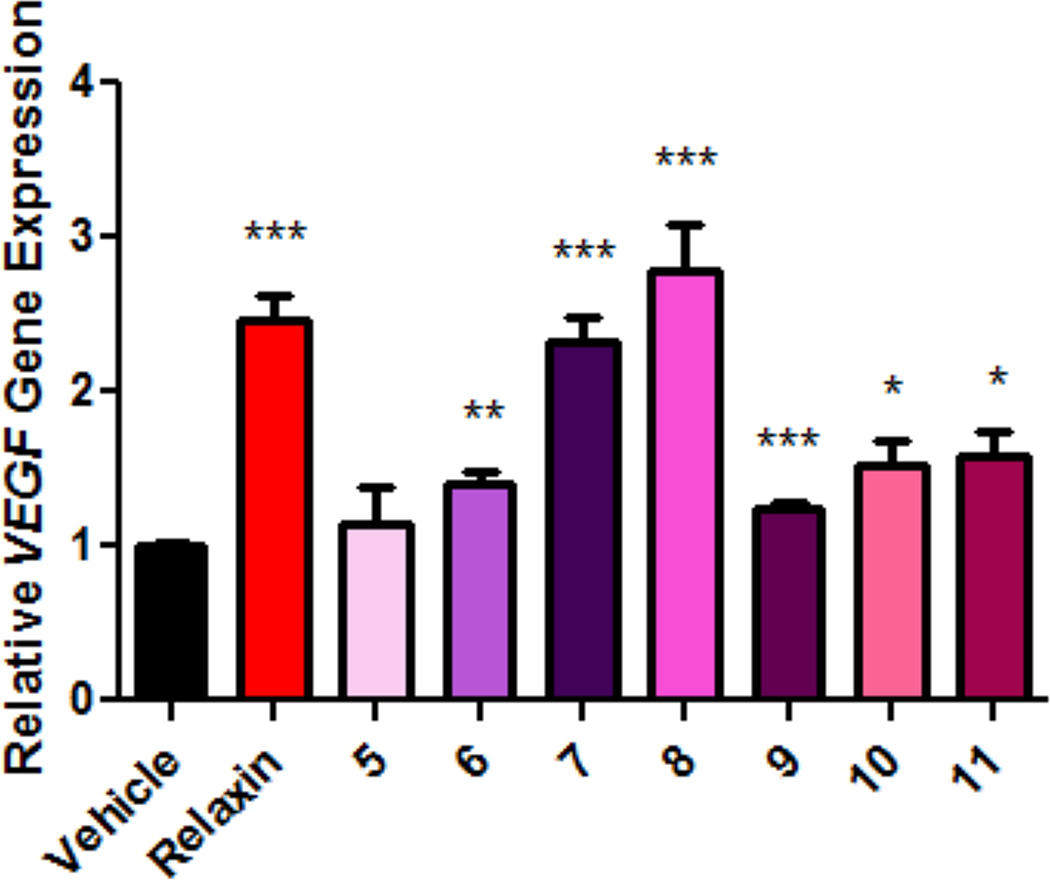
Beyond inducing elevated intracellular cAMP levels, we also evaluated the ability of the most potent compounds 5–11 to activate the transcription of the known relaxin target gene, VEGF, in THP1 cells23,24. The addition of 10 ng/mL (1.66 nM) of relaxin hormone induces a significant increase (2.4 fold) in the relative expression of VEGF mRNA as measured by quantitative real-time PCR. Similar effects were obtained in cells stimulated with compound (Fig. 3).
Figure 3. Activation of VEGF gene expression in THP1 cells.
THP1 cells were treated with compound vehicle control (0.58% DMSO), relaxin (10 ng/mL; 1.66 nM) or seven representative compounds 5–11 at 250 nM for 2 h. The level of VEGF gene expression was measured by quantitative real-time PCR and normalized to GAPDH expression and vehicle control as 1 (n=4 for each). All analogs other than compound 5 show significant (*P<0.05; **P<0.01; ***P<0.001) up-regulation of VEGF gene expression between treatment groups and vehicle control. All bars represent the mean values ± s.e.m.; statistical analysis was performed using two-tailed, Student’s t-test.
