Abstract
Microtubule nucleation within cells is catalyzed by γ-tubulin ring complexes localized at specific microtubule-organizing centers. In this issue, Muroyama et al. (2016. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201601099) reveal heterogeneity in the composition and function of these complexes, with wide implications for how cells organize their microtubule arrays.
Microtubules are filamentous polymers assembled from α/β-tubulin dimers that can grow and shrink rapidly or remain stable. Microtubules are absolutely essential for cell viability, helping to form structures involved in cell division, cell polarity and motility, cell-to-cell signaling, and intracellular transport. They can polymerize spontaneously in vitro, but within cells microtubule polymerization requires the catalytic activity of other proteins and protein complexes. The best studied of these catalysts is the γ-tubulin ring complex (γ-TuRC), a large ∼2.1-megadalton protein complex that is recruited to various microtubule-organizing centers (MTOCs), such as the centrosome. As its name suggests, the γ-TuRC forms a helical ring-like structure with a diameter and pitch that closely matches that of a microtubule (Kollman et al., 2010). γ-Tubulin is the most abundant protein within the γ-TuRC and forms direct interactions with α-tubulin at the base of the microtubule. It is now widely accepted that γ-TuRCs provide an end-on template to catalyze microtubule nucleation (Fig. 1; Lin et al., 2014; Oakley et al., 2015; Petry and Vale, 2015).
Figure 1.
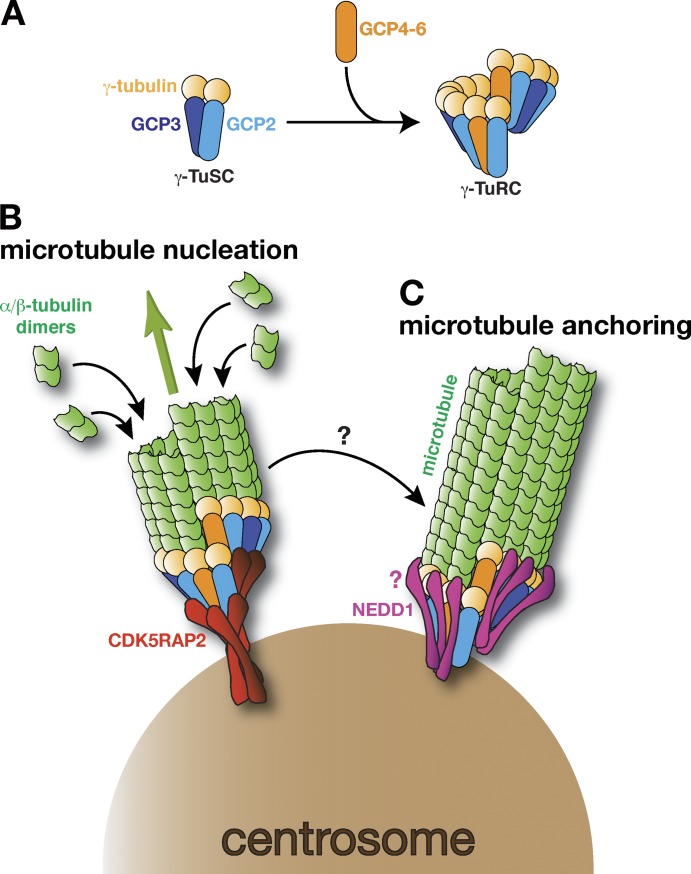
γ-TuRCs bound to different tethering proteins have different functions in mouse keratinocytes. (A) A schematic of a γ-TuSC comprising γ-tubulin, GCP2, and GCP3, and the larger γ-TuRC, which includes GCP4–6. During γ-TuRC formation, γ-TuSCs associate laterally into helical ring structures and it is thought that some of the GCP2/3 molecules are replaced with GCP4–6. How other non-GCPs associate with γ-TuRCs is not known and is not depicted here. (B) A schematic of a γ-TuRC bound by CDK5RAP2 (red), which is catalyzing (green arrow) the formation of a new microtubule (green) from the centrosome (brown). This process is called microtubule nucleation. (C) A schematic of a γ-TuRC bound by NEDD1 (pink), which is anchoring a microtubule at the centrosome. It is likely that the CM1 domain located in the N terminus of CDK5RAP2 binds directly to the GCP ring, but how NEDD1 binds to the γ-TuRC and how this allows the γ-TuRC to remain associated with (i.e., anchor) the microtubule remains unclear (denoted by the pink question mark). Whether a microtubule nucleated by a CDK5RAP2-bound γ-TuRC is transferred to a NEDD1-bound γ-TuRC also remains unclear (as denoted by the black arrow with a question mark).
