Abstract
Objectives
The introduction of generic second-generation antipsychotics (SGAs), starting with risperidone in July 2008, could reduce antipsychotic spending and cost-related use barriers. This study examines associations between generic risperidone use and spending and adherence after introduction among Medicare Advantage (MA) beneficiaries.
Study Design
Historic cohort study
Methods
The study included MA beneficiaries receiving SGA treatment prior to July 2008. We examined antipsychotic spending using linear models; adherence (proportion of days covered ≥80%) using logistic models; and nonpersistence (time to first gap in antipsychotic use >30 days) in 2009 using Cox proportional hazard models, comparing beneficiaries with versus without generic use, adjusting for individual and plan characteristics.
Results
Between July 2008 and December 2009, 22.8% of beneficiaries had ≥1 fill of generic risperidone: 73% of those previously using branded risperidone and 6.7% of those previously using other SGAs. Beneficiaries in private fee-for-service (PFFS) versus HMO plans had lower rates of generic use (HR, 0.73 [0.56–0.96]); however, cost-sharing levels were not associated with generic use. Compared with beneficiaries who continued using other SGAs, those who switched from other SGAs to generic risperidone in 2008 had lower out-of-pocket spending (−$214 [−$314 to −$115]), higher adherence (OR, 2.34 [1.62–3.40]) and lower rates of nonpersistence (HR, 0.56 [0.46–0.69]) in 2009.
Conclusions
Generic use was concentrated among patients previously using branded risperidone. HMO plans appeared to be more effective at encouraging generic use than unmanaged PFFS plans; however, patient financial incentives had limited influence on switching. Additional opportunity remains to encourage greater generic SGA use, as well as to reduce spending, and potentially improve treatment adherence and outcomes.
Précis
This study examines the plan characteristics associated with generic risperidone use and spending and adherence outcomes associated with use among Medicare Advantage Part D beneficiaries.
Antipsychotics are among the top selling classes of drugs in the United States, largely driven by spending on second-generation antipsychotics (SGA). Antipsychotics are one of 6 protected drug classes within the Medicare Part D prescription drug program, meaning that plans are required to include all or substantially all drugs within the class on plan formularies. Medicare spending on antipsychotics was $5.9 billion in 2009, second only to antihyperlipidemics.1
Several commonly used SGAs have recently lost patent protection, starting with Risperdal (risperidone) in July 2008, and then Zyprexa (olanzapine), Geodon (ziprasidone) and Seroquel (quetiapine) in 2011 and 2012. The entry of new generic SGAs could result in substantial cost savings for Medicare and other payers. The Congressional Budget Office estimated that the overall Part D savings attributable to generic substitution in 2007 were approximately $33 billion.2
However, realizing savings associated with the availability of generic antipsychotics could be more challenging than in other therapeutic classes. Physicians and patients are often hesitant to change psychotropic drug regimens for stable patients due to concerns about lack of tolerability or effectiveness of new regimens.3–7 Market reports suggest that the entry of generic risperidone did not decrease the market share of other SGAs, suggesting limited generic substitution across molecules (ie, non-bioequivalent substitution).8,9 Even for bioequivalent substitution of the same molecule, some studies note concerns about potential reductions in adherence due to patients’ anxieties regarding changes in the name, packaging, or appearance of the drug.10,11 Conversely, greater generic substitution could improve adherence by reducing the out-of-pocket costs associated with treatment12; however, little is known about the effects of switching to generic SGAs on spending or adherence.
We examined changes in SGA treatment choices among Medicare Advantage (MA) beneficiaries in the first 18-months after generic risperidone introduction (July 2008–December 2009) and Part D plan characteristics associated with generic use. We also examined the associations between generic risperidone use and antipsychotic spending and adherence.
METHODS
Study Population
This study included noninstitutionalized beneficiaries enrolled in MA prescription drug plans offered by a national carrier, including health maintenance organizations (HMOs), preferred provider organizations (PPOs), and private fee-for-service (PFFS) plans. Identifying information on the plans included in the study has been removed, including markets served and exact details on cost-sharing and plan structures.
MA-HMO plans have the most closed physician networks, whereas PPOs have more open networks. Until 2011, PFFS plans were not required to have formal provider networks and included the same physicians as in traditional Medicare (96% of eligible physicians, nationally).13 Because plans with tighter network structures have a greater range of tools with which to influence physician behavior, we hypothesized that generic use would be greater in HMO versus PFFS plans. Examples of commonly used tools include providing feedback to physicians on their relative rates of generic use and drug spending, and use of physician financial incentives to encourage generic drug use.
Antipsychotics are a protected class under Part D; thus, plans had to include all drugs within the class in their formularies, but could vary tier placement and/or cost-sharing amounts for drugs within the class. We hypothesized that greater cost-sharing for brand relative to generic drugs would be associated with higher rates of generic risperidone use.
All study plans had a similar formulary for antipsychotics in terms of tier placement, but co-payment levels for these tiers varied across plans. In 2007, most SGAs were preferred brand drugs (tier 2). Following the introduction of generic risperidone in 2008, risperidone was on tier 1 and Risperdal (risperidone) became nonformulary, or a nonpreferred brand, with a try/fail requirement for generic risperidone. Most of the other commonly used SGAs remained preferred brands without utilization management requirements, while some SGAs became nonpreferred brands.