Lead compound 8 increases cell impedance in HEK293 cells
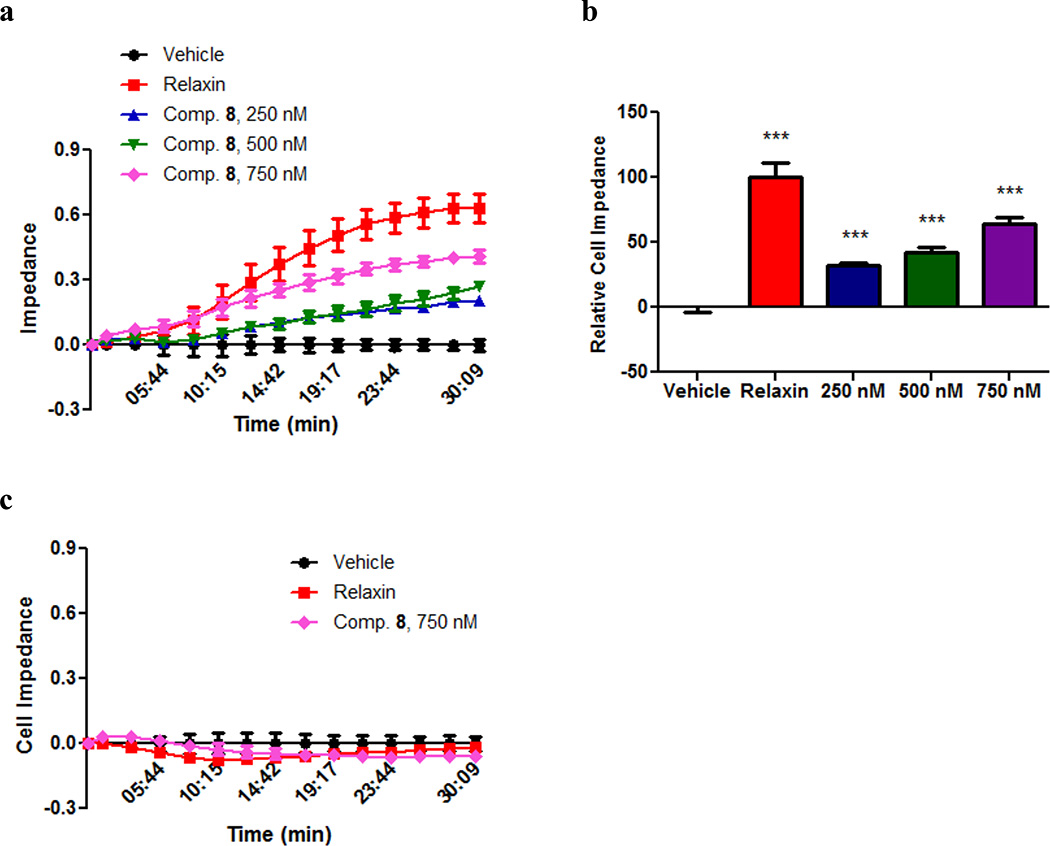
Cell-substrate impedance was measured using xCELLigence Analyzer (Roche Diagnostics), which allows for continuous time-resolved measurement of cellular index without additional labeling25. Cells are treated with compound for 30 minutes. Given this short incubation time, changes in cellular density are unlikely to contribute to the overall effect. The results indicate that compound 8 treatment generated a dose-dependent response similar to that of relaxin hormone (Fig. 4a,b). Neither treatment affects cell impedance in the corresponding parental HEK293 cells (Fig. 4c), indicating that changes in cell impedance are mediated through RXFP1.
Figure 4. Compound 8 exhibits concentration-dependent increase of cellular impedance in RXFP1 transfected HEK293 cells.
(a) Effect of relaxin (10 ng/mL; 1.66 nM), compared to compound 8 at 250 nM, 500 nM, and 750 nM on cell impedance in HEK293 cells stably transfected with RXFP1 (n=4 for each). The values at each point were normalized to the values of vehicle treatment. The subset of data points was used for the graph drawing. All points represent the mean values ± s.e.m. (b) Relative cell impedance normalized to the relaxin treatment group (100) at 30 min after addition of relaxin or compound 8. Columns represent the mean values ± s.e.m.. Differences, evaluated by two-tailed, Student’s t-test, between treatment groups and vehicle control are significant (***P<0.001). (c) Relaxin (10 ng/mL; 1.66 nM) and compound 8 at 750 nM did not affect cell impedance in parental HEK293 cells.