Two categories of γ-tubulin complexes exist: a γ-tubulin small complex (γ-TuSC), comprizing two molecules of γ-tubulin and one each of γ-tubulin complex protein 2 (GCP2) and GCP3; and the larger γ-TuRC, estimated to contain up to six or seven other core proteins (Teixidó-Travesa et al., 2010; Fig. 1 A). Both complexes are found in the cytosol of most eukaryotic cells, but only γ-TuRCs are potent microtubule nucleators (Oegema et al., 1999). This finding was for many years at odds with the knowledge that budding yeast cells contain only γ-TuSCs but can nucleate microtubules perfectly well. Seminal work from Kollman et al. (2010), however, showed that yeast γ-TuSCs are driven to form γ-TuRCs after binding to an anchoring protein concentrated at the yeast MTOC. Using electron microscopy, they generated a detailed density map of the γ-TuRC showing that the γ-tubulin molecules were almost in the correct position to directly contact the base of a microtubule. When a hinge region in GCP3 was artificially moved, the γ-tubulin molecules were brought into correct alignment and the in vitro nucleating ability of the γ-TuRC was greatly enhanced (Kollman et al., 2015). These studies revealed how γ-TuRC activity might be regulated and provided a structural framework for the γ-TuRC likely to be conserved among different species. In support of this conservation, the crystal structure of human GCP4 (not found in budding yeast) can be spatially mapped into the yeast γ-TuRC density map (Guillet et al., 2011). GCP4, 5, and 6 are structural homologues of GCP2 and 3, and so this finding suggests that, when present, molecules of GCP4–6 likely replace some molecules of GCP2/3 within the helical ring (consistent with stoichiometric measurements of the γ-TuRC [Murphy et al., 2001; Choi et al., 2010] and with the finding that GCP4 can bind directly to γ-tubulin [Guillet et al., 2011]). Depletion of GCP4, 5, or 6 reduces the levels of cytosolic γ-TuRCs and the recruitment of γ-TuRCs to certain MTOCs, but does not hinder the viability of organisms such as Drosophila melanogaster, fission yeast, or Aspergillus nidulans (Teixidó-Travesa et al., 2012). Thus, the formation of functional γ-TuRCs does not necessarily require the additional γ-TuRC proteins found in higher eukaryotes, suggesting that these extra proteins might provide specificity to γ-TuRC assembly, localization, or activity in organisms and cell types that contain multiple MTOCs.
Despite these recent advances in our understanding of γ-TuRC structure, it remains unclear whether these complexes are heterogeneous in structure. Differences between species must exist, as not all species contain all known γ-TuRC components (Teixidó-Travesa et al., 2012), but what about within species? There is limited direct evidence but a previous study found that γ-TuRCs purified from human cells using a fragment of the γ-TuRC anchoring protein CDK5RAP2 (discussed in more detail below) lack certain known γ-TuRC components (Choi et al., 2010). There is also indirect evidence of heterogeneity, as the depletion of different γ-TuRC proteins can have different phenotypic effects. For example, only certain γ-TuRC proteins are required for oocyte polarization in Drosophila (Vogt et al., 2006; Reschen et al., 2012). Nevertheless, no single study has directly addressed γ-TuRC heterogeneity, until now.
In this issue, Muroyama et al. demonstrate that γ-TuRCs can differ in both composition and function. They identified a fraction of γ-TuRCs in mouse keratinocytes that function to nucleate microtubules, while a separate fraction functioned to anchor microtubules. These functional differences resulted from the complex associating with different proteins: γ-TuRCs bound to a protein called CDK5RAP2 nucleate microtubules (Fig. 1 B), whereas γ-TuRCs bound to a protein called NEDD1 (also called GCP-WD) anchor microtubules (Fig. 1 C). Whether or not these differences are specific to mouse keratinocytes is not clear, but the results highlight the importance of not simply grouping γ-TuRCs into a single category, even within the same cell type.
Muroyama et al. (2016) began by assessing microtubule organization and nucleation at centrosomes from either proliferative or differentiating mouse keratinocytes. Keratinocytes originate from stem cells in the basal layer of the epidermis and then differentiate through several stages until they are shed from the outermost layer of the skin. As keratinocytes differentiate, their centrosomes lose the ability to organize microtubules, allowing noncentrosomal microtubule arrays to form that ultimately help keratinocytes associate to generate a barrier against infection (Sumigray et al., 2012). Muroyama et al. (2016) were interested in the mechanisms that control centrosome inactivation. They found that although centrosomes from proliferative keratinocytes could both nucleate and organize microtubules, centrosomes from differentiated keratinocytes could only nucleate microtubules. Intriguingly, this change in centrosome behavior correlated with changes in centrosome composition: whereas γ-tubulin and NEDD1 were lost rapidly from the centrosome, CDK5RAP2 was lost more slowly.
