Abstract
Caffeine has been proposed to have several beneficial effects on obesity and its related metabolic diseases; however, how caffeine affects adipocyte differentiation has not been elucidated. In this study, we demonstrated that caffeine suppressed 3T3-L1 adipocyte differentiation and inhibited the expression of CCAAT/enhancer binding protein (C/EBP)α and peroxisome proliferator-activated receptor (PPAR)γ, two main adipogenic transcription factors. Anti-adipogenic markers, such as preadipocyte secreted factor (Pref)-1 and Krüppel-like factor 2, remained to be expressed in the presence of caffeine. Furthermore, 3T3-L1 cells failed to undergo typical mitotic clonal expansion in the presence of caffeine. Investigation of hormonal signaling revealed that caffeine inhibited the activation of AKT and glycogen synthase kinase (GSK) 3 in a dose-dependent manner, but not extracellular signal-regulated kinase (ERK). Our data show that caffeine is an anti-adipogenic bioactive compound involved in the modulation of mitotic clonal expansion during adipocyte differentiation through the AKT/GSK3 pathway. [BMB Reports 2016; 49(2): 111-115]
Keywords: Adipogenesis, AKT, Caffeine, Glycogen synthase kinase, Mitotic clonal expansion
INTRODUCTION
Obesity is a strong risk factor for metabolic disorders including hypertension, dyslipidemia, cardiovascular disease, and type 2 diabetes, and has become a worldwide public health problem (1, 2). Obesity is associated with an excessive growth and expansion of adipose tissue mass through increases in both the number and size of fat cells (3). Therefore, many studies have been performed to identify critical processes for either reducing lipid accumulation or inhibiting adipocyte formation to limit obesity and its metabolic consequences.
3T3-L1 is a well-established preadipocyte cell line for the study of adipogenesis (4). Upon a hormonal stimulation with 1-methyl 3-isobutylxanthine, dexamethasone, and insulin, growth-arrested 3T3-L1 preadipocytes synchronously reenter the cell cycle, known as mitotic clonal expansion, and then express adipocyte-specific genes for terminal differentiation (5, 6). Adipogenic differentiation is regulated by a cascade of several transcription factors including CCAAT/enhancer-binding protein (C/EBP) family members and peroxisome proliferator-activated receptor (PPAR)γ (7, 8). During mitotic clonal expansion, both C/EBPβ and C/EBPδ are temporally expressed and regulate cell proliferation (9, 10). This process eventually leads to the expression of C/EBα and PPARγ, two pleiotropic transcriptional activators that coordinately induce terminal differentiation to a mature adipocyte phenotype by regulating adipocyte-specific genes. The transition from mitotic clonal expansion to terminal differentiation involves several mechanisms, including the phosphorylation (11, 12), dimerization (13), and reactive oxygen species (ROS)-mediated activation (5) of C/EBPβ. Also, factors regulating the adipogenic process, either positively or negatively, have been identified such as preadipocyte secreted factor (Pref)-1 (14) and Krüppel-like factors (15-17).
Caffeine (1,3,7-trimethylxanthine) is a plant alkaloid found in coffee, chocolate, and tea, widely consumed by humans worldwide. Several studies have shown that caffeine reduces body weight and adipose tissue weight in animal models (18). Moreover, caffeine has a variety of effects on metabolism; it increases energy expenditure by inhibiting the phosphodiesterase-induced degradation of intracellular cAMP, results in reduced food intake, alters intracellular calcium levels, increases cAMP-dependent protein kinase (PKA), and inhibits phosphatidylinositol-3-kinase (PI3K)/AKT activity (19). It has recently been demonstrated that caffeine suppresses intracellular lipid accumulation (20, 21), inhibits insulin-stimulated glucose uptake, and increases lipolysis in adipose cells (22). These results indicate that caffeine plays a role in reducing lipid accumulation; however, how caffeine affects adipogenesis has not been clearly elucidated.
In this study, we show that caffeine inhibits the expression of C/EBPβ, C/EBPα, and PPARγ during 3T3-L1 preadipocyte differentiation. Caffeine exerted its inhibitory effects on adipocyte differentiation by regulating the expression of G1-S phase-specific cell cycle markers, thereby blocking mitotic clonal expansion. Our results demonstrate that caffeine has an anti-adipogenic effect on 3T3-L1 preadipocytes that involves the AKT/glycogen synthase kinase (GSK) 3β pathway.