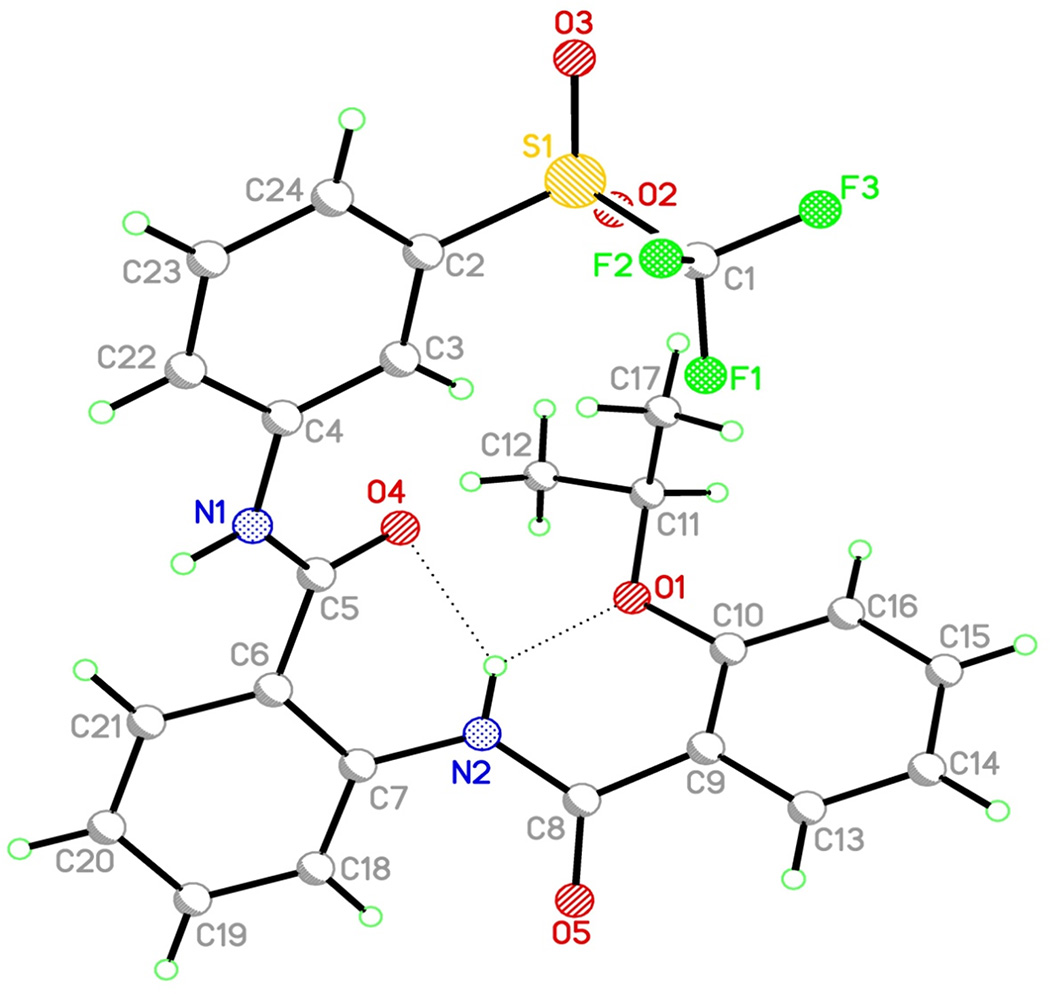
3D conformation and stability of the lead compound 8
The minimum energy conformation of compound 8 was determined in solid state by X-ray crystallography (Fig. 5, Supplementary Table S3) and in solution by variable temperature NMR and Nuclear Overhauser effect spectroscopy studies (Supplementary Fig. S2). The two intramolecular hydrogen binding interactions found in compound 8 rigidify its 3D conformation, possibly contributing to its remarkable stability. Moreover, stability studies using mouse and human plasma demonstrated that compound 8 has excellent plasma stability in vitro (Supplementary Table S4), with no significant decrease after two hours of exposure.
Figure 5. Three dimensional conformation of compound 8 determined by X-ray diffraction crystallography.
Two intramolecular hydrogen binding interactions (dash lines) are identified with a bond length of 2.70 Å and 2.72 Å, respectively. Full parameters are also provided (Supplementary Table S3).
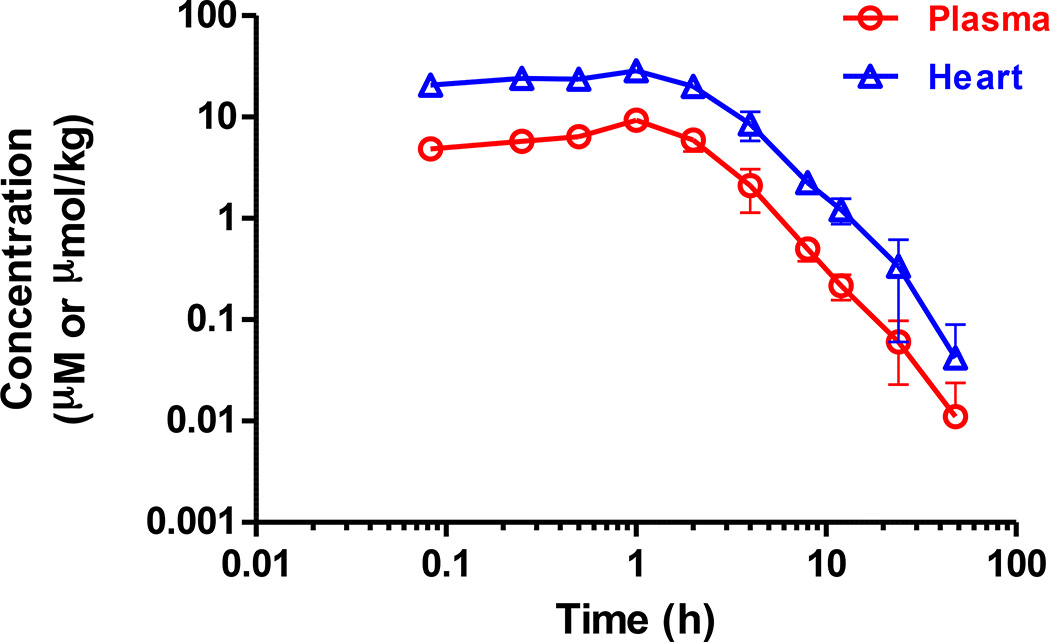
In vivo pharmacokinetic of the lead compound 8 in mice
We measured the plasma and heart concentration of compound 8 in C57BL/6 mice after a single intraperitoneal (IP) administration at a dose of 30 mg/kg (Fig. 6, Supplementary Table S5). Compound 8 has a long half-life in both plasma (T1/2 = 8.56 h) and heart (T1/2 = 7.48 h). In addition, exposure in heart was generally three to four times higher than plasma levels. Furthermore, no abnormal clinical behaviors or acute toxicity were observed in animals throughout the study.
