Abstract
Pancreatic ductal adenocarcinoma (PDAC) ranks fourth among cancer-related deaths in the United States and for patients with unresectable disease, treatment options are currently limited and lack curative potential. Preclinical mouse models of PDAC that recapitulate the biology of human pancreatic cancer offer an opportunity for the rational development of novel treatment approaches that may improve patient outcomes. With the recent success of immunotherapy for subsets of patients with solid malignancies, interest is mounting regarding how to utilize immunotherapy for the treatment of PDAC. Here, we discuss the value of genetic mouse models for informing the immunobiology of PDAC and describe their application for investigating novel immunotherapeutics. In addition, we present several variants of these models, which may be used in drug development and to inform unique aspects of disease biology and therapeutic responsiveness.
Keywords: pancreatic ductal adenocarcinoma, immunotherapy, genetically engineered mouse model, KPC, T cell exclusion, inflammation, macrophage
Introduction
Immunotherapy has recently demonstrated significant benefit for the treatment of some patients with advanced cancer. Most notably, immune checkpoint inhibitors that target cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) (Hodi et al., 2010; Postow et al., 2015; Schadendorf et al., 2015) and the programmed cell death protein 1 pathway (PD-1/PD-L1) (Ansell et al., 2015; Garon et al., 2015; Le et al., 2015a; Motzer et al., 2015; Robert et al., 2015a; Robert et al., 2015b; Topalian et al., 2012, Lesokhin et al., 2015) have produced major tumor regressions leading to durable long-term responses in a subset of patients. For example, in metastatic melanoma approximately 20% of patients respond to therapeutic blockade of CTLA-4 with remissions lasting >5 years (Schadendorf et al., 2015). However, while these responses to immunotherapy can be robust and durable leading to improved overall survival, not all patients respond, and responses are often transient (Beatty and Gladney, 2015). For example, in non-small cell lung cancer the response rate with anti-PD-1 blockade with nivolumab was approximately 19% with a 12.5 month median duration of response (Garon et al., 2015). Similarly, in advanced renal cell carcinoma, the response rate to nivolumab was 25% with a median progression-free survival of 4.6 months (Motzer et al., 2015). This immune resistance that is seen to immunotherapy is especially evident in pancreatic ductal adenocarcinoma (PDAC) in which immunotherapies have not yet reliably produced clinical benefit (Brahmer et al., 2012; Royal et al., 2010), despite promising findings demonstrating the potential of the immune system to be harnessed in this disease (Le et al., 2013; Le et al., 2015b). The biology that underlies the poor responsiveness of PDAC and other malignancies to immunotherapy remains ill-defined. As a result, studying immune resistance mechanisms exploited by cancer will be critical to effectively designing and implementing immune-targeted strategies capable of impacting the natural history of this almost uniformly lethal disease.
Prior to the development of genetically engineered mouse models (GEMMs) of PDAC, tumor transplantation models were the primary mechanism for investigating the capacity of immunotherapy to impact tumor growth in vivo. Transplantation models utilize human or mouse pancreatic cancer cells that are implanted into recipient mice. For example, in a xenograft model system, human pancreatic tumor cell lines derived from patients are implanted into mice via subcutaneous, intravenous, peritoneal, or orthotopic injection (Ding et al., 2010; Singh et al., 2010). This approach offers the opportunity to investigate the biology of human PDAC cells in vivo and to assess the responsiveness of PDAC, in this setting, to cytotoxic therapies. However, in order to avoid immune rejection of transplanted human tumor cells due to species mismatch, xenograft models utilize immunodeficient mice. Thus, a major drawback of this model is the lack of a competent immune system which limits its value in the investigation of immunotherapeutics. Moreover, tumor cells used in these xenograft models have often been passaged extensively in vitro thereby limiting tumor cell clonal heterogeneity and thus, potential biological relevance. While recent advances with patient-derived xenograft tumor models (Hidalgo et al., 2014) and humanized mice (Rongvaux et al., 2014) can be used to preserve many features of tumor heterogeneity and to introduce human leukocyte populations, respectively, these models are also challenging logistically and can be costly to develop which together has limited their broad application within the research community. A mainstay in the study of cancer immunology has been the use of murine-derived cancer cell lines implanted into syngeneic mice. This model system, unlike xenograft models, allows for investigations to be conducted in an immunocompetent host and therefore, can be informative of immune mechanisms of anti-tumor immunity and immune resistance. However, most transplantation models do not effectively model tumor development and the induction of immune tolerance that occurs in patients. In fact, mere implantation of tumor cells, often passaged extensively in vitro, can induce a “vaccine effect” leading to immune activation and alterations of the tumor microenvironment that may not be characteristic of human disease (Beatty and Paterson, 2000). For example, tumor cells commonly grow more slowly when implanted into immune competent mice compared to immunodeficient mice (Gubin et al., 2014). This finding also reflects the capacity of cancer cells to adapt to immune pressure, a process termed immunoediting, in which cancer clones that acquire the capacity to evade immune elimination emerge and dominate tumor outgrowth (Schreiber et al., 2011). For these reasons and as described below, genetically engineered mice that spontaneously develop PDAC are particularly appealing for immunotherapeutic drug discovery given that tumors arise in the context of a competent and fully intact immune system. However, these models also present with significant challenges in terms of their use and maintenance as well as with interpretation of findings given the stochastic and heterogeneous nature of tumors that arise spontaneously (Beatty et al., 2011; Hingorani et al., 2005; Olive and Tuveson, 2006). Here, we report on the use of genetic mouse models of PDAC and variants of these models that can be used to strategically study and advance the development of immunotherapeutic drugs.