We focused on beneficiaries with at least 1 SGA fill between January and June 2008, and examined SGA use patterns after the introduction of generic risperidone in July 2008. Beneficiaries were censored upon disenrollment or death. Because we focused, in part, on the relationship between cost-sharing levels and drug choices, we excluded beneficiaries receiving Part D low income subsidies (LIS) who had minimal cost-sharing. In sensitivity analyses among LIS beneficiaries, findings were consistent.
Data Sources
We linked Part D Event files, medical claims, plan information, and beneficiary characteristics for all subjects. To identify the type of SGA use, we used the Medi-Span Electronic Drug File (version 2) and FDA Orange Book data.14, 15
Generic Risperidone Use and Part D Plan Characteristics
We examined the cumulative proportion of beneficiaries with any generic risperidone use between July 2008 and December 2009. We used Cox proportional hazard models to examine the plan characteristics associated with time to first generic risperidone use. We stratified both analyses by whether beneficiaries were previously using brand risperidone or other SGAs before generic introduction.
We classified beneficiaries’ plan types as HMO, PPO or PFFS; and beneficiaries’ benefit designs as enhanced or basic Part D—enhanced plans included coverage for generics during the standard coverage gap (eg, after $2,510 in total drug spending in 2008) and basic plans had no gap coverage. We also examined the relative cost of brand versus generic drugs during the initial coverage period prior to the gap, and focused on the preferred brand (tier 2) versus generic (tier 1) co-payment differential because the most commonly used SGAs were tier 2 drugs throughout the study period.
These models adjusted for beneficiary age, gender, race/ethnicity, Part D comorbidity risk score (RxHCC), mental health diagnoses, reason for Medicare entitlement (disabled vs aged), and prior drug spending. As a measure of beneficiaries’ stability on their SGA regimens, we adjusted for the length of time beneficiaries were receiving the last SGA used prior to July 2008. Among non-risperidone users, we also adjusted for the generic name of the last SGA used prior to July 2008.
Generic Substitution, Spending and Adherence
We examined the association between SGA drug use patterns and antipsychotic spending (total and out-of-pocket) and treatment adherence and persistence. Our primary analyses examine variations in these outcomes in 2009 based on 2008 switching patterns. We focused on 4 SGA use patterns, as defined by the last SGA used prior to July 2008 and SGA use between July and December 2008: 1) bioequivalent generic substitution (brand risperidone to generic risperidone); 2) non-bioequivalent generic substitution (non-risperidone SGA to generic risperidone); 3) non-bioequivalent brand switching (non-risperidone SGA to other SGA brand); and 4) no change (same non-risperidone SGA brand throughout 2008). Beneficiaries with no SGA fills between July and December 2008 were excluded from these analyses (19.9% and 23% of brand risperidone and non-risperidone users, respectively). Because Part D Event files do not capture data on uncovered drugs, we reliably cannot assess brand risperidone use after July 2008.
Antipsychotic Spending, Adherence, and Nonpersistence
We examined total and out-of-pocket antipsychotic drug spending in 2009. We examined adherence monthly (January 2008–December 2009) and annually (2009) using the proportion of days covered (PDC). The PDC was calculated as the sum of available days’ supply of all antipsychotics divided by the total days in each time period. We defined nonpersistence as having a gap in antipsychotic supply >30 days.
Analyses
To examine the associations between patterns of SGA use (eg, generic substitution) in 2008 and annual 2009 drug spending and adherence (ie, PDC >80%), we use linear and logistic regression models, respectively, adjusting for plan characteristics and covariates described above, as well as antipsychotic PDC levels in 2008. To examine the association between patterns of SGA use and nonpersistence in 2009, we used Cox proportional hazard models, adjusting for the same covariates.
To examine changes in monthly adherence levels by SGA use patterns, we used linear fixed effects (within-person) regression models to estimate changes in monthly PDC relative to the month prior to generic introduction (June 2008). These models are robust to potential measured and unmeasured time-stable confounders; we adjusted for time-varying covariates, including calendar month, beneficiaries’ Part D risk scores, and tier 2 versus tier 1 co-payment differentials.
RESULTS
Study Population Characteristics
Among all subjects, 24.3% used brand risperidone prior to generic introduction in July 2008. Overall, 41.3% had schizophrenia or bipolar disorder, 46.1% were disabled, and about 35.4% and 57.1% were in HMO and PFFS plans, respectively (Table 1). Mean cost-sharing levels were approximately $3 (tier 1) and $25 (tier 2).
Table 1.