Figure 6. In vivo pharmacokinetic profile of compound 8.
Mean plasma and heart concentration-time profiles of compound 8 ± s.e.m. after a single intraperitoneal (IP) dose of 30 mg/kg in male C57BL/6 mice (n = 3). No abnormal clinical observation was found during the in life phase. The IP dosing formulation solution was prepared in 10% NMP + 10% Solutol HS15 + 10% PEG400 + 70% saline. Full pharmacokinetic data and parameters are also provided (Supplementary Table S5).
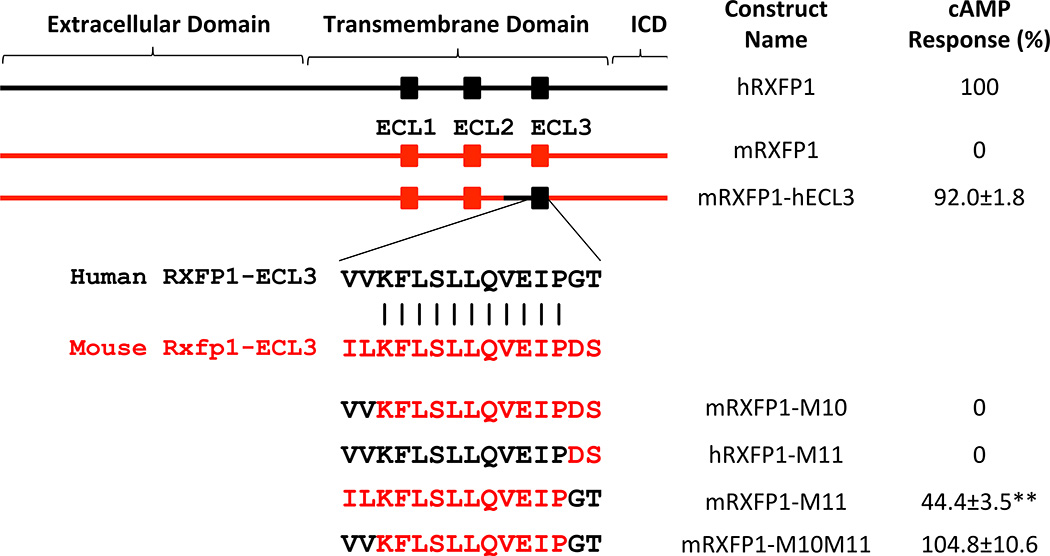
RXFP1 region responsible for activation by compound 8
The majority of preclinical studies with relaxin peptide have been performed on mouse and rat models. Therefore, we analyzed the performance of our series of agonists on HEK293 cells transfected with mouse RXFP1 (89% identity to human sequence) and found that the compounds are significantly better agonists for the human RXFP1 receptor than for the mouse clone (Fig. 7). We took advantage of this difference in species activity to map out the regions of the human receptor that determine such specificity. We tested a number of chimeric mouse/human RXFP1 constructs and found that the human receptor region of transmembrane helix 5 to extracellular loop 3 (ECL3) is required for the activation by compound 8. Importantly, all receptor constructs responded to relaxin. Further site-specific mutagenesis studies demonstrated that the substitution of mouse ECL3 sequence with the human ECL3 resulted in full activation of the mouse receptor after treatment with compound 8. Conversely, applying mouse 659GT660/659DS660 mutations to ECL3 of human receptor completely abolished compound 8 induced cAMP activation; whereas a mouse construct with the 659DS660/ 659GT660 equivalent mutation recovered partial activity (Fig. 7).
Figure 7. Identification of RXFP1 region responsible for activation by compound 8.
Human RXFP1 (for clarity denoted hRXFP1 in black) is fully activated (100%) after treatment with relaxin (15 nM) or compound 8 (66 µM). Mouse RXFP1 (denoted mRXFP1 in red) does not respond to compound 8 (marked as 0%) at 66 µM. RXFP1 contains extracellular, transmembrane, and intracellular (ICD) domains. Using chimeric mouse-human receptors the region responsible for RXFP1 activation by compound 8 was mapped to the part containing extracellular loop 3 (ECL3) of the transmembrane domain. Alignment of hRXFP1 and mRXFP1 shows two pairs of divergent amino acids within ECL3. The N-terminal IL to VV substitution in the mouse construct (mRXFP1-M10) did not rescue mouse receptor response, whereas C-terminal GT to DS substitution in human RXFP1 (hRXFP1-M11) abolished its compound 8 dependent activation. The mouse construct with (mRXFP1-M11) mutant was partially active and the mouse receptor with humanized ECL3 (mRXFP1-M10M11) was fully active after stimulation with compound 8. The cAMP response to compound 8 (66 µM) in cells transfected with a specific construct was normalized to the response of the same cells to relaxin (15 nM). The results represent the average of 3 independent experiments ± s.e.m. repeated in quadruplicates. **P<0.01 vs mRXFP1 by Student’s t-test.
