Abstract
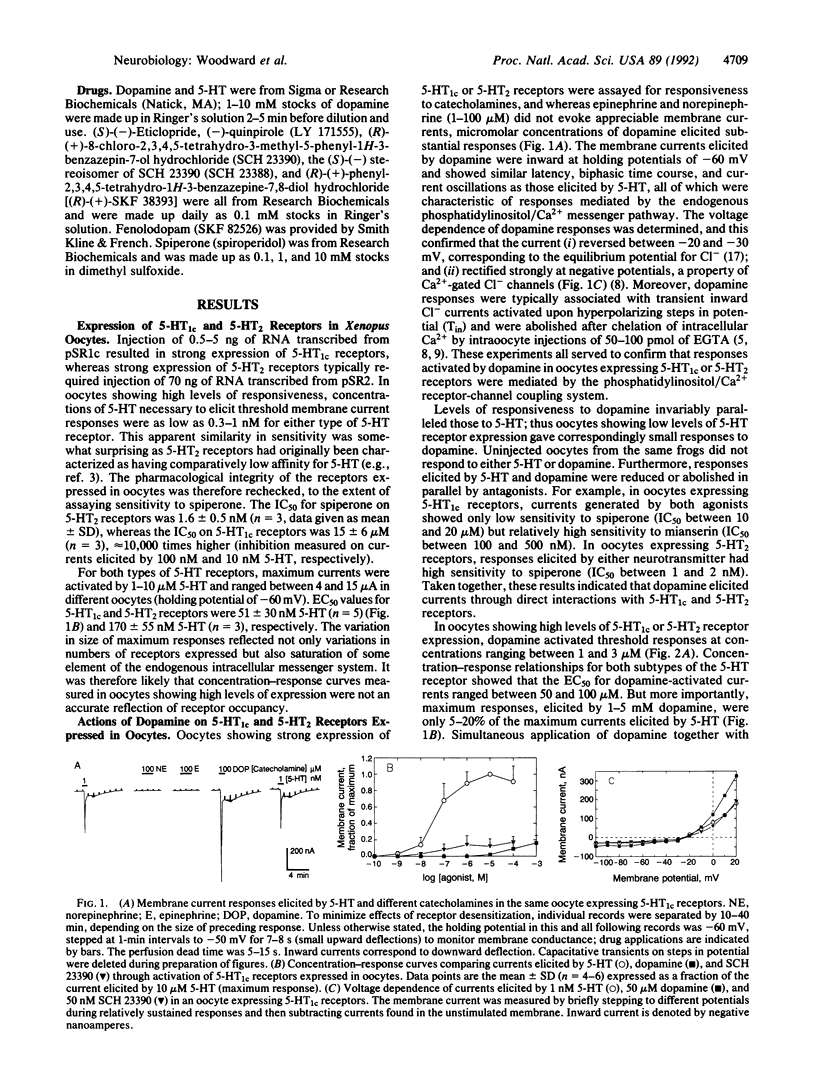
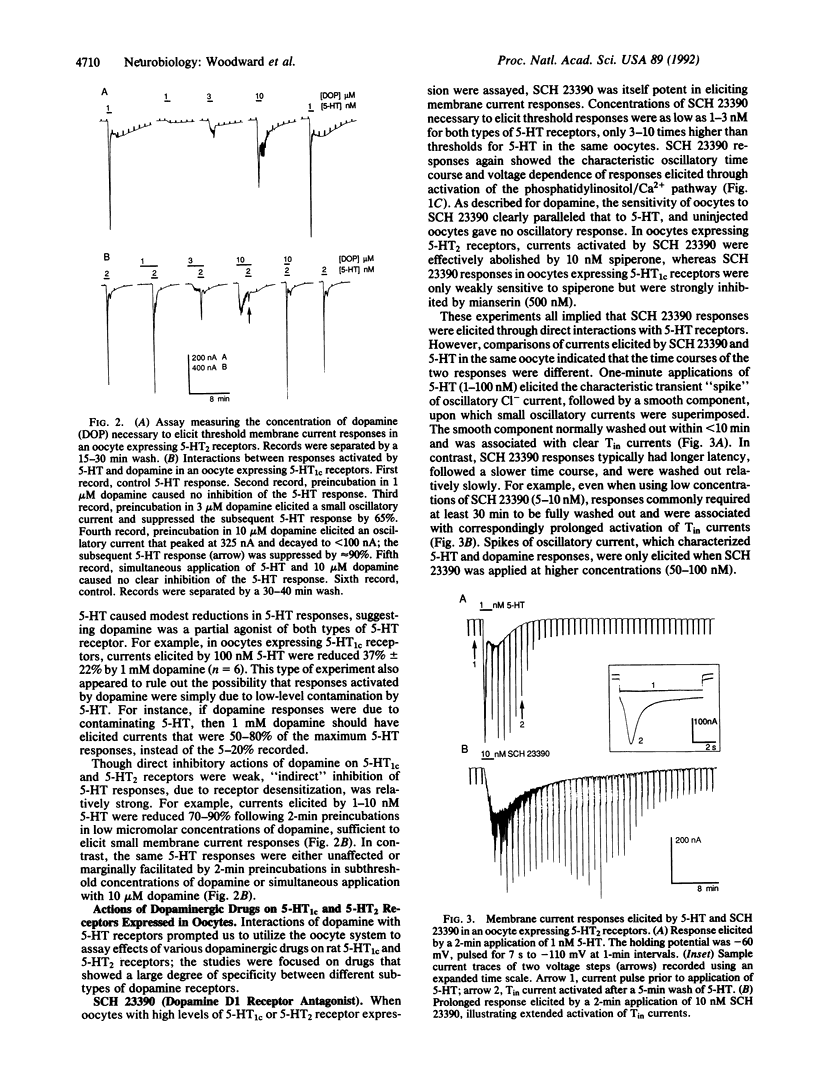
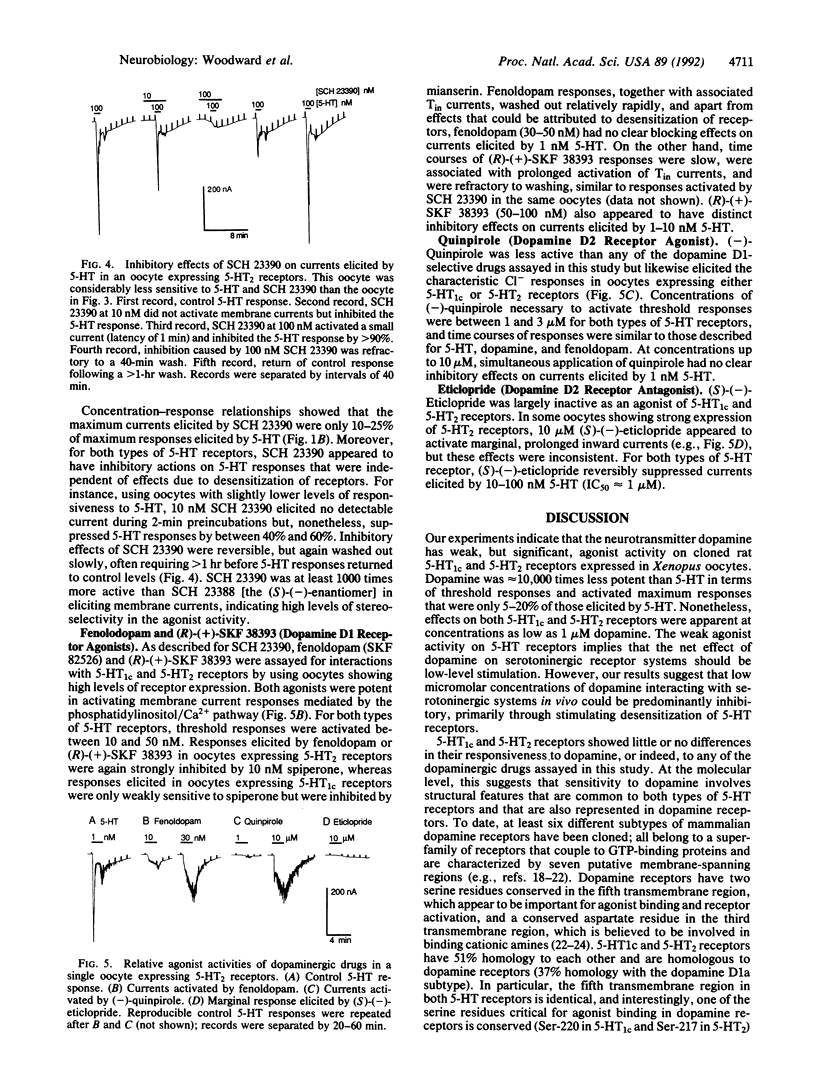
Using electrophysiological techniques, we studied interactions of dopamine and selected dopaminergic drugs with serotonin (5-hydroxytryptamine; 5-HT) receptors expressed in Xenopus oocytes by RNAs transcribed from cloned cDNAs. Oocytes showing strong expression of 5-HT1c and 5-HT2 receptors became weakly responsive to the neurotransmitter dopamine, which, like 5-HT, elicited Cl- currents through activation of the phosphatidylinositol/Ca2+ messenger pathway. The two types of 5-HT receptors showed similar sensitivity to dopamine; threshold responses were activated at concentrations as low as 1 microM. However, maximum dopamine responses were only 5-20% of maximum responses activated by 5-HT. The dopamine D1 receptor antagonist SCH 23390 was a potent agonist on 5-HT1c and 5-HT2 receptors. SCH 23390 elicited currents at concentrations as low as 1 nM, but maximum responses were again only 5-20% of those activated by 5-HT. Fenoldopam, a dopamine D1 receptor agonist, also interacted with 5-HT1c and 5-HT2 receptors, eliciting threshold responses between 10 and 20 nM. Our experiments raise the possibility that low micromolar concentrations of dopamine can cause weak activation and concomitant desensitization of serotoninergic systems in vivo and demonstrate that benzazepines can interact with 5-HT receptors at nanomolar concentrations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyson S. J., Alexander A. Net production of cerebrospinal fluid is decreased by SCH-23390. Ann Neurol. 1990 Jun;27(6):631–635. doi: 10.1002/ana.410270608. [DOI] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Serotonin receptors induced by exogenous messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Aug 22;219(1214):103–109. doi: 10.1098/rspb.1983.0062. [DOI] [PubMed] [Google Scholar]