Study Population Characteristics
| Type of SGA use prior to July 2008 |
||||
|---|---|---|---|---|
| All | Non- risperidone SGA |
Brand risperidone |
||
| 100% | 75.7% | 24.3% | ||
| Age, Mean (SD) | 65.5 (14.8) | 65.2 (14.7) | 70.2 (14.6) | |
| Part D RxHCC Risk Score, Mean (SD) | 1.22 (0.43) | 1.24 (0.43) | 1.18 (0.42) | |
| Female | 61.4% | 61.3% | 61.7% | |
| Race: | White | 87.3% | 88.1% | 84.7% |
| Black | 8.6% | 7.8% | 11.2% | |
| Asian | 0.3% | 0.3% | 0.4% | |
| Hispanic | 2.3% | 2.2% | 2.5% | |
| Other1 | 1.5% | 1.6% | 1.2% | |
| Reason for Medicare Entitlement: | Aged | 53.9% | 50.4% | 64.9% |
| Disability | 46.1% | 49.6% | 35.1% | |
| Diagnosis: | Schizophrenia | 17.3% | 16.9% | 18.6% |
| Bipolar | 24.0% | 26.6% | 15.7% | |
| Other mental health diagnosis | 44.2% | 43.9% | 45.0% | |
| Dementia | 11.2% | 9.3% | 17.1% | |
| No mental health diagnosis | 3.3% | 3.3% | 3.6% | |
| Part D coverage:2 | Basic | 67.3% | 68.2% | 64.7% |
| Enhanced | 32.7% | 31.8% | 35.3% | |
| Medicare Advantage plan type: | HMO | 35.4% | 33.6% | 40.8% |
| PFFS | 57.1% | 58.4% | 53.1% | |
| PPO | 7.5% | 8.0% | 6.1% | |
| Cost-sharing:2 | Mean Tier 1 copay 2008 (SD) | $3.34 (2.27) | $3.40 (2.22) | $3.16 (2.38) |
| Mean Tier 2 copay 2008 (SD) | $24.90 (10.04) | $25.21 (9.75) | $24.07 (10.85) | |
| Reached coverage gap threshold in 20073 | 59.5% | 61.0% | 55.0% | |
HMO indicates health maintenance organization; PPO, preferred provider organization; PFFS, private fee-for-service; RxHCC, prescription drug hierarchical condition categories; SGA, second-generation antipsychotic.
This table presents the study population characteristics among beneficiaries with at least 1 SGA fill between January and June 2008.
Other race includes missing, Other and Native American.
A random factor (± 5%) was applied to the percentages of beneficiaries enrolled in basic versus enhanced plans; HMO, PFFS, and PPO plans; and the mean tier 1 and tier 2 co-pays in 2008 to mask the identity of the plans included in our study.
The coverage gap threshold in 2007 was $2400 in total drug spending. The proportion of beneficiaries reaching the coverage gap threshold was calculated among those enrolled in 2007
Generic SGA Use
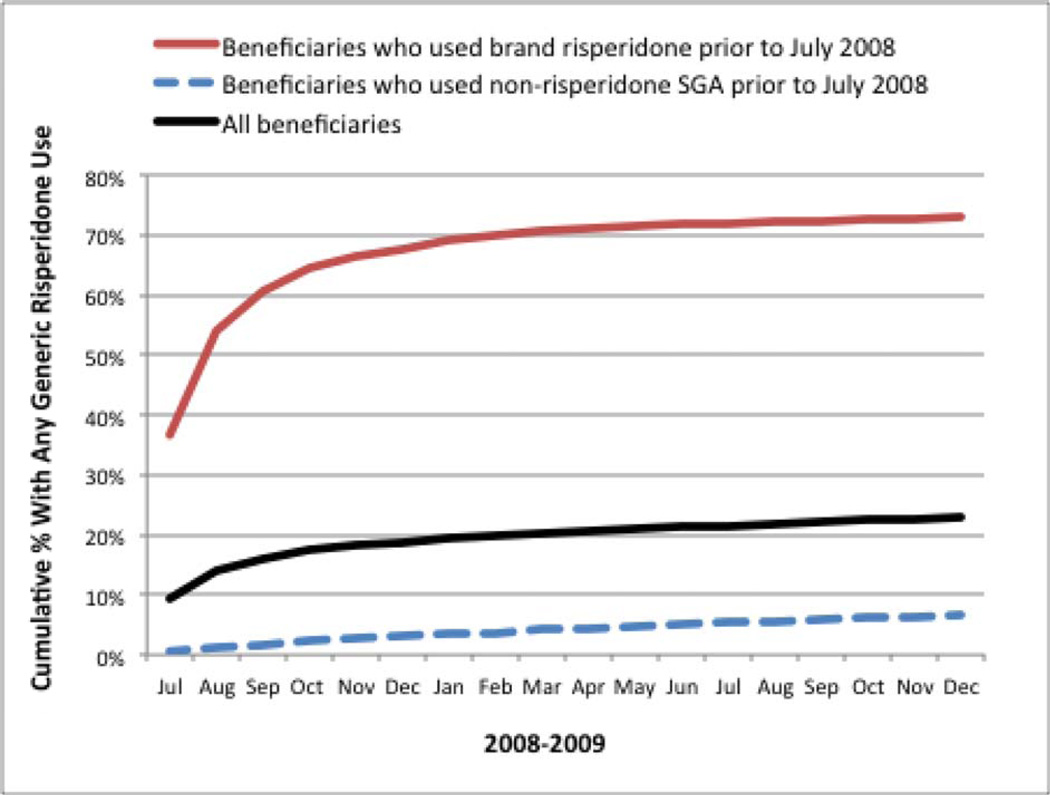
Figure 1 presents the cumulative percent of beneficiaries who used generic risperidone between July 2008 and December 2009. Among those using brand risperidone prior to July 2008, 67.7% used generic risperidone by December 2008 and 73% by December 2009. Among those using other brand SGAs prior to July 2008, 6.7% used generic risperidone by December 2009.