Discussion
We have identified the first novel series of small molecule agonists of RXFP1 through a screening of > 350, 000 compound libraries in qHTS format with >100-fold selectivity over RXFP2 receptor (Fig. 1). Through extensive medicinal chemistry efforts, a pool of lead compounds with improved potency and selectivity were discovered (Fig. 2). Analog 3 was obtained by replacement of the cyclohexyl group in hit compound 1 with a phenyl ring. Analog 4 was obtained from 3 by introduction of an ortho-methoxy group within this benzoic substituent. Meta-substituents with increased field-defect values on the aniline ring yield compounds 4 to 6. Last, compounds 7–11 were produced from analogue 6 through the introduction of longer aliphatic chains within the ortho-alkoxy-substituent of the benzoic ring. Optimized compounds show a remarkable increase in activity from the high micromolar EC50’s of the initial hits to potencies below 50 nM (11, EC50 = 47 nM, max. response = 98%). The activity of all our analogs was further tested in cAMP response assays against RXFP2 and also counter-screened against AVPR1B. Cytotoxicity was also evaluated by measuring cellular adenosine triphosphate (ATP) levels 72 h after treatment. Most of analogs were inactive against RXFP2 and AVPR1B, and had low cytotoxicity. These analogs also show a remarkable rodent and human microsomal stability, especially important in light of the poor metabolic stability of the recombinant hormone26, with intrinsic half-lives greater than 100 minutes and at least 100-fold potency separation between RXFP1 activities and cytotoxicity in the same cells, measured by overall ATP cellular levels as an indicator of cell growth and viability (Table 1). In addition, no activity with RXFP3, another member of the relaxin family of receptors, was detected for compound 8 (personal communication, Ross Bathgate).
The cAMP EC50 of the molecules decreases only by a factor of two between RXFP1 transfected HEK293 cells and THP1 cells endogenously expressing this receptor. Differences in EC50’s among transfected and native cell lines have been previously reported for G protein-coupled receptors, and explained as function of receptor reserve which is a measurement of the receptor occupancy required to mediate the response and amplification of the signal27. Transfected cell lines, with an elevated number of receptor transcripts, produce a higher expression of the receptor on the cell surface and might require less amount of compound to elicit an equivalent functional signal. Moreover, transfection efficiency is cell type dependent. Thus, differences in EC50’s depend on the cell type and expression of the receptor in transfected cells.
Although VEGF stimulation seems to be dependent on cAMP activation23, some differences in compound activity were observed between assays measuring cAMP elevation and VEGF stimulation. The strongest effect on VEGF activation was obtained after treatment with compounds 7 and 8. In addition to cAMP elevation, RXFP1 activation by relaxin hormone is known to stimulate other pathways including protein kinase A, protein kinase C and extracellular signal-regulated protein kinases 1 and 228. These other pathways might also contribute to the modulation of VEGF transcriptional activation. Differences in functional selectivity between members of our series or between relaxin hormone and our compounds might impact VEGF expression. Our group has previously described significant differences in functional selectivity with compounds belonging to a specific chemical class for other G protein-coupled receptors29. As a further confirmation that the compound effects were RXFP1-dependent, we recapitulated previous work showing that RXFP1 activation by compound 8 increases the cell impedance of transfected cells25.
Balancing the potency, efficacy, and in vitro metabolic stability properties of our analogs, we chose compound 8 for full mouse in vivo pharmacokinetic (PK) studies investigating its levels in plasma and heart upon drug administration. Upon a single intraperitoneal (IP) dose of compound 8 at 30 mg/kg in male C57BL/6 mice, the concentration of compound 8 in heart reached 28.6 µM (Cmax) within 1 h, and concentrations above its EC50 of 200 nM in THP1 cells was maintained for a period of 12 h in plasma and more than 24 h in heart. Oral gavage and intravenous administration recapitulate the overall pharmacokinetics properties of compound 8 (Supplementary Table S6), indicating good metabolic stability with an extended exposure, and preferential distribution toward the heart. The oral bioavailability was low (F ≈ 14%), which we attribute to poor solubility and suboptimal formulation. Nevertheless, excellent exposure was observed at this dose. Additional formulation studies or introducing solubilizing moieties into the molecule might further improve oral bioavailability.
According to the two-domain model of relaxin binding to RXFP1, the native hormone first interacts with leucine-rich repeats of extracellular domain, and then with the extra-cellular loops of the transmembrane domain, most probably with ECL230–32. This follows by an effect on the intracellular N-terminus located low density lipoprotein class A domain that is necessary for the activation of the RXFP1 signaling. Receptors without this domain or with a mutated domain including both mutations at the calcium binding asparagine or substituted with glutamic acid (D58E) bind relaxin normally but do not signal33. Surprisingly, compound 8 can still induce cAMP production with this latter D58E mutant, indicating that the receptor activation by the small molecule does not require a functional lipoprotein domain (Supplementary Fig. S3). The evaluation of compound 8 up to a concentration of 30 µM was unable to displace the binding of [125I]-human relaxin to human RXFP1 (Supplementary Table S7). This data, in addition to our mutagenesis studies, suggests that our small molecule agonists likely interact with an allosteric site at the ECL3 loop and act non-competitively with the natural hormone to activate RXFP1. In summary, we present the first synthetic small molecule alternative to relaxin hormone. The described molecules show good potency, selectivity and functional activity in cell-based assays. Our structural studies show that our agonists appear to function through a novel allosteric mechanism. Optimized compounds display excellent in vitro ADME properties and in vivo pharmacokinetic properties with high levels of exposure for extended period of time. These molecules represent the first bioavailable small-molecule agonists of human RXFP1 and a promising series to further investigate relaxin biology and evaluate the therapeutic benefits of RXFP1 activation in chronic settings.