NEDD1 and CDK5RAP2 are large proteins involved in recruiting γ-TuRCs to MTOCs. NEDD1 copurifies with γ-TuRCs from the cytosol but, unlike GCP4–6, it is not required for γ-TuRC assembly (Haren et al., 2006; Lüders et al., 2006). It is therefore viewed as a more peripheral member of the γ-TuRC, used to tether the complex to MTOCs. CDK5RAP2 contains a centrosomin motif 1 (CM1) domain that is well conserved in proteins involved in γ-TuRC recruitment across species ranging from yeast to humans (Sawin et al., 2004). In contrast to NEDD1, CM1-domain proteins, such as CDK5RAP2, do not readily copurifiy with γ-TuRCs, but instead localize to MTOCs before γ-TuRC binding. Given that the rapid loss of NEDD1 from keratinocyte centrosomes correlated with the loss of centrosomal microtubule organization, Muroyama et al. (2016) speculated that NEDD1 might be specifically responsible for anchoring microtubules at the centrosome.
To test this idea, the authors assessed the effect of knocking down NEDD1 or CDK5RAP2 on centrosomal γ-tubulin recruitment, microtubule nucleation, and microtubule anchoring. Depleting NEDD1 strongly reduced the centrosomal levels of γ-tubulin without affecting the rate of centrosomal microtubule nucleation. Conversely, depleting CDK5RAP2 had little effect on the centrosomal levels of γ-tubulin, but strongly reduced the rate of centrosomal microtubule nucleation. Moreover, even though centrosomes could still nucleate microtubules after NEDD1 depletion, they lost their ability to retain these microtubules. Collectively, these results suggest that most γ-TuRCs are tethered to keratinocyte centrosomes by NEDD1; whereas these NEDD1-associated γ-TuRCs function to anchor microtubules, CDK5RAP2-associated γ-TuRCs function to nucleate microtubules.
To test this hypothesis directly, Muroyama et al. (2016) purified γ-TuRCs from keratinocytes by exogenously expressing GST-tagged fragments of NEDD1 or CDK5RAP2 that contained the known γ-TuRC binding domains (termed GST-NγBD or GST-CγBD, respectively), and then tested the ability of these complexes to nucleate microtubules in vitro. During purification, the GST fragments dissociated from the γ-TuRCs, but this allowed the authors to perform “add-back” experiments. When the purified γ-TuRCs were mixed only with purified tubulin, they produced very few microtubules. Strikingly, adding back the GST-CγBD fragment increased the number of microtubules eightfold, whereas adding back GST-NγBD had no effect. Moreover, the GST-CγBD fragment had the same positive effect when added to GST-NγBD–purified γ-TuRCs, showing that the GST-NγBD–purified γ-TuRCs are not fundamentally incapable of nucleating microtubules and suggesting that the binding of CDK5RAP2 to γ-TuRCs promotes microtubule nucleating activity.
Consistent with NEDD1 and CDK5RAP2 associating with different types of γ-TuRCs, NEDD1 was not present in GST-CγBD–purified complexes and CDK5RAP2 was not present in GST-NγBD–purified complexes. Given that endogenous CDK5RAP2 does not readily copurify with γ-TuRCs, it was perhaps not surprising that CDK5RAP2 was not present in GST-NγBD–purified complexes. More surprising was that NEDD1 was not present in GST-CγBD–purified complexes. This result has been reported previously (Choi et al., 2010) and suggests that either a fraction of cytosolic γ-TuRCs do not contain NEDD1, that endogenous NEDD1 is readily lost during GST-CγBD purification, or that the GST-CγBD fragments bind and catalyze the assembly of γ-TuSCs into γ-TuRCs in the cytosol, with NEDD1 being excluded from these complexes. This latter possibility is reminiscent of MTOCs in budding yeast, where protein fragments containing the CM1 domain bind to γ-TuSCs and catalyze their assembly into γ-TuRCs (Kollman et al., 2010). Either way, the data suggest that the binding of NEDD1 and CDK5RAP2 to γ-TuRCs may be mutually exclusive, although whether they bind to the same region of the γ-TuRC remains to be established.