Genetic mouse models of PDAC
Analyses of tumor samples collected from patients have revealed that the genomic landscape of PDAC is complex, marked by high chromosomal instability and frequent alterations in a select group of genes including KRAS, TP53, SMAD4, CDKN2A, and ARID1A, with relatively few somatic coding mutations (Waddell et al., 2015). Based on this knowledge, multiple mouse models of PDAC have been developed and we refer the reader to several recent reviews that discuss in detail the development of these models which incorporate alterations in proto-oncogenes (e.g., KRAS) and tumor suppressor genes (e.g., TP53, SMAD4, and CDKN2A) that targeted specifically to the mouse pancreas (Perez-Mancera et al., 2012; Westphalen and Olive, 2012). Two of the most commonly mutated genes in PDAC are the KRAS proto-oncogene and the TP53 tumor suppressor gene (Biankin et al., 2012; Hruban et al., 2001; Jones et al., 2008; Waddell et al., 2015). KRAS mutations are observed in 80–90% of all human PDACs with mutations in TP53 detected in 50–75% of PDACs. As a result, one of the most studied genetically engineered mouse models of PDAC incorporates mutations in Kras and Trp53 that are targeted specifically to the mouse pancreas using Cre-Lox technology (Hingorani et al., 2005). Here, we focus on the LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) mouse model of PDAC as a platform for investigating immunotherapy because (i) it reproduces many of the key features of the immune microenvironment observed in human PDAC including a robust inflammatory reaction and exclusion of effector T cells, (ii) it is the most extensively studied genetic model of PDAC for evaluation of immunotherapy, and (iii) it has reproduced clinical observations seen in PDAC patients treated with several immune oncology drugs including CD40 agonists and anti-PDL1 antibodies (Beatty et al., 2011; Beatty et al., 2015; Feig et al., 2013; Keenan et al., 2014; Stromnes et al., 2015). However, the approaches that we describe for studying immunotherapy and the immunobiology of PDAC may also be applied to other PDAC models. For discussion on additional genetically engineered mouse models of PDAC, we direct readers to recent reviews on this topic (Colvin and Scarlett, 2014; DeCant et al., 2014).
The KPC mouse model of PDAC was first described in 2005 and incorporates, through Cre-Lox technology, the conditional activation of mutant endogenous alleles of the Kras and Trp53 genes (Hingorani et al., 2005). Specifically, an activating point mutation (G12D) in Kras and a dominant negative mutation in Trp53 (R172H) are conditionally activated in the mouse pancreas by breeding LSL-KrasG12D/+; LSL-Trp53R172H/+ mice to Pdx-1-Cre mice that express Cre recombinase under the expression of the pancreas-specific Pdx-1 promoter. Cre-mediated recombination acts to excise the loxP-flanked stop codon (LSL), an event that occurs only in cells expressing Cre, thereby leading to conditional expression of mutant Kras and Trp53 genes specifically in the mouse pancreas.
The routine breeding of KPC mice requires first crossing LSL-KrasG12D/+(K/+) mice with LSL-Trp53R172H/+(P/+) mice to generate LSL-KrasG12D/+; LSL-Trp53R172H/+(K/+P/+) mice (Fig. 1A). K/+P/+ mice are then crossed with P/+ mice to generate KrasG12D/+; LSL-Tp53R172H/R172H(K/+P/P) mice. While both K/+P/+ and K/+P/P male mice can be used to cross with homozygous Pdx-1-Cre female mice to generate KPC mice, the efficiency of this cross with K/+P/P mice is increased by 2-fold and therefore, is the preferred breeding strategy (Fig. 1A). However, the maintenance of K/+P/P mice can be challenging given their shortened lifespan (range 10–20 weeks of age) and propensity to develop tumors (e.g. lymphoma) due to homozygous inactivation of the Trp53 gene.
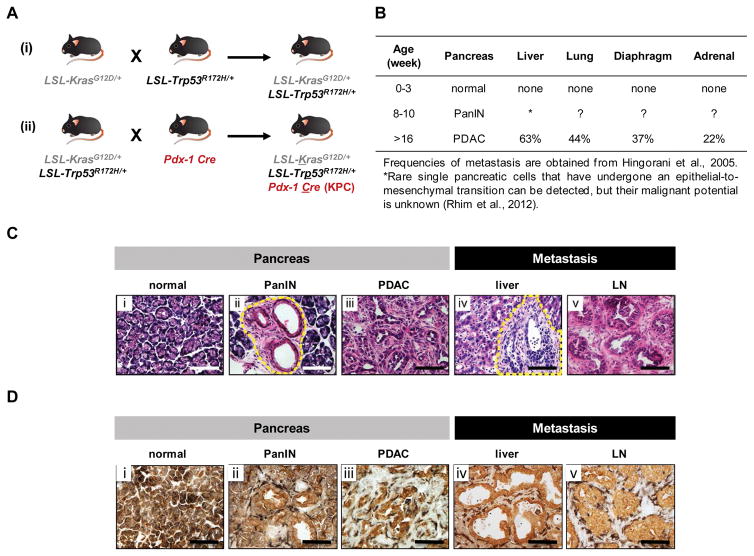
Figure 1. KPC murine model of spontaneous PDAC.
(A) Breeding strategy for generation of KPC mice. (B) Disease progression in KPC mice. (C) Histology (40× field) of (i) normal pancreas, (ii) pancreas of a 10-week-old KPC mouse showing pancreatic intraepithelial neoplasia (PanIN), (iii) primary pancreatic tumor, and (iv) liver and (v) lymph node showing metastatic tumor. Images representative of at least 5 mice per group. Dashed lines indicate PanIN in image (ii) and metastatic tumor in image (iv). (D) Shown is two color immunohistochemistry to demonstrate the spatial relationship between epithelial cells (EpCAM, brown) and macrophages (F4/80, black) in (i) normal pancreas, (ii) PanIN, (iii) PDAC, and metastatic lesions including (iv) liver and (v) an adjacent pancreatic lymph node.
The original KPC model was produced on a mixed genetic background (129Sv and C57BL/6) (Hingorani et al., 2005). Each of the three strains of mice (i.e. K/+, P/+, and Pdx-1-Cre mice) are commercially available on this mixed background from Jackson Laboratories (LSL-KrasG12D, stock No: 008179; LSL-Trp53R172H, stock No: 008652; Pdx-1 Cre, stock No: 014647) and NCI Mouse Repository (LSL-KrasG12D, strain No: 01XJ6; LSL-Trp53R172H, strain No: 01XAF; Pdx-1 Cre, strain No: 01XL5). In our laboratory, all three strains of mice have been backcrossed to the C57BL/6J genetic background for at least 10 generations. In doing so, we have not observed any compromise in tumor penetrance, kinetics of disease onset, or the phenotype of lesions that emerge, such as their propensity to metastasize. Moreover, a pure genetic background allows the opportunity to conduct adoptive cell transfer studies (i.e. the transfer of cells from one mouse to another, including the transfer of cells that have been altered or engineered with novel properties) (Rosenberg et al., 2008). Recently, Stromnes et al. investigated the adoptive transfer of T cells as a novel immunotherapeutic approach for the treatment of PDAC in KPC mice (Stromnes et al., 2015). These types of studies are fundamental to the investigation of immune mechanisms of action and for evaluation of novel therapies, such as engineered T cells.