- Hicks P. E., Schoemaker H., Langer S. Z. 5HT-receptor antagonist properties of SCH 23390 in vascular smooth muscle and brain. Eur J Pharmacol. 1984 Oct 15;105(3-4):339–342. doi: 10.1016/0014-2999(84)90628-9. [DOI] [PubMed] [Google Scholar]
- Iorio L. C., Barnett A., Leitz F. H., Houser V. P., Korduba C. A. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983 Aug;226(2):462–468. [PubMed] [Google Scholar]
- Julius D., Huang K. N., Livelli T. J., Axel R., Jessell T. M. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci U S A. 1990 Feb;87(3):928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Julius D. Molecular biology of serotonin receptors. Annu Rev Neurosci. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Monnier de Gouville A. C., Lawson K., Thiry C., Cavero I. SK&F 87516, a close analog of fenoldopam, is a partial agonist at dopamine-1 and alpha-2 receptors and produces stimulation of 5-hydroxytryptamine-2 receptors in the cardiovascular system of the rat. J Pharmacol Exp Ther. 1991 Mar;256(3):1049–1056. [PubMed] [Google Scholar]
- Lefebvre R. A., Guenaneche F., De Beurme F. A. Effects of the dopamine receptor agonists, fenoldopam and quinpirole, in the rat stomach. Eur J Pharmacol. 1990 Aug 21;185(1):69–79. doi: 10.1016/0014-2999(90)90212-o. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Hoffman B. J., Snutch T. P., van Dyke T., Levine A. J., Hartig P. R., Lester H. A., Davidson N. cDNA cloning of a serotonin 5-HT1C receptor by electrophysiological assays of mRNA-injected Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4332–4336. doi: 10.1073/pnas.84.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert H., Snutch T. P., Dascal N., Lester H. A., Davidson N. Rat brain 5-HT1C receptors are encoded by a 5-6 kbase mRNA size class and are functionally expressed in injected Xenopus oocytes. J Neurosci. 