RESULTS
Caffeine inhibits 3T3-L1 differentiation in a dose-dependent manner
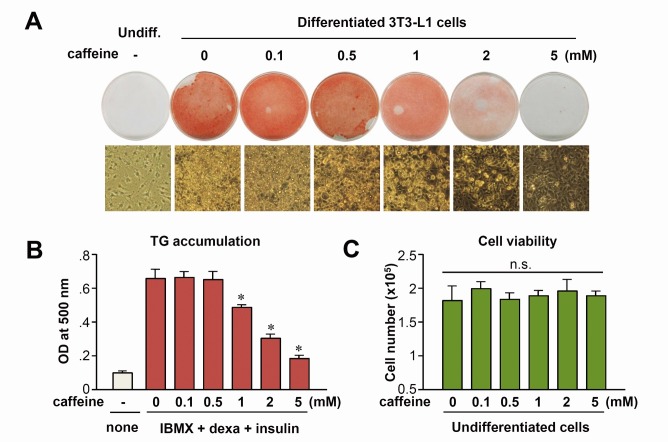
To elucidate the effects of caffeine and its underlying molecular mechanisms, we first tested the effect of caffeine on adipocyte differentiation in 3T3-L1 cells. 3T3-L1 cells were incubated in adipogenic differentiation medium in the presence of increasing concentrations of caffeine for 8 days, resulting in a dose-dependent inhibition of adipocyte differentiation; at 5 mM of caffeine, differentiation was almost completely suppressed (Fig. 1A). To quantify the magnitude of adipocyte differentiation, oil-red-O-stained cells were extracted in isopropyl alcohol and measured at 500 nm using a spectrophotometer. Consistent with the results of microscopic examination, the amount of oil-red-O stain eluted from cells was significantly decreased in samples treated with >1 mM caffeine (Fig. 1B). As shown in Fig. 1C, caffeine did not significantly affect cell viability at concentrations up to 5 mM. Thus, caffeine was used in a range of non-cytotoxic concentrations (0.1 to 5 mM) in subsequent experiments.
Fig. 1. Caffeine inhibits adipocyte differentiation in a dose-dependent manner. (A) Two-day post-confluent 3T3-L1 preadipocytes incubated with the hormonal inducers (IBMX, dexamethasone, and insulin) in the absence or presence of caffeine. After 2 days, all groups were fed with FBS, DMEM, and insulin for another 2 days and then with FBS and DMEM. After 8 days of differentiation, lipid accumulation was detected by oil-red-O staining. Representative photomicrographs (×400) are shown for each treatment group. (B) Quantitative analysis of adipocyte differentiation, assessed by spectrophotometric measurement of oil-red-O-stained adipocytes. *P < 0.01 vs. 0 mM caffeine group. (C) Cell count was performed in 3T3-L1 preadipocytes in which cells were incubated with the indicated concentration of caffeine for 48 h in growth medium in the absence of MDI. n.s., not significant.
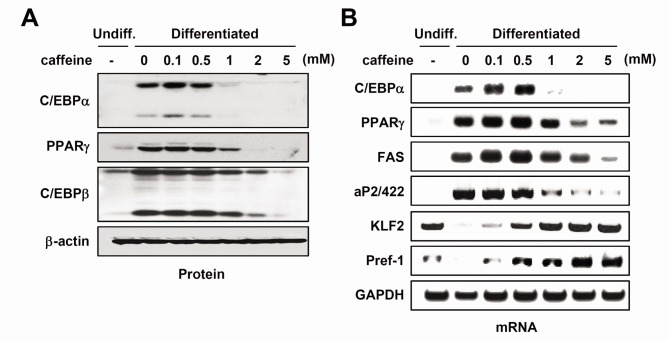
Adipogenesis, the process by which adipocyte precursors develop into mature adipocytes, is regulated by a group of specific transcription factors such as PPARγ and C/EBPα (4). To confirm whether diminished accumulation of triglyceride after caffeine exposure was accompanied by a change in protein expression for adipocyte-specific genes, whole cell lysates were obtained and subjected to western blot analysis. Our results showed that the protein expression of C/EBPβ, C/EBPα, and PPARγ were decreased after caffeine treatment in a dose-dependent manner (Fig. 2A). The mRNA expression of adipocytespecific genes, such as aP2/422 and FAS, was also diminished. In contrast, inhibitory factors of adipogenesis remained high (KLF2 and Pref-1) with comparable treatment (Fig. 2B). These results suggest that caffeine inhibits adipogenic differentiation accompanied by the suppression of C/EBPα and PPARγ expression. Interestingly, C/EBPβ expression was not affected by 1 mM caffeine treatment, at which concentration the expression of C/EBPα, PPARγ, aP2/422, KLF2, and Pref-1 was affected, suggesting that C/EBPβ expression is not a primary target for caffeine.