Figure 1. Cumulative Percent of Beneficiaries With Generic Risperidone Use, 2008–2009.
SGA indicates second-generation antipsychotic.
This figure presents the cumulative percent of beneficiaries with at least 1 fill of generic risperidone between July 2008 and December 2009, overall, and among those who previously used brand risperidone or other brand SGAs.
In multivariate analyses, greater cost-sharing differentials for generic versus brand drugs and enhanced Part D coverage were not significantly associated with generic use rates (Table 2). In contrast, enrollment in PFFS versus HMO plans was associated with lower rates of non-bioequivalent generic substitution (HR, 0.73; 95% CI, 0.56–0.96); there were no significant differences in bioequivalent generic substitution rates.
Table 2.
Association Between Part D Plan Characteristics and Time to First Generic Risperidone Use
| Antipsychotic use prior to July 2008 | ||||
|---|---|---|---|---|
| Non-risperidone SGA1 | Brand risperidone | |||
| HR | (95% CI) | HR | (95% CI) | |
| Plan cost-sharing levels: | ||||
| Enhanced benefits (vs. Basic) | 1.04 | (0.78, 1.37) | 1.12 | (0.96, 1.30) |
| Tier 2-Tier 1 copay differential: +$10 | 0.98 | (0.88, 1.09) | 0.99 | (0.93, 1.05) |
| Medicare Advantage plan type: | ||||
| PFFS (vs. HMO) | 0.73 | (0.56, 0.96) | 0.95 | (0.83, 1.10) |
| PPO | 0.91 | (0.67, 1.24) | 0.99 | (0.82, 1.19) |
HMO indicates health maintenance organization; HR, hazard ratio; PFFS, private fee-for-service; PPO, preferred provider organization; SGA, second-generation antipsychotic.
This table presents the results of Cox proportional hazard models, also adjusted for age, gender, race/ethnicity, Part D comorbidity risk score, mental health diagnoses, reason for Medicare entitlement (disabled vs aged), reaching the coverage gap threshold in 2007, and length of time on last antipsychotic use prior to July 2008.
In analyses among beneficiaries previously using other brand SGAs, the models also adjusted for the last antipsychotic used prior to July 2008. Bolded values indicate P <.05.
Antipsychotic Spending
Compared with beneficiaries who continued using the same non-risperidone SGA before and after generic introduction, those who switched to risperidone in 2008 (non-bioequivalent generic substitution) had similar 2009 total spending, but lower out-of-pocket antipsychotic spending (difference = −$214; [−$314 to −$115]). In contrast, those who switched to a different brand SGA in 2008 (non-bioequivalent brand switching) had higher total and out-of-pocket spending in 2009. Beneficiaries who switched from brand to generic risperidone (bioequivalent substitution) had lower total antipsychotic spending (difference = −$943 [−$1,042 to −$843]) and out-of-pocket spending (difference = −$445 [−$485 to −$404]).
Antipsychotic Adherence and Nonpersistence
Beneficiaries who switched antipsychotics, either to generic risperidone or other brand SGAs, had higher odds of adherence in 2009 versus those who remained on the same brand SGA: OR, 1.72 [1.48–2.00] for bioequivalent generic substitution; OR, 2.34 [1.62–3.40] for non-bioequivalent generic substitution; OR, 1.43 [1.04–1.96] for non-bioequivalent brand switching (Table 3). These beneficiaries also had lower rates of nonpersistence in 2009, defined as the time to first gap in SGA use >30 days, compared with those who continued using the same brand SGA in 2008.
Table 3.
Differences in Antipsychotic Spending and Adherence Associated With SGA Drug Use Patterns
| Total Antipsychotic Spending in 2009 |
Out-of-Pocket Antipsychotic Spending in 2009 |
|||||
|---|---|---|---|---|---|---|
| Adj. mean |
Diff ($) |
(95% CI) | Adj. mean |
Diff ($) |
(95% CI) | |
|
No change (stay on same non- risperidone brand SGA) |
$1,639 | -- | Ref | $541 | -- | Ref |
|
Bioequivalent generic substitution (brand risperidone → generic risperidone) |
$696 | −943 | (−1,043, −843) | $96 | −445 | (−485, −404) |
|
Non-bioequivalent generic substitution (non-risperidone SGA → generic risperidone) |
$1,542 | −97 | (−343, 148) | $327 | −214 | (−314, −115) |
|
Non-bioequivalent brand switching (non-risperidone SGA → other brand SGA) |
$3,008 | 1,369 | (1,162, 1,577) | $922 | 381 | (297, 465) |
| Adherence in 2009 (PDC≥80%) |
Non-persistence in 20091 (Time to first gap in use ≥31 days) |
|||||
|---|---|---|---|---|---|---|
| Adj. % | OR | (95% CI) | Unadj. %2 |
HR | (95% CI) | |
|
No change (stay on same non- risperidone brand SGA) |
43.1% | 1.00 | Ref | 64.0% | 1.00 | Ref |
|
Bioequivalent generic substitution (brand risperidone → generic risperidone) |
52.1% | 1.72 | (1.48, 2.00) | 50.4% | 0.76 | (0.70, 0.83) |
|
Non-bioequivalent generic substitution (non-risperidone SGA → generic risperidone) |
57.0% | 2.34 | (1.62, 3.40) | 53.1% | 0.56 | (0.46, 0.69) |
|
Non-bioequivalent brand switching (non-risperidone SGA → other brand SGA) |
49.0% | 1.43 | (1.04, 1.96) | 50.0% | 0.64 | (0.53, 0.78) |
Adj indicates adjusted; diff, differential; HR, hazard ratio; OR, odds ratio; PDC, proportion of days covered; SGA, second-generation antipsychotic; “→” switch to.