Methods
General methods for chemistry
Full experimental details and characterization data for all new compounds are included in the Supplementary Methods.
Measurements of cAMP concentration
cAMP assay was performed using HTRF cAMP HiRange kit (CisBio, Bedford, MA). The THP1 cells and HEK293 (ATCC, Manassas, VA) cells stably7,34 or transiently transfected with human RXFP1, RXFP2, or AVPR1B receptor were stimulated with the compounds or forskolin for 30 minutes at 37 °C, 5% CO2, after which, 8 µL/well of each HTRF detection reagent (diluted according to assay kit directions in HTRF lysis buffer) was added. The plates were incubated for 30 minutes at room temperature, and the signal was read on a ViewLux (PerkinElmer, Waltham, MA) or a FLUOstar Omega (BMG Labtech, Cary, NC) plate readers. Nonlinear regressions to the Hill equation were performed using Prism software (GraphPad Software, San Diego, CA).
ATP cytotoxicity assay in HEK293-RXFP1 cells
A cytotoxicity assay to measure the effect of compounds on cell viability was performed by measuring ATP levels (ATPLite™, Promega, Madison, WI). Cells were incubated with compounds for 72 h in growth media (DMEM 10% FBS, 1× Pen/Strep, 0.5 mg/mL of G418) in 384-well format. After compound incubation, the levels of ATP in each well were measured with the addition of the ATPLite assay reagent. Nonlinear regressions to the Hill equation were performed using Prism software.
Aqueous solubility and metabolic stability measurement
Kinetic solubility analysis in phosphate buffered saline solution was performed via a fee-for-service type of contract at Analiza based upon quantitative nitrogen detection as described (www.analiza.com). One sample was supplied as a DMSO dissolved stock (10 mM). A final DMSO concentration of 2.0% and maximum theoretical compound concentration of 200 µM was achieved by diluting a 6 µL aliquot of DMSO stock with 294 µL of 1× phosphate buffered saline using Hamilton Starlet liquid handling and incubated directly in a Millipore solubility filter plate. Following 24 h incubation at ambient temperature (22.0–23.0 °C), the sample was vacuum filtered. The filtrate was injected into the nitrogen detector for quantification on Analiza’s Automated Discovery Workstation. The results are reported here in both µM and µg/mL. Three separate on-board performance indicating standards were assayed in triplicate with supplied compounds and the results were within the acceptable range. Mouse liver microsomal (MLM) stability analysis was performed via a fee-for-service type of contract by Pharmaron (www.pharmaron.com). The detailed protocols can be found in the Supplementary Table S2.
VEGF expression analysis
The VEGF stimulation in THP1 cells was analyzed by quantitative real-time PCR. 400,000 THP1 cells (0.4 mL at 1×106 cells/mL) in test media (RPMI-1640 without phenol red, 0.5% FBS, 1× Pen/Strep, 0.05 mM of 2-mercaptoethanol) were seeded in each well on a 24-well plate. After 24 h at 37 °C, 5% CO2, relaxin, compounds, or vehicle were added for 2 h. The cells were harvested and RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. cDNA was synthesized using Verso cDNA kit (Thermo Scientific, Waltham, MA) according to manufacturer’s protocol. The VEGF and GAPDH gene expression were analyzed using Roche LightCycler 480 (Roche Diagnostics, Indianapolis, IN) with the appropriate set of primers and probes spanning different exons. The relative fold change in VEGF mRNA level was calculated by the comparative Ct (2−ΔΔCt) method using GAPDH expression for normalization. The experiments were repeated three times in quadruplicates. The data were analyzed by Student’s t-test.
Cell impedance assay in HEK293-RXFP1 cells
The cell line stably transfected with RXFP1 receptor and the parental HEK293 cells were used for the cell impedance assay. Cellular impedance was measured using a Roche DP RCTA xCELLigence Analyzer (Roche Diagnostics, Indianapolis, IN) on E-Plates as described before35. Delta Cellular Indices were calculated as the change of impedance at a given time t, from the time of compound addition (CIcompound): ΔCIt=CIt−CIcompound. Impedance at each time point was then normalized to the average of quadruplicate CI of cells treated with vehicle (V1, V2, V3, and V4), to calculate normalized delta Cell Index NΔCI= (CIt−CIcompound)/Average [ΔCIV1, ΔCIV2, ΔCIV3, ΔCIV4]. 20,000 cells were added per well in a volume of 100 µL test media and allowed to sediment at room temperature for 30 minutes. The plate was placed into xCELLigence RTCA DP Instrument in the CO2 incubator overnight to allow the cells to attach. Relaxin (10 ng/mL; 1.66 nM), vehicle, or compound at different concentrations (250, 500, and 750 nM) were added to the wells and the cellular impedance was measured every 30 seconds for 30 min. The graphs depicted in this manuscript were prepared in Prizm software using a subset of data points acquired by the xCELLigence software. The data were analyzed by two-tailed, Student’s t-test using Prism software.