Fig. 2. The effect of caffeine on the expression of adipogenic marker genes. Two-day post-confluent 3T3-L1 preadipocytes were induced to differentiate with IBMX, dexamethasone, and insulin in the absence or presence of indicated concentrations of caffeine. (A) Cell lysates were obtained after 48 h of induction and subjected to Western blot analyses using specific antibodies against each protein. Immunoblots are a representative image of three independent experiments. (B) mRNA expression was analyzed using RT-PCR.
Caffeine inhibits the mitotic clonal expansion process in 3T3-L1 preadipocytes
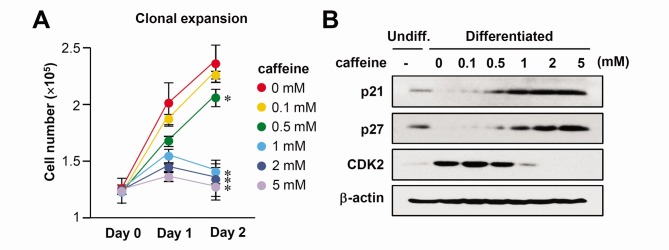
To examine the role of caffeine in adipogenesis, we assessed the effect of caffeine on the proliferation of 3T3-L1 preadipocytes stimulated by the hormonal inducers of IBMX, dexamethasone, and insulin, designated as MDI. When 2 day post-confluent cells are incubated in a medium containing MDI, the cell number increases over time for up to 2 days, because growth-arrested 3T3-L1 cells reenter the cell cycle and undergo approximately two rounds of cell division, called mitotic clonal expansion. The treatment of cells with 0.1 and 0.5 mM caffeine resulted in no change of mitotic clonal expansion but caffeine concentrations of 1, 2, and 5 mM significantly abolished the increase in cell numbers at day 2 compared with MDI alone (Fig. 3A).
Fig. 3. The effect of caffeine on the mitotic clonal expansion process of adipocyte differentiation. Two-day post-confluent 3T3-L1 cells were incubated with the hormonal inducers in the absence or presence of various concentrations of caffeine. (A) Cell numbers were counted at the indicated time points after initiation of MDI-induced differentiation. Cells were trypsinized, aliquots were taken in triplicate, and then cells were counted using an ADAM cell counter. *P < 0.01 vs. 0 mM caffeine group. (B) The protein levels of p21, p27, and cdk2 in these cells during differentiation (Day 2) were detected by immunoblot assay using specific antibodies. β-actin was used as a loading control.
As previously reported (5), mitotic clonal expansion results in the detection of dividing cells (G2/M) after 24 h of induction. To examine how cell cycle events during mitotic clonal expansion are affected by caffeine, cells treated with various concentrations of caffeine were subjected to flow cytometry. While the cells with MDI synchronously entered S phase after 16 h of induction, cells with MDI plus caffeine clearly showed a decreased S phase population (Supplementary Fig. 1), indicating that caffeine blocks mitotic clonal expansion. We next examined whether caffeine altered the expression of cell cycle regulatory proteins. Caffeine treatment increased the expression of p21 and p27, the major regulators arresting cell cycle progression at the G1/S checkpoint, whereas CDK2, the essential factor for G1/S transition, was downregulated (Fig. 3B). These results suggest that caffeine disturbed the mitotic clonal expansion at the level of G1/S transition of the cell cycle.
In order to confirm the caffeine effect on adipogenesis, another preadipogenic cell line, 3T3-F442A cells, were tested. As shown in Supplementary Fig. 2, 3T3-F442A cells also showed similar results upon caffeine treatment, evident by oil-red-O staining, the expression of adipogenic markers, cell count, and FACS analysis.