Muroyama et al. (2016) also tested the function of the different types of γ-TuRCs in vivo. In a clever approach, they artificially targeted γ-TuRCs to the cell cortex by expressing fusions of the NEDD1- or CDK5RAP2–γ-TuRC binding domains to a desmosome-targeting domain (DP-NγBD and DP-CγBD, respectively). Strikingly, although similar levels of γ-tubulin were recruited to the cell cortex in both cases, microtubules were organized and nucleated from the cortex only after expression of DP-CγBD. Even more revealing was the fact that DP-CγBD expression led to the recruitment of endogenous NEDD1 to the cortex and that knockdown of NEDD1 in these cells inhibited cortical microtubule organization, without affecting cortical microtubule nucleation. These results confirm that in keratinocytes CDK5RAP2-bound complexes nucleate microtubules, whereas NEDD1-bound γ-TuRCs anchor microtubules. Whether microtubules nucleated by CDK5RAP2–γ-TuRCs are transferred to NEDD1–γ-TuRCs for anchoring remains unclear, but the recruitment of NEDD1 to cortical CDK5RAP2-bound complexes indicates that CDK5RAP2 and NEDD1 might be in close proximity at MTOCs and this may foster cooperative microtubule organization.
The authors then showed that cell cycle exit, rather than a specific differentiation pathway, drives the observed changes in centrosome composition during differentiation. It remains unclear, however, why NEDD1 is lost before CDK5RAP2. Cellular levels of NEDD1, but not of γ-tubulin or CDK5RAP2, are reduced during keratinocyte differentiation, indicating that the specific loss of NEDD1 from centrosomes might be partly a result of protein degradation. An intriguing possibility is therefore that cell cycle exit initiates the targeted destruction of NEDD1 to drive the loss of centrosomal microtubule-organizing activity without affecting microtubule nucleation, which could be important for the generation of noncentrosomal microtubule arrays.
Muroyama et al. (2016) have, for the first time, revealed the existence of functionally distinct γ-TuRCs. Given that CDK5RAP2 and other CM1 domain proteins normally bind γ-TuRCs only at MTOCs, their data might help explain why freely diffusing γ-TuRCs do not nucleate microtubules in the cytosol, something that would prevent tight spatiotemporal control of microtubule formation. However, previous studies have shown that NEDD1, which is associated with cytosolic γ-TuRCs, is important for microtubule nucleation in U2OS, HeLa, and Arabidopsis thaliana cells (Haren et al., 2006; Lüders et al., 2006; Walia et al., 2014) Thus, specific types of γ-TuRCs appear to function differently in different cell types. The ability of NEDD1-associated γ-TuRCs to nucleate microtubules might be regulated by posttranslational modifications that occur only at MTOCs in specific cell types. Consistent with this, NEDD1 phosphorylation at ser405 is required for microtubule nucleation around the chromatin in HeLa cells, but not for microtubule nucleation from centrosomes (Pinyol et al., 2013). Thus, although the study by Muroyama et al. (2016) is unlikely to have revealed a conserved function for NEDD1 in microtubule anchoring rather than nucleation, it has opened our eyes to the notion that different types of γ-TuRCs exist and have varying functions in different cell types.
It remains important to find out if γ-TuRC heterogeneity is more widespread, both in terms of γ-TuRC composition and how other cell types might use γ-TuRC heterogeneity to generate different microtubule arrays. There is already evidence that a fraction of γ-TuRCs in human embryonic kidney cells does not contain GCP6 (Choi et al., 2010), and it is conceivable that variable GCP composition could help define γ-TuRC function or localization. Although this is speculative, future studies will undoubtedly reveal further γ-TuRC heterogeneity and its role in establishing complex microtubule arrays.
Acknowledgments
I would like to thank Corinne Phillips and Peter Lawrence for critical but constructive comments on the manuscript.
P.T. Conduit was funded by a Wellcome Trust/Royal Society Sir Henry Dale Fellowship (105653/Z/14/Z).
The author declares no competing financial interests.