Study of chimeric and site-specific mutant receptors
Full length human RXFP1 receptor in pCR3.1 was used for production of site-specific and chimeric constructs. Mouse RXFP1 cDNA clone was a kind gift from Dr. Bathgate, R. A. D. (Howard Florey Institute, Melbourne, Australia). Chimeric receptors containing parts of mouse or human sequence were generated using overlap PCR. Site-specific human and mouse mutant receptors were generated by conventional method using long-range PCR with overlapping primers containing mutated nucleotide sites, then digested by DpnI and transformed into competent cells. The cDNA inserts of the resulted clones were completely sequenced to confirm the correct substitutions. Transient transfections of HEK293 cells were performed using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Cells transiently expressing the receptors were used within 48 h of transfection in cAMP assay as described above. Cells expressing each construct were treated with different concentrations of relaxin or compound 8 in the same experiment in quadruplicates. Each construct was tested at least three times.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Sherwood, O. D. at the University of Illinois at Urbana-Champaign for providing porcine relaxin; Dr. Bryant-Greenwood, G. D. at the University of Hawaii and Dr. Kern, A. at the Scripps Research Institute-Florida for providing HEK293 cells stably transfected with RXFP1; Dr. Hsu, S. Y. at Stanford University for HEK293 cells stably transfected with RXFP2; and Dr. Bathgate, R. A. D. at Howard Florey Institute, Melbourne for the mouse RXFP1 construct and RXFP3 data. The authors also thank Drs. Xu, X. and Wang, A. at NCATS for assessment of oral bioavailability in mice and Dr. Rheingold, A. L. at UCSD for the X-ray analysis of compound 8. This research was supported by the Molecular Libraries Initiative of the NIH Roadmap for Medical Research (U54MH084681 and R03MH085705 to A.I.A.), the Intramural Research Program of the National Human Genome Research Institute (NHGRI) and National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), and the Faculty Research Support Program of the Florida International University.
Footnotes
Author contributions
J.X. designed, synthesized and characterized all new compounds. Z.H. and I.U.A performed secondary assays and structural mutagenesis studies. C.Z.C., R.E.J., M.F. and W.Z. adapted the assay and conducted the qHTS and confirmatory assays. N.S. and X.H. provided data analysis and model docking study support. A.I.A. and J.J.M. guided the design and proposed follow up experimentation. J.X., N.S., A.I.A. and J.J.M. drafted the manuscript. All authors discussed the results, analyzed the data and commented on the manuscript.
Supplementary Information accompanies this paper at http://www.nature.com/naturecommunications
Competing financial interests: The authors declare no competing financial interests.
Accession codes: The X-ray crystallographic coordinates for structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 933133. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
References
- 1.Roger VL, et al. Executive Summary: Heart Disease and Stroke Statistics—2011 Update. Circulation. 2011;123:459–463. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hisaw FL. Experimental relaxation of the pubic ligament of the guinea pig. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 1926;23:661–663. [Google Scholar]
- 3.Fevold HL, Hisaw FL, Meyer RK. The relaxative hormone of the corpus luteum. Its purification and concnetration. J. Am. Chem. Soc. 1930;52:3340–3348. [Google Scholar]
- 4.Baylis C. Relaxin may be the "elusive" renal vasodilatory agent of normal pregnancy. Am. J. Kidney Dis. 1999;34:1142–1144. doi: 10.1016/S0272-6386(99)70024-7. [DOI] [PubMed] [Google Scholar]
- 5.Jeyabalan A, Shroff SG, Novak J, Conrad KP. The vascular actions of relaxin. Adv. Exp. Med. Biol. 2007;612:65–87. doi: 10.1007/978-0-387-74672-2_6. [DOI] [PubMed] [Google Scholar]
- 6.Bathgate RA, et al. Relaxin family peptides and their receptors. Physiol. Rev. 2013;93:405–480. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 7.Kern A, Hubbard D, Amano A, Bryant-Greenwood GD. Cloning, expression, and functional characterization of relaxin receptor (leucine-rich repeat-containing g protein-coupled receptor 7) splice variants from human fetal membranes. Endocrinology. 2008;149:1277–1294. doi: 10.1210/en.2007-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Westhuizen ET, Summers RJ, Halls ML, Bathgate RAD, Sexton PM. Relaxin receptors - new drug targets for multiple disease states. Curr. Drug Targets. 2007;8:91–104. doi: 10.2174/138945007779315650. [DOI] [PubMed] [Google Scholar]
- 9.Teerlink JR, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 10.Metra M, et al. Effect of Serelaxin on Cardiac, Renal, and Hepatic Biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) Development Program: Correlation With Outcomes. J. Am. Coll. Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Unemori E, Sibai B, Teichman SL. Scientific rationale and design of a phase I safety study of relaxin in women with severe preeclampsia. Ann. N. Y. Acad. Sci. 2009;1160:381–384. doi: 10.1111/j.1749-6632.2009.03838.x. [DOI] [PubMed] [Google Scholar]