The KPC model has significant advantages over previously described xenograft and implantation models of PDAC for the following reasons. First, the KPC model recapitulates many of the salient clinical (e.g. ascites development, bowel and biliary obstruction, and cachexia) and histopathological features (e.g. cellular morphology, poor vascularity, fibrosis, and metastatic spread) of human disease (Beatty et al., 2011; Feig et al., 2013; Hingorani et al., 2005; Jacobetz et al., 2013; Olive et al., 2009; Provenzano et al., 2012). Second, KPC mice are immunocompetent and provide a useful platform for studying interactions between the immune system and tumor cells. In particular, the tumor microenvironment in the KPC model demonstrates a striking inflammatory infiltrate with a scarcity of effector T cells (Bayne et al., 2012; Beatty et al., 2011; Beatty et al., 2015; Keenan et al., 2014). This immunobiology seen in tumors of KPC mice mirrors observations in human PDAC and thus, offers an opportunity to understand mechanisms driving inflammatory cell recruitment/biology and T cell exclusion. Third, in contrast to xenograftand syngeneic transplantation models of PDAC, tumors arise spontaneously with development of defined histopathologic stages of progression that mirror human disease (Hingorani et al., 2003; Hingorani et al., 2005; Hruban et al., 2001). Lastly, low passage tumor cell lines can be easily derived from tumor-bearing KPC mice for use in in vitro and in vivo studies that can inform preclinical drug development (Bayne et al., 2012; Beatty et al., 2015; Feig et al., 2013; Olive et al., 2009).
KPC mice display disease progression that closely resembles human disease (Fig. 1B). The pancreas of newly born KPC mice is normal and devoid of neoplastic cells (Fig. 1C). However, by 8 to 10 weeks of age, KPC mice harbor precursor lesions, or pancreatic intraepithelial neoplasia (PanIN), within the pancreas (Fig. 1C). At this early stage of PDAC development, a strong inflammatory response marked by infiltration of F4/80+ cells can be seen which persists from disease conception through invasion and metastasis (Fig. 1D). In addition, during PanIN progression, single pancreatic cells can be seen to have undergone an epithelial-to-mesenchymal transition in which cells lose epithelial markers such as E-cadherin; upregulate mesenchymal markers such as Zeb1 and Fsp1; and become more spindle- and fibroblast-like in their morphology. With this transition, pancreatic cells can also undergo hematogenous dissemination with rare single pancreatic cell detection in the liver (Rhim et al., 2012). This process is promoted by the inflammatory reaction associated with the disease (Rhim et al., 2012). However, the precise mechanism(s) by which inflammatory cells regulate pancreatic cancer metastasis remain ill-defined. Nonetheless, similar findings occur in patients with pancreatic cystic lesions in the absence of an obvious clinical diagnosis of cancer (Rhim et al., 2014). Together, these findings suggest that hematogenous dissemination of malignant cells in PDAC may occur early during disease progression and potentially explain the high relapse rate seen in patients undergoing surgical resection with curative intent (Barugola et al., 2009; Mann et al., 2006; Siegel et al., 2015).
By 16 weeks of age, most KPC mice have developed locally invasive PDAC that is accompanied by a dense desmoplastic reaction. At this time point, the majority of mice show evidence of tumor development and biliary obstruction as detected by abdominal ultrasonography. With disease progression, mice can also develop cachexia, jaundice, weight loss, and malignant ascites due to peritoneal spread of disease. In addition, PDAC tumors that arise spontaneously in KPC mice can metastasize to multiple organs including the liver, lymph nodes, lung, diaphragm, and adrenal glands (Fig. 1C) (Hingorani et al., 2005). This proclivity of PDAC to metastasize and the anatomic distribution of metastases seen in the KPC model mirrors tumor dissemination in human PDAC and therefore, offers a valuable opportunity to investigate PDAC metastasis.
Genetic variants of the KPC model have also been developed to study malignant cell invasion and metastasis in PDAC. These models show a similar disease phenotype including the kinetics of disease onset but incorporate lineage tracing markers such as yellow fluorescent protein (YFP). For example, by including a LSL-Rosa26-YFP transgene into the KPC model to generate KPCY mice, cells of the pancreata (both benign and malignant) can be monitored due to YFP expression (Rhim et al., 2012). With this approach, circulating YFP+ pancreatic cells have been detected in the circulation of KPCY mice beginning early during PDAC development and their frequency increases with disease progression as well as inflammation. A variant of this strategy has also been developed to enable a multicolor lineage-tracing system to monitor the clonality of metastatic lesions (Maddipati and Stanger, 2015). This approach uses a “Confetti” lineage-labeling system, in which Cre-mediated recombination produces stochastic expression of one of four fluorescent proteins in a cell. Findings from these mice, termed “KPCX”, have suggested that PDAC metastases may be polyclonal and support a role for clonal diversity in the evolution of metastatic disease. This observation has profound implications regarding the potential intra- and inter-lesional clonal heterogeneity of PDAC lesions. For example, in KPC mice, individual PDAC lesions almost invariably demonstrate a non-uniform distribution of leukocytes such that some regions of a tumor may show an increased presence of leukocyte populations whereas other areas may be completely devoid of cellular subsets (e.g. eosinophils). Similarly, tumor cell morphology and fibrosis deposition can vary significantly across individual lesions (i.e. intra-lesional heterogeneity). This variability can also be seen between lesions (i.e. inter-lesional heterogeneity) even within the same host (Hingorani et al., 2005). While the therapeutic implications of this heterogeneity remains ill-defined in PDAC, it may explain the variability of treatment responses observed with some therapeutics such as chemotherapy in combination with a CD40 agonist (Beatty et al., 2013).
Another variant of the KPC model is the KC model which incorporates only an activating point mutation (G12D) in Kras that is conditionally expressed in the pancreas due to Cre recombinase expression driven by the pancreas-specific Pdx-1 promoter (Hingorani et al., 2003). In KC mice, the full spectrum of disease from PanIN to invasive PDAC can be seen although disease progression is slower with only a subset of mice showing progression to invasive and metastatic cancer by 1 year of age (Hingorani et al., 2003). Nonetheless, these mice have been particularly useful for evaluating the development of precursor PanIN lesions and strategies to delay progression to invasive PDAC. From these studies, components of the immune system, including IL-6, STAT3 and myeloid cells, have been identified as critical determinates for driving PanIN to PDAC progression (Fukuda et al., 2011; Lesina et al., 2011; Liou et al., 2015).
Immunobiology of human and mouse PDAC
Genetically engineered mouse models of PDAC provide an opportunity to investigate cross-talk between the immune system and neoplastic cells from disease conception through invasion and metastasis. These models can also be used to investigate the impact of immunotherapy at multiple stages of disease pathogenesis with the intent to (i) prevent disease occurrence, (ii) delay the progression of precursor lesions to invasive cancer, and (iii) treat local and metastatic disease. However, the value of these models in this setting is predicated on their capacity to reproduce immunobiology observed in human disease.