References
- Choi Y.-K., Liu P., Sze S.K., Dai C., and Qi R.Z.. 2010. CDK5RAP2 stimulates microtubule nucleation by the γ-tubulin ring complex. J. Cell Biol. 191:1089–1095. 10.1083/jcb.201007030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet V., Knibiehler M., Gregory-Pauron L., Remy M.-H., Chemin C., Raynaud-Messina B., Bon C., Kollman J.M., Agard D.A., Merdes A., and Mourey L.. 2011. Crystal structure of γ-tubulin complex protein GCP4 provides insight into microtubule nucleation. Nat. Struct. Mol. Biol. 18:915–919. 10.1038/nsmb.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren L., Remy M.-H., Bazin I., Callebaut I., Wright M., and Merdes A.. 2006. NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172:505–515. 10.1083/jcb.200510028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman J.M., Polka J.K., Zelter A., Davis T.N., and Agard D.A.. 2010. Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry. Nature. 466:879–882. 10.1038/nature09207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman J.M., Greenberg C.H., Li S., Moritz M., Zelter A., Fong K.K., Fernandez J.-J., Sali A., Kilmartin J., Davis T.N., and Agard D.A.. 2015. Ring closure activates yeast γTuRC for species-specific microtubule nucleation. Nat. Struct. Mol. Biol. 22:132–137. 10.1038/nsmb.2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-C., Neuner A., and Schiebel E.. 2014. Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence. Trends Cell Biol. 25:296–307. [DOI] [PubMed] [Google Scholar]
- Lüders J., Patel U.K., and Stearns T.. 2006. GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8:137–147. 10.1038/ncb1349 [DOI] [PubMed] [Google Scholar]
- Muroyama A., Seldin L., and Lechler T.. 2016. Divergent regulation of functionally distinct γ-tubulin complexes during differentiation. J. Cell Biol. 10.1083/jcb.201601099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.M., Preble A.M., Patel U.K., O’Connell K.L., Dias D.P., Moritz M., Agard D., Stults J.T., and Stearns T.. 2001. GCP5 and GCP6: two new members of the human γ-tubulin complex. Mol. Biol. Cell. 12:3340–3352. 10.1091/mbc.12.11.3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B.R., Paolillo V., and Zheng Y.. 2015. γ-Tubulin complexes in microtubule nucleation and beyond. Mol. Biol. Cell. 26:2957–2962. 10.1091/mbc.E14-11-1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema K., Wiese C., Martin O.C., Milligan R.A., Iwamatsu A., Mitchison T.J., and Zheng Y.. 1999. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144:721–733. 10.1083/jcb.144.4.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry S., and Vale R.D.. 2015. Microtubule nucleation at the centrosome and beyond. Nat. Cell Biol. 17:1089–1093. 10.1038/ncb3220 [DOI] [PubMed] [Google Scholar]
- Pinyol R., Scrofani J., and Vernos I.. 2013. The role of NEDD1 phosphorylation by Aurora A in chromosomal microtubule nucleation and spindle function. Curr. Biol. 23:143–149. 10.1016/j.cub.2012.11.046 [DOI] [PubMed] [Google Scholar]
- Reschen R.F., Colombié N., Wheatley L., Dobbelaere J., St Johnston D., Ohkura H., and Raff J.W.. 2012. Dgp71WD is required for the assembly of the acentrosomal Meiosis I spindle, and is not a general targeting factor for the γ-TuRC. Biol. Open. 1:422–429. 10.1242/bio.2012596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K.E., Lourenço P.C.C., and Snaith H.A.. 2004. Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr. Biol. 14:763–775. 10.1016/j.cub.2004.03.042 [DOI] [PubMed] [Google Scholar]
- Sumigray K.D., Foote H.P., and Lechler T.. 2012. Noncentrosomal microtubules and type II myosins potentiate epidermal cell adhesion and barrier formation. J. Cell Biol. 199:513–525. 10.1083/jcb.201206143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixidó-Travesa N., Villén J., Lacasa C., Bertran M.T., Archinti M., Gygi S.P., Caelles C., Roig J., and Lüders J.. 2010. The γTuRC revisited: a comparative analysis of interphase and mitotic human γTuRC redefines the set of core components and identifies the novel subunit GCP8. Mol. Biol. Cell. 21:3963–3972. 10.1091/mbc.E10-05-0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixidó-Travesa N., Roig J., and Lüders J.. 2012. The where, when and how of microtubule nucleation - one ring to rule them all. J. Cell Sci. 125:4445–4456. 10.1242/jcs.106971 [DOI] [PubMed] [Google Scholar]
- Vogt N., Koch I., Schwarz H., Schnorrer F., and Nüsslein-Volhard C.. 2006. The γTuRC components Grip75 and Grip128 have an essential microtubule-anchoring function in the Drosophila germline. Development. 133:3963–3972. 10.1242/dev.02570 [DOI] [PubMed] [Google Scholar]
- Walia A., Nakamura M., Moss D., Kirik V., Hashimoto T., and Ehrhardt D.W.. 2014. GCP-WD mediates γ-TuRC recruitment and the geometry of microtubule nucleation in interphase arrays of Arabidopsis. Curr. Biol. 24:2548–2555. 10.1016/j.cub.2014.09.013 [DOI] [PubMed] [Google Scholar]