These analyses included all beneficiaries enrolled in 2009 with at least 1 SGA fill after the introduction of generics between July and December 2008.
We examined antipsychotic drug spending in 2009 using linear regression models, adherence in 2009 (PDC ≥80%) using logistic regression models, and non-persistence (ie, time to first gap in use of more than 30 days) using Cox proportional hazard models.
All models are adjusted for plan type (PFFS, PPO, HMO), coverage type (enhanced vs basic), tier 2 versus tier 1 co-pay differentials, beneficiary gender, age, race/ethnicity, Part D comorbidity risk score, mental health diagnoses, reason for Medicare entitlement, reaching the Part D coverage gap threshold in 2007 ($2700 in total spending), days on last antipsychotic prior to July 2008, 2009 person-months of enrollment, and total antipsychotic PDC in 2008.
The nonpersistence model excluded beneficiaries with no days of supply in 2009 (11.6%).
Among beneficiaries that had a gap in use ≥31 days, the unadjusted mean time to nonpersistence was 133 (no change), 131 days (bioequivalent substitution), 154 days (nonbioequivalent substitution), and 145 days (non-bioequivalent brand switching). Bolded values indicate P <.05.
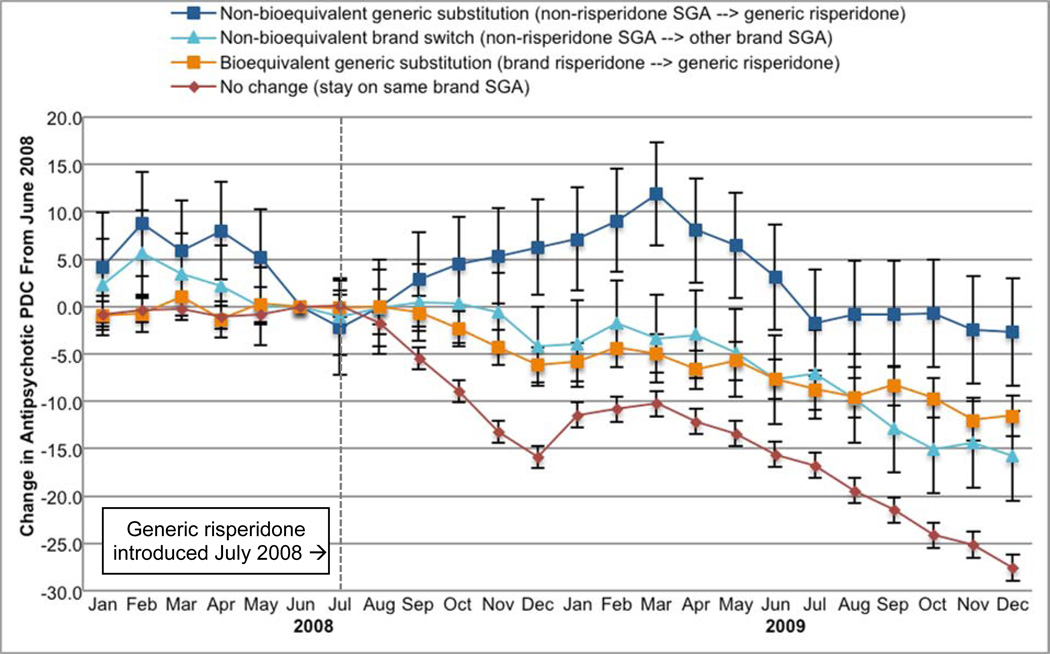
Figure 2 presents within-person changes in the PDC by antipsychotics in each month in 2008 and 2009 relative to June 2008—the month prior to generic introduction. Declines in use toward the end of each calendar year are consistent with the pattern of an increasing number of beneficiaries reaching the coverage gap and losing drug coverage. Those with non-bioequivalent generic substitution had the smallest relative reductions in SGA use by the end of 2009 compared with other beneficiaries (−2.6 percentage points in PDC; 8.3, 3.0), including those who switched to a different brand SGA (−15.7 percentage points; −20.4 to −10.9); those who stayed on the same brand SGA throughout 2008 had the largest reductions in use by the end of 2009 (−27.5 percentage points; −28.9 to −26.1).
Figure 2. Monthly Changes in Antipsychotic Use Before and After Generic Risperidone Introduction by SGA Drug Use Patterns.
PDC indicates proportion of days covered; SGA, second-generation antipsychotic.
This figure displays the adjusted monthly change in antipsychotic proportion of days covered relative to June 2008, the month prior to generic risperidone introduction, estimated using fixed effect (within-person) linear models and adjusted for time changing covariates (risk score and tier 1 and tier 2 co-payment differential); time stable covariates drop out of the model.
Bars indicate 95% confidence intervals.