The immunobiology of human PDAC has mainly been studied using primary pancreatic tissue obtained at the time of surgery from patients presenting with surgically resectable disease (Chang et al., 2011; De Monte et al., 2011; Fukunaga et al., 2004; Ino et al., 2013; Lutz et al., 2014). Thus, analysis of the immune microenvironment has largely been restricted to a small subset (approximately 20%) of patients that are diagnosed with PDAC. From these studies, the immune microenvironment of PDAC has been found to be complex with multiple leukocyte populations present including macrophages, neutrophils, eosinophils, and mast cells. The degree of recruitment of these cell types can also vary significantly between tumors. Lymphocytes, including B and T cells, can also be found within some PDAC lesions although their presence is often confined to the tumor margin and sometimes limited to clustering rather than diffuse infiltration. This general exclusion of T cells from the bulk of the tumor tissue is consistent with the premise that PDAC is a poorly immunogenic tumor capable of establishing a site of immune privilege (Beatty et al., 2015). This contrasts tumors commonly classified as “immunogenic”, such as melanoma and renal cell carcinoma, where effector T cell infiltration of tumor tissue is more frequently observed.
Because developing tumors in KPC mice have a propensity to metastasize, KPC mice can be used to investigate the immunobiology of primary and metastatic lesions. In contrast, obtaining sufficient material to investigate leukocyte recruitment and biology in primary and metastatic lesions from patients with surgically unresectable PDAC has been difficult due to poor anatomic accessibility of lesions and the limited material that can be obtained from a core biopsy. As a result, there is a lack of understanding of the immunobiology of PDAC and the degree of heterogeneity that may exist between lesions even in the same patient. These studies, though, can be more readily performed in genetic mouse models of PDAC.
PDAC lesions arising spontaneously in the KPC mouse model reproduce the leukocyte complexity that has been observed in human PDAC (Bayne et al., 2012; Beatty et al., 2011; Beatty et al., 2015; Feig et al., 2013). Similar to human disease, primary PDAC lesions in KPC mice are marked by a strong infiltration of F4/80+macrophages (Fig. 2A and C). Myeloid cells can be found in close proximity to PDAC cells. Their phenotype, though, is dependent on their surrounding microenvironment such that many cellular subsets are likely to be present within tumors despite the common tendency in the literature to describe these cells based on a few surface markers (e.g. CD11b, F4/80, Gr-1, MHC II, CD206, CD163) and presence or absence of molecules (e.g. iNOS and Arg1) that are associated with findings observed primarily in in vitro culture conditions (e.g. M1 versus M2 macrophages (Murray et al., 2014)). In general, it is thought that myeloid cells associated with PDAC, similar to other solid malignancies, act to enhance tumor growth and metastasis by promoting tumor cell migration and invasion into local tissues (Liou et al., 2015).
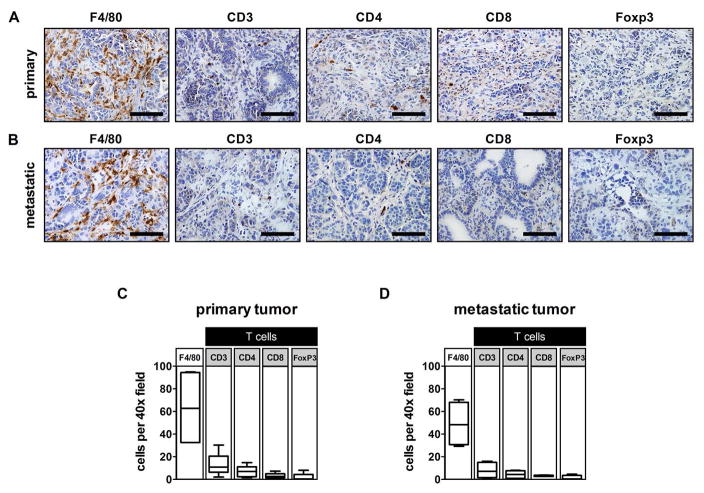
Figure 2. Primary and metastatic tumors in KPC mice show a strong recruitment of F4/80+ myeloid cells with a weak infiltration of CD3+ T cells.
Representative immunohistochemistry images (40× field) showing F4/80, CD3, CD4, CD8, and FoxP3 staining of (A) primary pancreatic tumor and (B) metastatic tumor in the liver of KPC mice. Quantification of cell types identified in (C) primary pancreatic tumor (n=4–5 per group) and (D) metastatic tumor in the liver (n=4 per group). Scale bars in (A) and (B) indicate 100 μm.
In contrast to the strong recruitment of myeloid cells to tumors, T cell infiltrates are usually observed in significantly lower frequencies (Fig. 2A and C) with few effector T cells capable of recognizing and eliminating tumor cells. Of the T cell subsets found, Foxp3+regulatory T cells (Tregs) are most prevalent and seen commonly infiltrating precursor PanIN lesions (Keenan et al., 2014). In addition, IL-17 secreting CD4+ T cells and γδT cells (Rei et al., 2015) have been identified in the desmoplastic reaction that surrounds PanIN lesions (McAllister et al., 2014). Together, these T cell subsets have been implicated as key promoters of PanIN initiation and progression to PDAC due to their capacity to be immunosuppressive and provide growth factors (e.g. IL-17) for PanIN development. For example, Tregs are well-recognized for their capacity to suppress tumor-specific T cell immunity by releasing immunosuppressive cytokines (e.g. IL-10 and TGF-β) that impede effector T cell activity (Josefowicz et al., 2012; Roychoudhuri et al., 2015). Moreover, inhibiting the activity of IL-17 producing cells using IL-17 antagonists and depleting Tregs in combination with a vaccine have shown efficacy in genetic models of PDAC (Keenan et al., 2014; McAllister et al., 2014). Together, these findings provide a strong rationale for the development and translation of therapies aimed at redirecting the immune response to PDAC, and have provided key insights into a role for the natural immune reaction to PDAC in promoting rather than inhibiting disease progression.