Caffeine inhibits AKT/GSK3β signaling in differentiating 3T3-L1 preadipocytes
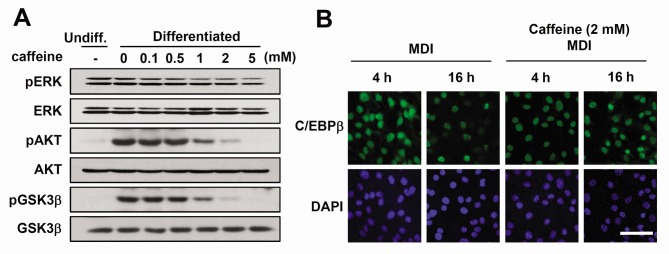
Hormonal inducers, IBMX, dexamethasone, and insulin trigger the activation of several signaling pathways during adipogenesis (4). Among them, insulin or insulin-like growth factor (IGF)-I activates ERK and/or AKT signaling for the initial progression of adipogenesis, including mitotic clonal expansion (23, 24). Because caffeine inhibited the mitotic clonal expansion and adipocyte differentiation, we investigated whether caffeine affects these signaling pathways. When caffeine was added to differentiating 3T3-L1 cells, the phosphorylation of AKT and its downstream signal GSK3β (25) was reduced (Fig. 4A). However, ERK was not significantly affected by caffeine treatment (Fig. 4A). These results suggest that caffeine inhibits the AKT/GSK3β-mediated signaling pathway stimulated by MDI.
Fig. 4. The effect of caffeine on signaling molecules involved in mitotic regulation. Two-day post-confluent 3T3-L1 cells were incubated with the hormonal inducers in the presence or absence of various concentrations of caffeine for 48 h. (A) Total cell lysates were prepared and analyzed by Western blot using the indicated phospho-specific antibodies. Western blot analysis of each total protein was done in parallel. Immunoblots are a representative image of three independent experiments. (B) Immunofluorescence analysis of C/EBPβ. 3T3-L1 preadipocytes were induced to differentiate. After 4 h or 16 h, cells were fixed and subjected to immunofluorescence analysis with antibody against C/EBPβ and 4',6-diamidino-2-phenylindole. Fluorescence images were obtained by confocal microscopy. Scale bar = 50 μm.
While the expression of C/EBPβ remains unchanged (Fig. 2A), it is possible that the activation process of C/EBPβ might be affected by caffeine. C/EBPβ activation involves a serial event resulting in the characteristic "punctate" pattern at 16 h of differentiation in immunofluorescent staining (Fig. 4B), which was attenuated by the treatment of caffeine. This suggests that caffeine negatively affects the C/EBPβ activation process, as well as AKT signaling pathway.
DISCUSSION
The use of an anti-adipogenic agent derived from natural sources could be helpful in the prevention of obesity. Blocking of adipocyte differentiation is one of the anti-obesity strategies that fall under the category of modulating fat storage. In recent years, many groups have explored novel compounds derived from natural resources for their effect on adipogenic differentiation and gene regulation, often associated with obesity. For example, caffeine intake decreased diet-induced insulin resistance (20), inhibited lipid accumulation, and stimulated breakdown of accumulated triglyceride in adipocytes (21). Among them, a study by Nakayabashi et al. (21) concluded that 0.5 mM caffeine had no obvious effect on adipocyte differentiation per se; however, in our study, we identified caffeine (>1 mM) as an effective anti-adipogenic compound in cultured 3T3-L1 preadipocytes, preventing the adipocyte differentiation process by showing more comprehensive experiments; (1) oil-red-O staining, (2) differentiation markers including the negative regulators such as KLF2 and Pref-1, and (3) mitotic clonal expansion by cell count, FACS analysis, and the expression of p21/p27.