- 12.Tozzi CA, et al. Recombinant human relaxin reduces hypoxic pulmonary hypertension in the rat. Pulm. Pharmacol. Ther. 2005;18:346–353. doi: 10.1016/j.pupt.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida T, et al. Relaxin ameliorates salt-sensitive hypertension and renal fibrosis. Nephrol. Dial. Transplant. 2012;27:2190–2197. doi: 10.1093/ndt/gfr618. [DOI] [PubMed] [Google Scholar]
- 14.Samuel CS, et al. Relaxin remodels fibrotic healing following myocardial infarction. Lab. Invest. 2011;91:675–690. doi: 10.1038/labinvest.2010.198. [DOI] [PubMed] [Google Scholar]
- 15.Du X-J, Bathgate RAD, Samuel CS, Dart AM, Summers RJ. Cardiovascular effects of relaxin: from basic science to clinical therapy. Nat. Rev. Cardiol. 2010;7:48–58. doi: 10.1038/nrcardio.2009.198. [DOI] [PubMed] [Google Scholar]
- 16.Teichman SL, Unemori E, Teerlink JR, Cotter G, Metra M. Relaxin: review of biology and potential role in treating heart failure. Curr. Heart Failure Rep. 2010;7:75–82. doi: 10.1007/s11897-010-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teerlink JR, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicenter, randomized, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. 2009;373:1429–1439. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 18.Segal MS, et al. Relaxin increases human endothelial progenitor cell NO and migration and vasculogenesis in mice. Blood. 2012;119:629–636. doi: 10.1182/blood-2011-04-346007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin CP, Brady LS, Insel TR, Collins FS. NIH Molecular Libraries Initiative. Science. 2004;306:1138–1139. doi: 10.1126/science.1105511. [DOI] [PubMed] [Google Scholar]
- 20.Inglese J, et al. Quantitative high-throughput screening: A titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc. Nat. Acad. Sci. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CZ, et al. Identification of small-molecule agonists of human relaxin family receptor 1 (RXFP1) by using a homogenous cell-based cAMP assay. J. Biomol. Screen. 2013 doi: 10.1177/1087057112469406. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansch C, et al. Aromatic substituent constants for structure-activity correlations. J. Med. Chem. 1973;16:1207–1216. doi: 10.1021/jm00269a003. [DOI] [PubMed] [Google Scholar]
- 23.Unemori EN, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum. Reprod. 1999;14:800–806. doi: 10.1093/humrep/14.3.800. [DOI] [PubMed] [Google Scholar]
- 24.Unemori EN, et al. Relaxin induces vascular endothelial growth factor expression and angiogenesis selectively at wound sites. Wound Repair Regen. 2000;8:361–370. doi: 10.1111/j.1524-475x.2000.00361.x. [DOI] [PubMed] [Google Scholar]
- 25.Shemesh R, et al. Activation of relaxin-related receptors by short, linear peptides derived from a collagen-containing precursor. Ann. N. Y. Acad. Sci. 2009;1160:78–86. doi: 10.1111/j.1749-6632.2009.03827.x. [DOI] [PubMed] [Google Scholar]
- 26.Dschietzig T, Bartsch C, Baumann G, Stangl K. Relaxin-a pleiotropic hormone and its emerging role for experimental and clinical therapeutics. Pharmacol. Ther. 2006;112:38–56. doi: 10.1016/j.pharmthera.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Wilson CN, Mustafa SJ, editors. Adenosine Receptors In Health And Disease. [In: Handb. Exp. Pharmacol., 2009; 193] Springer GmbH; 2009. [PubMed] [Google Scholar]
- 28.Summers RJ, Halls ML, van der Westhuizen ET. In: Encyclopedia of Signaling Molecules. Sangdun Choi., editor. Vol. 362. New York: Springer; 2012. pp. 1635–1643. [Google Scholar]
- 29.McCoy JG, et al. Selective Modulation of Gq/Gs pathways by Naphtho Pyrano Pyrimidines As Antagonists of the Neuropeptide S Receptor. ACS Chem. Neurosci. 2010;1:559–574. doi: 10.1021/cn100040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudo S, et al. H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J. Biol. Chem. 2003;278:7855–7862. doi: 10.1074/jbc.M212457200. [DOI] [PubMed] [Google Scholar]
- 31.Halls ML, et al. Multiple binding sites revealed by interaction of relaxin family peptides with native and chimeric relaxin family peptide receptors 1 and 2 (LGR7 and LGR8) J. Pharmacol. Exp. Ther. 2005;313:677–687. doi: 10.1124/jpet.104.080655. [DOI] [PubMed] [Google Scholar]
- 32.Halls ML, et al. Identification of binding sites with differing affinity and potency for relaxin analogues on LGR7 and LGR8 receptors. Annu. N. Y. Acad. Sci. 2005;1041:17–21. doi: 10.1196/annals.1282.004. [DOI] [PubMed] [Google Scholar]
- 33.Kern A, Agoulnik AI, Bryant-Greenwood GD. The low-density lipoprotein class A module of the relaxin receptor (leucine-rich repeat containing G-protein coupled receptor 7): its role in signaling and trafficking to the cell membrane. Endocrinology. 2007;148:1181–1194. doi: 10.1210/en.2006-1086. [DOI] [PubMed] [Google Scholar]
- 34.Hsu SY, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 35.Hodgson MC, et al. Decreased expression and androgen regulation of the tumor suppressor gene INPP4B in prostate cancer. Cancer Res. 2011;71:572–582. doi: 10.1158/0008-5472.CAN-10-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.