In our investigation of metastatic liver lesions detected in KPC mice, we have found that the leukocyte composition of metastases is similar to the primary tumor and marked by an abundance of F4/80+ macrophages and few effector T cells (Fig. 2B and D). This finding is consistent with recent reports suggesting that primary and metastatic lesions in human PDAC appear histologically similar (Whatcott et al., 2015). In our studies, we have also found that the capacity of PDAC to exclude T cells appears to be cell intrinsic as T cell exclusion is observed in tumors arising from KPC-derived tumor cell lines (i) implanted subcutaneously (Fig. 3A and D), (ii) injected orthotopically into the pancreas (Fig. 3B and E), and (iii) injected intrasplenically as a model of liver metastasis(Fig. 3C and F). Thus, the capacity of PDAC to establish a site of immune privilege (Beatty et al., 2015) may be, at least in part, cancer cell intrinsic and maintained even during metastasis. This phenomenon of immune privilege (i.e. the capacity to exclude T cells and/or evade immune elimination) can also be seen in many other malignancies such as colorectal cancer (Galon et al., 2006) and ovarian cancer (Zhang et al., 2003). Several mechanisms have been proposed to explain the lack of T cell infiltration into solid malignancies including insufficient T cell priming, lack of strong immunogenic antigens capable of being recognized by effector T cells, recruitment of regulatory cell populations (e.g. Tregs and myeloid suppressor cells), and alterations in tumor vasculature that prevent T cell extravasation into tissues (Joyce and Fearon, 2015). Understanding these mechanisms has significant translational implications.
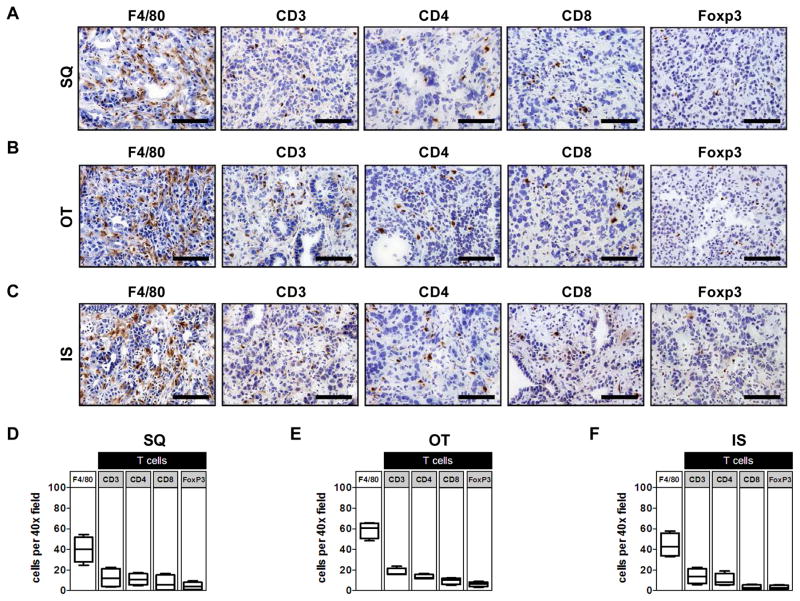
Figure 3. Leukocyte infiltration in implantable models of PDAC.
Low-passage KPC-derived PDAC cell lines were injected subcutaneously (SQ), orthotopically (OT), or intrasplenically (IS) into syngeneic C57BL/6J mice (6–8 weeks of age). Shown are representative immunohistochemistry images (40x field) showing F4/80, CD3, CD4, CD8, and FoxP3 staining from a (A) SQ tumor, (B) OT tumor arising in the pancreas, and (C) IS tumor that deposited in the liver. Quantification of leukocyte subsets detected by immunohistochemistry in (D) SQ tumor, (E) OT tumor, and (F) IS tumor (n=4 per group). Scale bars in (A), (B), and (C) denote 100 μm.
Immuno-oncology discovery using genetic models of PDAC
KPC mice can be used in a variety of experimental conditions to investigate the immunobiology of PDAC. For example, to study the evolution of immune responses during PDAC progression, mice can be evaluated at 4–6 (early PanIN), 8–10 (late PanIN), and 16–20 (PDAC and metastases) weeks of age. The most common method for monitoring tumor development in KPC mice involves the use of ultrasonography, which is both cost-effective and non-invasive (Olive and Tuveson, 2006). Ultrasonography provides a high resolution view of the entire abdominal cavity with the ability to quantify tumor volumes. This approach can be used to evaluate approximately 4–6 KPC mice per hour. However, when applied to a large mouse colony, this strategy may become time consuming and require dedicated facilities. As a result, alternative approaches may be necessary for individual laboratories seeking to incorporate this model into their studies. For this reason, it is often common practice to screen mice weekly by monitoring their weight and palpating their abdomen to detect the presence of a tumor prior to ultrasound analysis. As tumors do not typically become apparent by ultrasonography until approximately 12–14 weeks of age, screening is also usually initiated at this time.
The use of KPC mice in studies is approached in a similar manner as a clinical trial. This is because of (i) the stochastic nature of PDAC development in KPC mice, (ii) the complicated breeding strategy that is unlikely to produce sufficient numbers of mice to fill all experimental groups at the time of initiating an experiment and (iii) the need to incorporate specific eligibility criteria for mice to be enrolled in a study. For these reasons, KPC mice are enrolled into studies on a rolling basis. At the onset of a study, eligibility criteria should be defined for the study and mice should be block randomized into experimental groups to prevent bias. An example of eligibility criteria used in studies of KPC mice with advanced PDAC includes ultrasound evidence of a tumor measuring 50–150 mm3 and age greater than 10 weeks. Mice are excluded from enrollment if they demonstrate significant co-morbidities including greater than 10% weight loss, weakness, or evidence of a non-pancreatic tumor such as lymphoma, sarcoma, or hepatocellular carcinoma. In addition to defining eligibility criteria, end-of-study criteria should also be determined. For example, treatment studies can be conducted that assess response rate and/or survival. Window studies assessing the impact on tumor progression evaluated at 1 or 2 time points after treatment may be necessary if therapy cannot be repeatedly administered. In contrast, survival studies that simplify the need for serial imaging analysis can also be used and should incorporate specific endpoints and censoring criteria. For example, a KPC mouse may develop a sarcoma or lymphoma during the course of treatment requiring euthanasia for reasons other than pancreatic tumor progression. In addition, IACUC guidelines define clear criteria for euthanasia, which is important to consider in the study design. Finally, similar to a clinical trial, the impact of a treatment can be evaluated in KPC mice by repeated peripheral blood analysis and serum collection. This offers the opportunity to identify potential pharmacodynamic markers that may inform treatment efficacy, mechanism of action, and potential clinically-useful biomarkers. For example, Faca et al used a genetic mouse model of PDAC to conduct a mouse to human search of plasma proteins associated with pancreatic tumor development (Faca et al., 2008). Similarly, Melo et al. used a genetic mouse model of PDAC to validate the potential utility of tumor-derived exosomes (i.e. extracellular vesicles released by tumor cells that contain tumor biomaterial) as a non-invasive diagnostic and screening tool in PDAC (Melo et al., 2015). In addition to blood-derived samples used in these two studies, tissues can also be collected from mice. These tissues can either be analyzed in real-time or the tissue, cells, RNA, and DNA can be preserved using standard measures for subsequent analysis.