The mechanism of action of anti-adipogenic agents should be carefully assessed, and importantly, studies should determine the stage of adipogenesis at which such agents are active. Adipogenesis requires a network of transcription factors and signaling pathways, which contribute to the serial gene expression for adipocyte differentiation and maturation. C/EBPβ is expressed immediately after exposure to adipogenic inducers, whereas PPARα and C/EBPγ expression are acquired 36-48 h after exposure. In this regard, it should be noted that 1 mM caffeine did not affect C/EBPβ expression but repressed C/EBPα and PPARγ (Fig. 2A) expression. This suggests that caffeine works as an early adipogenic inhibitor, likely acting between C/EBPβ expression and C/EBPα/PPARγ expression. C/EBPβ requires an activation process after its expression; first C/EBPβ is sequentially phosphorylated (11), resulting in dimerization of the phosphorylated C/EBPβ, enabling it to bind its DNA binding element with the help of reactive oxygen species (5, 13). Therefore, in the presence of caffeine, C/EBPβ was expressed normally but might not be activated. This was proven by immunofluorescent study showing that caffeine attenuates the punctate pattern of C/EBPβ binding (Fig. 4B). Inhibitory factors of adipogenesis remained high with comparable treatment (Fig. 2B), also indicating that caffeine blocks an early adipogenic event. Consistently, caffeine inhibited the typical mitotic clonal expansion in our experiments (Fig. 3). We demonstrated that caffeine blocked the cell cycle at the G1/S transition and cell cycle protein analysis also supported G1 arrest by caffeine. Thus, our conclusion is that caffeine inhibits preadipocyte growth and differentiation at the initial stage, including C/EBPβ activation and mitotic clonal expansion of preadipocytes.
AKT mediates the signaling pathway of insulin or IGF-I in adipogensis, and in mature adipocytes, the activation of AKT is required for insulin-induced glucose transport as well as energy metabolism (24). Our results show that caffeine dose-dependently reduces the activation of AKT in 3T3-L1 adipocytes. It was reported that the involvement of AKT in the insulin or IGF-I signaling pathway is partly by phosphorylating GSK3β protein (26). Our data indicate that the treatment of caffeine decreases the phosphorylation of GSK3β in 3T3-L1 cells, suggesting that the AKT/GSK3β axis required for adipogenesis is disturbed by caffeine treatment. It is also possible that AKT-dependent inactivation of the Forkhead transcription factor (FKHR), which is essential for the progression of adipogenesis (27), is attenuated by caffeine. Taken together, our results revealed that caffeine-mediated suppression of adipogenesis is caused by attenuation of AKT and GSK3β phosphorylation resulting in decreased levels of C/EBPα and PPARγ.
Caffeine-inhibited adipocyte differentiation was recently observed in mesenchymal stem cells (28), but the detailed molecular basis of this inhibition was not determined. Our results demonstrate that caffeine significantly suppresses the expression of adipogenic genes, thereby reducing lipid storage and accumulation in 3T3-L1 adipocytes. It should be noted that the concentration of caffeine used in this study is far beyond a physiologic range. The plasma concentration of caffeine was reported to be 0.54-1.94 mg/L in human consuming 0-3 cups of coffee a day (29). However, higher concentration of caffeine (>1 mM) has been used in cell studies in vitro (30-32), as in the case of many other chemicals, hormones, and growth factors. For example, we use more than hundred folds of physiologic concentration of insulin for detecting glucose uptake, inducing differentiation, or observing an activation of signaling pathway. Thus, it is rather relevant to investigate the caffeine effect in vivo, fully considering a dose and duration of exposure together than to simply conclude it is only effective in high doses in cell culture system.
MATERIALS AND METHODS
For full details of all materials and methods, please refer to Supplementary Information
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grants 2011-0030086, 2015R1A2A2A01004345, and 2013R1A1A1059527, funded by the Korea government, Ministry of Science, ICT and Future Planning (MSIP).