DISCUSSION
This study focused on the effects of the introduction of the first widely used SGA, risperidone, in Medicare Part D. We examined the levels of generic risperidone use in the first 18 months of introduction among beneficiaries receiving ongoing SGA drug therapy. Fewer than 1 in 4 beneficiaries used generic risperidone by the end of the study period, and the vast majority of use was among those previously using brand risperidone. Generic risperidone use was associated with lower total and out-of-pocket antipsychotic spending, as well as higher levels of adherence and persistence to SGA therapy.
The high levels of bioequivalent generic substitution and the limited variation by MA plan type and cost-sharing levels suggest that these switches could happen automatically (eg, generic substitution at the pharmacy). Despite concerns about patient mistrust of generics, bioequivalent generic substitution did not adversely affect adherence, on average. Moreover, bioequivalent generic substitution was associated with savings in total and out-of-pocket antipsychotic spending.
We are not able to determine if beneficiaries used brand risperidone after July 2008 or stopped using SGAs entirely because Part D Event data do not capture uncovered drug use. We hypothesize, however, that levels of brand risperidone use were low. We found a similar percent of beneficiaries had no SGA fills after July 2008 across those who were previously using branded risperidone and other SGAs. Moreover, industry reports suggest that levels of brand Risperdal use were minimal after the introduction of generic risperidone.8
The Congressional Budget Office estimates that across all drug classes, Part D could have saved an additional $4 billion through non-bioequivalent generic substitution in 2007.2 However, we found that few beneficiaries (<7%) using other SGAs switched to generic risperidone. While physicians and patients are hesitant to switch SGAs if patients are stable on existing regimens, we found that even among those who actively switched SGAs only one-third switched to generic risperidone. It is possible that switching to risperidone might not have been clinically appropriate for all of these beneficiaries if some previously experienced poor tolerability or treatment failure with risperidone. In our study population, however, only 6% of beneficiaries who switched to a different brand SGA had any documented use of brand risperidone in the prior year, suggesting potential opportunities for greater non-bioequivalent generic substitution.
Encouraging more non-bioequivalent generic substitution among beneficiaries who require changes in their SGA drug regimen could yield clinical benefits in addition to cost savings. We found that beneficiaries who switched to generic risperidone from other brand SGAs had higher levels of adherence and lower rates of gaps in use in 2009. In addition, we found smaller relative reductions in SGA use over the first 18 months after generic introduction for beneficiaries who switched to generic risperidone compared with those who switched to other brand SGAs. Nevertheless, work is needed to evaluate the clinical effects of non-bioequivalent generic substitution. The recent availability of additional generic SGAs, including olanzapine, ziprasidone and quetiapine in 2011 and 2012, however, provide a greater range of therapeutic options for generic substitution. This is especially important given the recent proposal by CMS to remove the formulary protections for 3 of the 6 protected drug classes, including antipsychotics.16 Although CMS retracted this proposal after substantial backlash, statute allows for the protections for these classes to be revisited in the future.
We found that HMO plans with tighter and formal physician networks were more effective at encouraging non-bioequivalent generic substitution than PFFS plans without provider networks.12 The Medicare Improvements for Patients and Providers Act required most PFFS plans to establish provider networks starting in 2011, resulting in the withdrawal of many PFFS plans from the market.17,18 The older PFFS model, however, could more closely mirror the experience of beneficiaries enrolled in stand-alone prescription drug plans (PDPs), which cover about two-thirds of Part D beneficiaries.19
Contrary to our expectations, patient financial incentives, via cost-sharing, to use generic drugs had limited influence on generic substitution rates. These findings differ from evidence in other drug classes, such as hyperlipidemia and hypertension drugs, where incentive-based formularies have been linked with higher rates of switching to lower-tier drugs.20–22 Patient financial incentives could be less effective at encouraging generic use for psychotropic drugs and result in greater cost-shifting to patients, especially for those with serious mental illness where there is greater hesitancy to switch drug regimens if patients are stable.23,24
Limitations
This was a non-randomized study and there could be unmeasured differences across beneficiaries with different drug use patterns. In particular, beneficiaries with non-bioequivalent changes in their SGA regimens could have worse disease severity or recent exacerbations that could affect their proclivity to adhere to SGAs. In fact, we found higher levels of adherence among beneficiaries who switched to different brand SGA drugs compared with those who remained on the same brand SGA. Similarly, we found no significant difference in total antipsychotic spending for those who switched from other SGAs to generic risperidone compared with those who remained on the same SGA; this is likely due to the higher levels of antipsychotic use.
To address this potential confounding by indication, we compared within-person changes in adherence using fixed effects estimation, which is robust to time-stable confounders across the groups. In addition, we included comparisons between those with non-bioequivalent changes in their SGA drug regimens (ie, those who switched from other SGAs to generic risperidone vs those who switched to a different brand drug). These beneficiaries were more similar with respect to prior year drug spending, comorbidity risk scores, and disability (eAppendix Table). Consistent with our hypotheses, in the fixed effects analyses, we found those who switched from other SGAs to generic risperidone had smaller relative reductions over time in their antipsychotic adherence compared with those who switched to other brand SGAs. We also conducted parallel sensitivity analyses among the subgroup of beneficiaries with documented diagnoses of schizophrenia and bipolar disorder and found consistent results.