1987 Apr;7(4):1159–1165. doi: 10.1523/JNEUROSCI.07-04-01159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Mahan L. C., McVittie L. D., Gerfen C. R., Sibley D. R. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklaus K. J., McGonigle P., Molinoff P. B. [3H]SCH 23390 labels both dopamine-1 and 5-hydroxytryptamine1c receptors in the choroid plexus. J Pharmacol Exp Ther. 1988 Oct;247(1):343–348. [PubMed] [Google Scholar]
- O'Dowd B. F., Nguyen T., Tirpak A., Jarvie K. R., Israel Y., Seeman P., Niznik H. B. Cloning of two additional catecholamine receptors from rat brain. FEBS Lett. 1990 Mar 12;262(1):8–12. doi: 10.1016/0014-5793(90)80140-e. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. A transient inward current elicited by hyperpolarization during serotonin activation in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):279–292. doi: 10.1098/rspb.1985.0002. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Inositol trisphosphate activates a voltage-dependent calcium influx in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1987 Jun 22;231(1262):27–36. doi: 10.1098/rspb.1987.0033. [DOI] [PubMed] [Google Scholar]
- Parker I., Panicker M. M., Miledi R. Serotonin receptors expressed in Xenopus oocytes by mRNA from brain mediate a closing of K+ membrane channels. Brain Res Mol Brain Res. 1990 Jan;7(1):31–38. doi: 10.1016/0169-328x(90)90070-t. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Sleight A. J., McCarthy B. G., Pierce P. A., Schmidt A. W., Hekmatpanah C. R. The clinical utility of pharmacological agents that act at serotonin receptors. J Neuropsychiatry Clin Neurosci. 1989 Summer;1(3):253–262. doi: 10.1176/jnp.1.3.253. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Bach A. W., Wozny M., Taleb O., Dal Toso R., Shih J. C., Seeburg P. H. Structure and functional expression of cloned rat serotonin 5HT-2 receptor. EMBO J. 1988 Dec 20;7(13):4135–4140. doi: 10.1002/j.1460-2075.1988.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A. W., Peroutka S. J. 5-Hydroxytryptamine receptor "families". FASEB J. 1989 Sep;3(11):2242–2249. doi: 10.1096/fasebj.3.11.2673898. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Candelore M. R., Hill W. S., Sigal I. S., Dixon R. A. Identification of two serine residues involved in agonist activation of the beta-adrenergic receptor. J Biol Chem. 1989 Aug 15;264(23):13572–13578. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Candelore M. R., Rands E., Hill W. S., Dixon R. A. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988 Jul 25;263(21):10267–10271. [PubMed] [Google Scholar]
- Sunahara R. K., Guan H. C., O'Dowd B. F., Seeman P., Laurier L. G., Ng G., George S. R., Torchia J., Van Tol H. H., Niznik H. B. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991 Apr 18;350(6319):614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- Törk I. Anatomy of the serotonergic system. Ann N Y Acad Sci. 1990;600:9–35. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bunzow J. R., Guan H. C., Sunahara R. K., Seeman P., Niznik H. B., Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991 Apr 18;350(6319):610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Weinstock J., Wilson J. W., Ladd D. L., Brush C. K., Pfeiffer F. R., Kuo G. Y., Holden K. G., Yim N. C., Hahn R. A., Wardell J. R., Jr Separation of potent central and renal dopamine agonist activity in substituted 6-chloro-2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepines. J Med Chem. 1980 Sep;23(9):973–975. doi: 10.1021/jm00183a001. [DOI] [PubMed] [Google Scholar]