References
- 1.Friedman JM. Modern science versus the stigma of obesity. Nat Med. (2004);10:563–569. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. (2000);404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. (2008);453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 4.Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. (1994);14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 5.Lee H, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem. (2009);284:10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. (2005);40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- 7.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. (2006);4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. (1994);79:1147–1156. doi: 10.1016/0092-8674(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 9.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. (1991);5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 10.Lane MD, Tang QQ, Jiang MS. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun. (1999);266:677–683. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- 11.Tang QQ, Gronborg M, Huang H, et al. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci U S A. (2005);102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang QQ, Lane MD. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. (1999);13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JW, Tang QQ, Li X, Lane MD. Effect of phosphorylation and S-S bond-induced dimerization on DNA binding and transcriptional activation by C/EBPbeta. Proc Natl Acad Sci U S A. (2007);104:1800–1804. doi: 10.1073/pnas.0611137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smas CM, Sul HS. Pref-1, a Protein Containing Egf-Like Repeats, Inhibits Adipocyte Differentiation. Cell. (1993);73:725–734. doi: 10.1016/0092-8674(93)90252-L. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee SS, Feinberg MW, Watanabe M, et al. The Kruppel-like factor KLF2 inhibits peroxisome proliferatoractivated receptor-gamma expression and adipogenesis. J Biol Chem. (2003);278:2581–2584. doi: 10.1074/jbc.M210859200. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Kim HJ, Lee YJ, Lee MY, Choi H, Kim JW. Kruppel-like factor KLF8 plays a critical role in adipocyte differentiation. PLoS One. (2012);7:e52474. doi: 10.1371/journal.pone.0052474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oishi Y, Manabe I, Tobe K, et al. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. (2005);1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. (2002);40:1243–1255. doi: 10.1016/S0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. (2006);5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conde SV, da Silva TN, Gonzalez C, Carmo MM, Monteiro EC, Guarino MP. Chronic caffeine intake decreases circulating catecholamines and prevents diet-induced insulin resistance and hypertension in rats. Brit J Nutr. (2012);107:86–95. doi: 10.1017/S0007114511002406. [DOI] [PubMed] [Google Scholar]
- 21.Nakabayashi H, Hashimoto T, Ashida H, Nishiumi S, Kanazawa K. Inhibitory effects of caffeine and its metabolites on intracellular lipid accumulation in murine 3T3-L1 adipocytes. Biofactors. (2008);34:293–302. doi: 10.1002/biof.5520340405. [DOI] [PubMed] [Google Scholar]
- 22.Akiba T, Yaguchi K, Tsutsumi K, et al. Inhibitory mechanism of caffeine on insulin-stimulated glucose uptake in adipose cells. Biochem Pharmacol. (2004);68:1929–1937. doi: 10.1016/j.bcp.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 23.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci U S A. (2003);100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu JF, Liao K. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem. (2004);279:35914–95922. doi: 10.1074/jbc.M402297200. [DOI] [PubMed] [Google Scholar]
- 25.Sutherland C, Leighton IA, Cohen P. Inactivation of Glycogen-Synthase Kinase-3-Beta by Phosphorylation - New Kinase Connections in Insulin and Growth-Factor Signaling. Biochem J. (1993);296:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross SE, Erickson RL, Hemati N, MacDougald OA. Glycogen synthase kinase 3 is an insulin-regulated C/EBP alpha kinase. Mol Cell Biol. (1999);19:8433–8441. doi: 10.1128/MCB.19.12.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. (2003);4:119–129. doi: 10.1016/S1534-5807(02)00401-X. [DOI] [PubMed] [Google Scholar]
- 28.Su SH, Shyu HW, Yeh YT, Chen KM, Yeh H, Su SJ. Caffeine inhibits adipogenic differentiation of primary adipose-derived stem cells and bone marrow stromal cells. Toxicol in Vitro. (2013);27:1830–1837. doi: 10.1016/j.tiv.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 29.de Leon J, Diaz FJ, Rogers T, et al. A pilot study of plasma caffeine concentration in a US sample of smoker and nonsmoker volunteers. Prog Neuropshychopharmacol Biol Psychiatry. (2003);27:165–171. doi: 10.1016/S0278-5846(02)00348-2. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto T, He Z, Ma WY, et al. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res. (2004);64:3344–3349. doi: 10.1158/0008-5472.CAN-03-3453. [DOI] [PubMed] [Google Scholar]
- 31.Okano J, Nagahara T, Matsumoto K, Murawaki Y. Caffeine inhibits the proliferation of liver cancer cells and activates the MEK/ERK/EGFR signalling pathway. Basic Clin Pharmacol Toxicol. (2008);102:543–551. doi: 10.1111/j.1742-7843.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 32.Sinchai T, Plasen S, Sanvarinda Y, et al. Caffeine potentiates methamphetamineinduced toxicity both in vitro and in vivo. Neurosci Lett. (2011);502:65–69. doi: 10.1016/j.neulet.2011.07.026. [DOI] [PubMed] [Google Scholar]