Characterization of the immune microenvironment of tumors in mice has historically involved flow cytometry to detect lymphocytes (e.g. CD19+ B cells and CD3+ T cells including CD4+, CD8+ and Foxp3+ T cell subsets) and myeloid cells (e.g. F4/80+ macrophages and CD11b+ Gr-1+granulocytes and immature myeloid cells). With the capacity to now conduct multi-color flow cytometry, a detailed characterization of the phenotype of these leukocyte populations (i.e. surface molecule expression, signaling pathway activation, and cytokine production) is feasible. However, this approach can sometimes be misleading, particularly for tumors that develop spontaneously. For example, tumors arising in KPC mice often metastasize to the lymph nodes where they will form tumors that are then encased with a rim of lymphoid tissue. Developing tumors may also encapsulate lymph nodes so that separation of lymphoid tissue from a tumor is not feasible. Thus, while flow cytometric analysis of PDAC tumors may reveal an abundance of lymphoid cells in tumor tissue, histological examination may demonstrate a lack of lymphocyte infiltration into tumors with their presence confined to peri-tumoral lymph nodes. This exact scenario was seen in the analysis of CD40 immunotherapy in KPC mice where it was initially concluded that CD40 therapy induced potent activation of T cells in tumor tissue (Beatty et al., 2011). However, on histological examination it became apparent that activated T cells were not present within tumors, but rather were localized to adjacent lymph nodes. For this reason, it is recommended that immunohistochemistry or immunofluorescence microscopy be used to compliment studies using flow cytometry to investigate leukocyte infiltration into PDAC.
The value of genetic models for evaluating immunotherapy in PDAC
The potential of immunotherapy to provide clinical benefit for patients with PDAC has been observed now in several studies. For example, an allogeneic whole tumor cell vaccine (GVAX) combined with a mesothelin-specific Listeria-based vaccine was found to improve the overall survival of PDAC patients with chemotherapy-refractory disease in a recent multicenter, randomized, phase II study (Le et al., 2015b). In addition, GVAX in combination with anti-CTLA-4 immunotherapy has produced signs of clinical benefit in a subset of PDAC patients (Le et al., 2013). The immunological significance of these findings is strengthened by evidence demonstrating that GVAX vaccination in combination with anti-CTLA-4 therapy induces T lymphocyte infiltration into tumors and the development of tertiary-lymphoid aggregates suggestive of anti-tumor immune activity (Lutz et al., 2014).
Macrophages, immature myeloid cells, and granulocytes infiltrate PDAC and can be supportive of tumor progression and metastasis. Strategies to deplete these cells, block their recruitment to tissues, or redirect their biology are being evaluated preclinically as well as in ongoing clinical trials (Long and Beatty, 2013). In contrast, the exclusion of T cells from PDAC tumors poses a significant challenge to T cell immunotherapy, which relies on the capacity of tumor-specific T cells to infiltrate tumor tissue, recognize malignant cells and lyse them. Thus, poor infiltration of T cells into PDAC lesions may explain the lack of efficacy seen to date with immune checkpoint inhibitors.
The ability of the KPC model to mirror immunobiology seen in human PDAC forms the basis for its value in studying immunotherapy including strategies to modulate the inflammatory reaction to PDAC and invoke tumor-specific T cell immune responses. Genetically engineered mouse models can be used to conduct “co-clinical” trials in which therapeutic interventions administered to KPC mice match the treatment design of an ongoing human clinical trial in patients. In this regard, the KPC model has been used in combination with a clinical trial to inform the mechanistic underpinnings of tumor responses to immunotherapy with an antagonistic CD40 antibody (Beatty et al., 2011; Beatty et al., 2013). An obvious advantage of the KPC model compared to a clinical trial is the ability to include appropriate treatment control groups and to conduct mechanistic studies. Using this approach of simultaneous human and mouse studies, a CD40 agonist was found to induce peripheral blood monocytes to acquire anti-tumor and anti-fibrotic properties leading to tumor regressions in both KPC mice and humans (Beatty et al., 2011). This represented a novel mechanism of action for CD40 antibodies, which are best known for their capacity to “license” antigen presenting cells with T cell stimulatory properties (Diehl et al., 1999; French et al., 1999; Sotomayor et al., 1999).
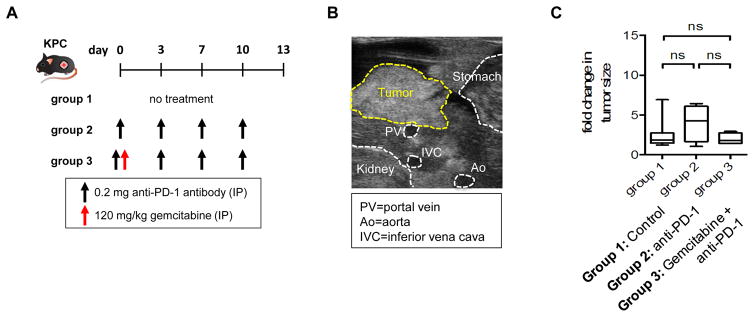
The KPC mouse model has also reproduced findings of immunotherapy seen in patients with PDAC. For example, limited efficacy in human PDAC has been observed with single agent immunotherapies, including anti-PD-L1 and anti-CTLA-4 antibodies (Brahmer et al., 2012; Royal et al., 2010). Feig et al. reported that anti-PD-L1 and anti-CTLA-4 antibodies also fail to impact tumor progression in KPC mice (Feig et al., 2013). We have similarly found that treatment of KPC mice with an anti-PD-1 antibody either alone or in combination with gemcitabine chemotherapy produces no impact on tumor outgrowth (Fig. 4). These similarities between mice and humans in the responsiveness of PDAC to immunotherapy strengthens the validity of the KPC model as a tool to unravel immune mechanisms that may regulate T cell exclusion and strategies to convert PDAC into an immunogenic tumor.
Figure 4. Effect of PD-1 blockade on tumor growth in KPC mice.
(A) Schematic of study design. Tumor-bearing KPC mice were randomized into 3 groups: (i) Group 1 (n=19): no treatment, (ii) Group 2 (n = 4): anti-PD-1 antibody (clone RMP1–14, 0.2 mg IP twice weekly), and (iii) Group 3 (n=4): gemcitabine (120 mg/kg IP once) plus anti-PD-1 antibody. Tumor size was assessed by ultrasound on days 0 and 13 after treatment. (B) Representative ultrasound image of primary pancreatic tumor in KPC mice. Ultrasonography was performed as previously described (Beatty et al., 2011; Sastra and Olive, 2013). Yellow dashed lines identify tumor border and white dashed lines indicate normal organs and blood vessels (Ao, Aorta; IVC, inferior vena cava; PV, portal vein). (C) Fold change in tumor size. ns, not significant by unpaired 2-tailed Student’s t test. IP=intraperitoneal injection.