Our study is limited to beneficiaries enrolled in MA Part D plans, and may not generalize to those in stand-alone prescription drug plans. Within our study population, there were low levels of PPO enrollment, limiting our ability to estimate the association between PPOs and generic uptake. HMOs and PFFS plans have different geographic patterns (ie, HMOs tend to be offered in urban markets and PFFS plans in rural areas); however, there is overlap between these plan types in small metropolitan areas. Lastly, utilization management requirements, such as prior authorization, can also influence drug choices. All subjects in this study faced similar utilization management requirements (none for the most commonly used SGAs).
Findings from this study also may not generalize to non-Medicare populations, including those in commercial insurance plans or Medicaid (eg, due to differences in the underlying patient 14 populations or the availability and effects of various policy levers to influence generic use). Understanding the implications of generic entry within Medicaid is especially important as SGAs comprised 15% of all Medicaid drug expenditures in 2009.25,26
CONCLUSIONS
In conclusion, generic use of SGAs was concentrated among patients previously using branded risperidone, and Medicare Part D HMO plans appeared to be more effective at encouraging generic use than PFFS plans without formal physician networks. While patient financial incentives had limited influence on switching, lower patient out-of-pocket drug costs associated with generic substitution appeared to be associated with improved patient adherence to SGA treatment. With the recent introduction of additional generic SGAs, considerable opportunity remains to reduce Medicare and beneficiary spending, and potentially improve treatment adherence and outcomes.
Supplementary Material
Take-Away Points.
Risperidone was the first widely used second-generation antipsychotic (SGA) available as a generic. We examined patterns of use in the first 18-months of introduction and found the following:
Use of generic risperidone was concentrated among those previously using branded risperidone (ie, bioequivalent substitution); there was limited therapeutic (non-bioequivalent) substitution from other brand SGAs.
Rates of generic risperidone take-up were greater in Medicare Advantage HMO plans than in private-fee-for-service plans; however, patient financial incentives had limited influence on generic use.
Beneficiaries who switched to generics had lower out-of-pocket spending and higher levels of drug adherence compared with beneficiaries who remained on brand SGA therapy.
Acknowledgments
Source of Funding: The National Institute of Mental Health (R01MH090284) provided funding for this study.
Dr Fung received a grant from NIMH, which funded this study. Dr Newhouse is on the board of Aetna, which sells Part D plans, and also owns Aetna stock.
Footnotes
This is the pre-publication version of a manuscript that has been accepted for publication in The American Journal of Managed Care (AJMC). This version does not include post-acceptance editing and formatting. The editors and publisher of AJMC are not responsible for the content or presentation of the prepublication version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to it (eg, correspondence, corrections, editorials, etc) should go to www.ajmc.com or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Author Disclosures: Dr Nierenberg’s disclosures/conflicts of interest are available as eAppendix 2. The remaining authors report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (VF, ABB, JH, AAN, MBL); acquisition of data (VF, MP, JH); analysis and interpretation of data (VF, JPN, MP, ABB, BF, JH, MBL); drafting of the manuscript (VF, AAN); critical revision of the manuscript for important intellectual content (VF, JPN, ABB, BF, JH, AAN, MBL); statistical analysis (VF, JPN, MP, BF, MBL); provision of patients or study materials (VF, JH); obtaining funding (VF); administrative, technical, or logistic support (VF, MP); and supervision (VF).
REFERENCES
- 1.A Data Book: Healthcare Spending and the Medicare Program. Washington, DC: Medicare Payment Advisory Commission; 2011. [Google Scholar]
- 2.The Congressional Budget Office website; [Accessed Sept 1, 2014]. Effects of using generic drugs on Medicare’s prescription drug spending [pub No. 4043] https://www.cbo.gov/sites/default/files/cbofiles/ftpdocs/118xx/doc11838/09-15-prescriptiondrugs.pdf. Published September 2010. [Google Scholar]
- 3.Roman B. Patients’ attitudes towards generic substitution of oral atypical antipsychotics. CNS Drugs. 2009;23(8):693–701. doi: 10.2165/00023210-200923080-00006. [DOI] [PubMed] [Google Scholar]
- 4.Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578–1592. doi: 10.1016/s0149-2918(03)80157-1. [DOI] [PubMed] [Google Scholar]
- 5.Desmarais JE, Beauclair L, Margolese HC. Switching from brand-name to generic psychotropic medications: a literature review. CNS Neurosci Ther. 2011;17(6):750–760. doi: 10.1111/j.1755-5949.2010.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margolese HC, Wolf Y, Desmarais JE, Beauclair L. Loss of response after switching from brand name to generic formulations: three cases and a discussion of key clinical considerations when switching. Int Clin Psychopharmacol. 2010;25(3):180–182. doi: 10.1097/YIC.0b013e328337910b. [DOI] [PubMed] [Google Scholar]