The KPC model has been studied for its ability to restrict the infiltration of T cells into tumor tissue. One mechanism that has been identified involves chemokine (C-X-C motif) ligand 12 (CXCL12), which is produced by fibroblasts within the tumor microenvironment. By binding to its receptor, CXCR4, which is present on tumor cells, CXCL12 has been suggested to inhibit T cell entry into PDAC in the KPC model (Feig et al., 2013). In this study, AMD3100, a CXCR4 inhibitor, induced T cell infiltration into PDAC and acted synergistically with an anti-PD-L1 antibody to induce rapid tumor regressions in KPC mice. Based on this finding, an ongoing clinical trial is investigating continuous infusion of AMD3100 in patients with advanced PDAC (NCT02179970). Overall, these findings identify the potential importance of the tumor microenvironment in restricting T cell infiltration into tumor lesions. However, leukocytes present outside of the tumor microenvironment may also be involved in actively establishing PDAC as a site of immune privilege. To this end, we have recently reported that the infiltration of T cells into PDAC can be regulated by extra-tumoral macrophages (Beatty et al., 2015). Depletion of macrophages residing outside of the tumor microenvironment using clodronate-encapsulated liposomes (CEL) was found to induce T cell infiltration into PDAC lesions and when administered in combination with gemcitabine chemotherapy and a CD40 agonist, T cell-dependent tumor regressions were observed in KPC mice. Together, these two studies highlight the potential of the KPC model for studying T cell exclusion and strategies to enhance the potential of immunotherapy in PDAC. Findings discovered in KPC mice may also have implications beyond PDAC given that other solid malignancies, such as ovarian cancer (Zhang et al., 2003) and colorectal cancer (Galon et al., 2006), can at times demonstrate T cell exclusion and often harbor strong inflammatory reactions that are hallmarks of the KPC model.
Application of genetic models of PDAC and their variants to preclinical development of immunotherapy
Because of the complexity of the KPC model, including breeding, the heterogeneity of tumors, and the stochastic nature of PDAC development, many investigators have sought out alternative approaches to genetic mouse models of PDAC, including orthotopic (Mitchem et al., 2013)and subcutaneous implantation models (Beatty et al., 2015; Winograd et al., 2015) as well as intrasplenic and intravenous injection models of metastasis (Costa-Silva et al., 2015; Soares et al., 2014). These models most commonly utilize PDAC cell lines, either commercially available or derived from syngeneic genetic models of PDAC. Tumor organoids can also be established from primary tumors and used in an orthotopic or subcutaneous implantation model (Boj et al., 2015; Hwang et al., 2015). In addition, pieces of primary tumor tissue can be excised and re-implanted into syngeneic mice (Beatty et al., 2015). Overall, these approaches can be cost-effective and while they do reduce the degree of tumor heterogeneity seen between mice in a group, they can still maintain a significant degree of tumor clonal diversity.
The immunobiology of PDAC implantation models using low-passage tumor cell lines derived from primary tumors arising spontaneously in KPC mice is similar in many aspects to the parental primary tumors. For example, by histological analysis implanted PDAC tumors demonstrate similar levels of fibrosis (Fig. 5) and leukocyte infiltration as is seen in primary tumors arising in the KPC model (Fig. 2 and 3). As a result, these models may be used to understand some key aspects by which (i) tumors regulate T cell exclusion, (ii) the myeloid compartment can be redirected to modulate the tumor microenvironment including vascularity and fibrosis, and (iii) tumors adapt to immune pressure.
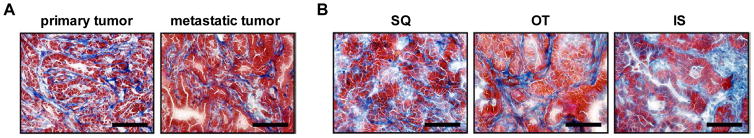
Figure 5. Tumor-associated fibrosis in primary and metastatic tumors in KPC mice and in implanted tumors.
(A) Representative Masson’s trichrome stain (40× field) of primary (left) and metastatic (right) tumor in KPC mice (n=4–5 per group). (B) Representative Masson’s trichrome stain (40× field) of SQ (left) and OT (middle) tumors, and metastatic tumor in the liver (right). Scale bars denote 100 μm.
Tumor cell lines derived from KPC mice are poorly immunogenic. As such, their in vivo growth rates in immunodeficient and immunocompetent mice are indistinguishable (Lo et al., 2015). This observation contrasts results reported for other more highly mutated tumors that have been used to establish the concept of immunoediting, a dynamic process in which tumor cells react to selective immune pressure by decreasing their immunogenicity leading to an acquired resistance to immune-mediated elimination (Matsushita et al., 2012). Furthermore, this observation raises the possibility that the biology of tumors, such as PDAC, which harbor few somatic mutations may be more appropriately studied using genetic models that also lack an abundance of somatic mutations. One argument against this rationale is the potential importance of neoantigens derived from somatic mutations, which may serve as immunogenic targets necessary for the success of currently available immunotherapeutic maneuvers. Neoantigens are proteins that become mutated during tumor development and lead to novel peptide sequences recognized by the immune system as foreign – thus, neoantigens enhance the immunogenicity of a cancer cell (Schumacher and Schreiber, 2015). However, shared antigens (i.e. non-mutated self-proteins that may be overexpressed or aberrantly expressed in tumor cells), while potentially less immunogenic than neoantigens, may also serve as potential immune targets (Le et al., 2015b; Lutz et al., 2014; Thomas et al., 2004). Certainly, we and others have found that T cells can be induced to mediate anti-tumor activity in the KPC model, which is based on only two mutations (Beatty et al., 2015; Feig et al., 2013; Keenan et al., 2014; Stromnes et al., 2015). However, the antigens targeted by T cells in PDAC remain ill-defined.
We have studied five routes of implantation of KPC-derived tumor cell lines (i.e. subcutaneous, intravenous, intrasplenic, intraperitoneal, and orthotopic) in the laboratory to investigate the impact of anatomic location on the immunobiology of PDAC and to establish models that can inform the role of immunotherapy aimed at targeting PDAC lesions residing in distinct anatomic compartments (Fig. 6). The major strength of subcutaneous injection over other routes of tumor cell implantation is the ease of this approach and the ability to monitor tumor development and growth using calipers. While PDAC tumors rarely metastasize to the skin in patients, this strategy can be informative for examining mechanisms and potential efficacy of immunotherapy. In addition, we have found that tumors implanted subcutaneously can metastasize to the lung and therefore, this model may be useful for studying PDAC lung metastasis.