- 7.Mofsen R, Balter J. Case reports of the reemergence of psychotic symptoms after conversion from brand-name clozapine to a generic formulation. Clin Ther. 2001;23(10):1720–1731. doi: 10.1016/s0149-2918(01)80139-9. [DOI] [PubMed] [Google Scholar]
- 8.Leonhauser M. Antipsychotics: multiple indications help drive growth. IMS Health website; [Accessed March 21, 2014]. http://www.imshealth.com/ims/Global/Content/Corporate/PressRoom/IMSintheNews/Documents/PM360_IMS_Antipsychotics_0112.pdf. Published January 2012. [Google Scholar]
- 9.Lenderts S, Kalali AH, Buckley P. Generic penetration in the retail atypical antipsychotic market. Psychiatry (Edgmont) 2010;7(3):9–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Meredith P. Bioequivalence and other unresolved issues in generic drug substitution. Clin Ther. 2003;25(11):2875–2890. doi: 10.1016/s0149-2918(03)80340-5. [DOI] [PubMed] [Google Scholar]
- 11.Treur M, Heeg B, Möller HJ, Schmeding A, van Hout B. A pharmaco-economic analysis of patients with schizophrenia switching to generic risperidone involving a possible compliance loss. BMC Health Serv Res. 2009;9:32. doi: 10.1186/1472-6963-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung V, Price M, Busch AB, et al. Adverse clinical events among Medicare beneficiaries using antipsychotic drugs: linking health insurance benefits and clinical needs. Med Care. 2013;51(7):614–621. doi: 10.1097/MLR.0b013e31829019c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boccuti C. Paying a visit to the doctor: current financial protections for Medicare patients when receiving physician services. Kaiser Family Foundation website; [Accessed April 17, 2014]. http://kff.org/medicare/issue-brief/paying-a-visit-to-the-doctor-current-financial-protections-for-medicare-patients-when-receiving-physician-services/. Published April 7, 2014. [Google Scholar]
- 14.Medi-Span Electronic Drug File (MED-File) v2. Wolters Kluwer website; [Accessed Oct 26, 2015]. http://www.wolterskluwercdi.com/medi-span-electronic-drug-file/ [Google Scholar]
- 15.U.S. Food and Drug Administration website; [Accessed Jul 15, 2014]. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. http://www.accessdata.fda.gov/scripts/cder/ob/. Last updated May 17, 2013. [Google Scholar]
- 16.Medicare Program. Contract Year 2015 Policy and Technical Changes to the Medicare Advantage and the Medicare Prescription Drug Benefit Programs; Proposed Rule. [Accessed: Oct 26, 2015];Federal Register. 79(7) http://www.gpo.gov/fdsys/pkg/FR-2014-01-10/pdf/2013-31497.pdf. Published: Jan 10, 2014. [PubMed] [Google Scholar]
- 17.Gold M, Jacobson G, Damico A, Neuman T. Medicare Advantage 2014 spotlight: plan availability and premiums. Kaiser Family Foundation website; [Accessed August 1, 2014]. http://kff.org/medicare/issue-brief/medicare-advantage-2014-spotlight-plan-availability-and-premiums/. Published November 25, 2013. [Google Scholar]
- 18.Frakt AB, Pizer SD, Feldman R. Payment reduction and Medicare private fee-for-service plans. Health Care Financing Review. 2009;30(3):15–24. [PMC free article] [PubMed] [Google Scholar]
- 19.Hoadley J, Cubanski J, Neuman T. Medicare Part D at Ten Years: The 2015 Marketplace and Key Trends, 2006–2015. The Kaiser Family Foundation; [Accessed: Oct 26, 2015]. http://kff.org/medicare/report/medicare-part-d-at-ten-years-the-2015-marketplace-and-key-trends-2006–2015/. Published Oct 5, 2015. [Google Scholar]
- 20.Huskamp HA, Deverka PA, Epstein AM, Epstein RS, McGuigan KA, Frank RG. The effect of incentive-based formularies on prescription-drug utilization and spending. N Engl J Med. 2003;349(23):2224–2232. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- 21.Kamal-Bahl S, Briesacher B. How do incentive-based formularies influence drug selection and spending for hypertension? Health Aff (Millwood) 2004;23(1):227–236. doi: 10.1377/hlthaff.23.1.227. [DOI] [PubMed] [Google Scholar]
- 22.Rector TS, Finch MD, Danzon PM, Pauly MV, Manda BS. Effect of tiered prescription copayments on the use of preferred brand medications. Med Care. 2003;41(3):398–406. doi: 10.1097/01.MLR.0000053022.47132.82. [DOI] [PubMed] [Google Scholar]
- 23.Hodgkin D, Parks Thomas C, Simoni-Wastila L, Ritter GA, Lee S. The effect of a three-tier formulary on antidepressant utilization and expenditures. J Ment Health Policy Econ. 2008;11(2):67–77. [PubMed] [Google Scholar]
- 24.Huskamp HA, Deverka PA, Epstein AM, et al. Impact of 3-tier formularies on drug treatment of attention-deficit/hyperactivity disorder in children. Arch Gen Psychiatry. 2005;62(4):435–441. doi: 10.1001/archpsyc.62.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdier JM, Zlatinov A. Trends and Patterns in the Use of Prescription Drugs Among Medicaid Beneficiaries: 1999 to 2009. [Accessed Aug 5, 2014];Medicaid Policy Brief. Brief 17.: Mathematica Policy Research. https://www.cms.gov/research-statistics-data-and-systems/computer-data-and-systems/medicaiddatasourcesgeninfo/downloads/max_ib17_rx.pdf. Published March 2013.
- 26.Slade EP, Simoni-Wastila L. Forecasting Medicaid Expenditures for Antipsychotic Medications. Psych Services. 2015;66(7):713–718. doi: 10.1176/appi.ps.201400042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.