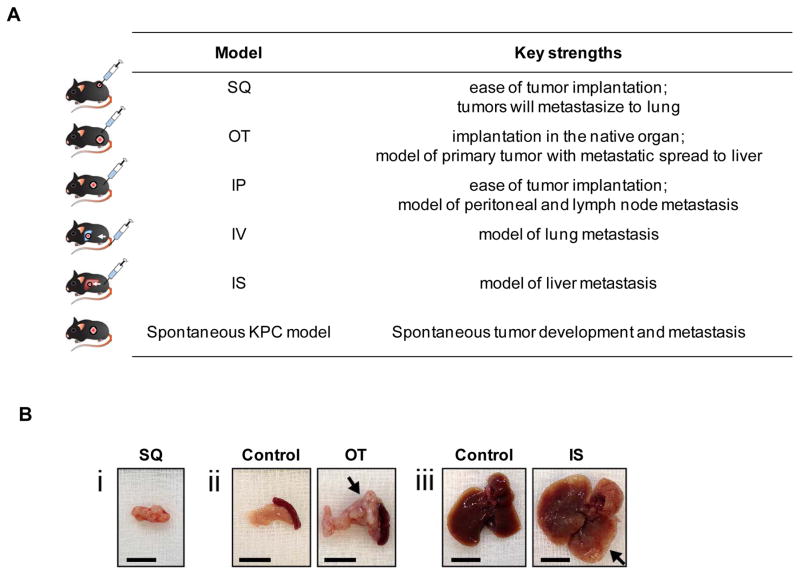
Figure 6. Implantation models of PDAC using KPC-derived tumor cell lines.
(A) Table showing various implantation models based on multiple routes of injection of KPC-derived PDAC cell lines, including subcutaneous (SQ), orthotopic (OT), intraperitoneal (IP), intravenous (IV) and intrasplenic (IS) routes of injection. (B) Representative images of (i) SQ tumor, (ii) normal pancreas with adjacent spleen of a control mouse (left) and pancreatic tumor with adjacent spleen from OT injected mouse (right), and (iii) normal liver from control mouse (left) and liver with numerous metastases from IS injected mouse (right). Scale bars denote 1 cm. Arrows indicate tumor mass.
Orthotopic implantation of PDAC cells is an attractive alternative to subcutaneous implantation because tumors will arise in their native organ and will metastasize to distant sites. However, monitoring of tumors implanted orthotopically requires imaging strategies similar to the KPC model, such as ultrasonography. Bioluminescence imaging can also be incorporated by engineering PDAC cell lines to express a luciferase reporter (Fig. 7). An advantage of bioluminescence is the ability to investigate the impact of therapeutic intervention on tumor viability rather than just tumor size, which in PDAC includes not only cellular content but also fibrosis.
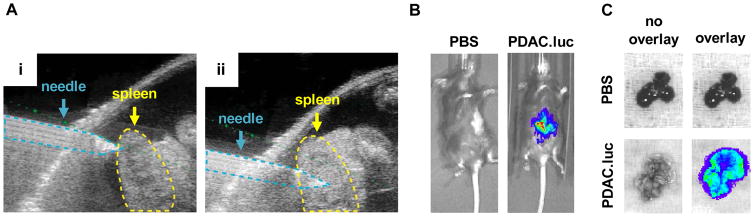
Figure 7. Bioluminescence imaging (BLI) for monitoring PDAC growth.
Syngeneic C57BL/6J mice were injected intrasplenically with PBS or 5×105 cells from a KPC-derived PDAC cell line engineered to express luciferase (PDAC.luc). Mice were imaged 3 weeks post-injection as previously described (Beatty et al., 2011; Sastra and Olive, 2013).(A) Representative ultrasound images of intrasplenic injection of tumor cells into the spleen, (i) pre- and (ii) post-injection. (B) Representative in vivo BLI images. For BLI imaging, D-luciferin substrate was injected intraperitoneally and images were acquired approximately 15 minutes post-injection using the Perkin Elmer IVIS Spectrum. (C) Representative ex vivo BLI images of the liver.
Intravenous, intraperitoneal and intrasplenic routes of administration of PDAC cell lines can be used to model lung metastasis, peritoneal and lymph node metastasis, and liver metastasis, respectively. With intrasplenic injection, we have used both ultrasound guided injections into the spleen as well as direct implantation involving surgery. Both strategies produce liver metastases. However, while the non-invasiveness of ultrasound-guided injections is attractive, peritoneal drop metastases can occur as well as tumor lesions in the spleen. These issues can be obviated by direct intrasplenic injection followed by surgical removal of the spleen. With this approach, tumor cells will exit the spleen via the splenic vein, enter the portal vein, and deposit in the liver forming metastases.
With each of these implantation models, we have found that the histological appearance and leukocyte complexity of developing tumors is similar to parental tumors arising spontaneously in KPC mice. These models also offer the opportunity to investigate, under controlled conditions, the capacity of immunotherapy to target PDAC in distinct anatomic locations where the phenotypic characteristics of the immune response may differ despite similarities in leukocyte recruitment. In addition, tumor cell lines can be genetically manipulated using Cas9/CRISPR technology or other gene knockdown approaches (e.g. shRNA) to study the role of tumor-derived molecules in regulating immunobiology and the efficacy of immunotherapy (Bayne et al., 2012).
However, it is important to understand that the responsiveness of implantable and genetic mouse models of PDAC to immunotherapy may vary dramatically despite the similar capacity of tumors in both models to exclude T cells and recruit a strong inflammatory response. For example, we have found that a vaccine comprising gemcitabine chemotherapy and a CD40 agonist can induce T cell-dependent tumor regressions of both KPC-derived tumor cell lines and KPC-derived primary tumor tissue injected subcutaneously (Beatty et al., 2015). However, when this same vaccine strategy is administered to KPC mice with spontaneously arising tumors, regressions are observed but are dependent on macrophages and occur even in the absence of T cells. Together, these models of PDAC have revealed distinct mechanisms by which the same immunotherapeutic approach may impact PDAC biology. However, when this treatment was administered to patients, findings suggestive of a T cell independent mechanism of action, similar to that seen in KPC mice, were observed (Beatty et al., 2011; Beatty et al., 2013). While these findings provide further validation for the KPC model of PDAC, it does not necessarily dismiss the potential utility of implantable models of PDAC. In contrast, we propose that implantable models may be quite useful for studying how PDAC may adapt to immune pressure imposed by tumor-specific T cells. In addition, we have found that implantable models can be invaluable for studying the capacity of macrophages to be redirected to alter the tumor microenvironment, in particular the fibrotic reaction that surrounds PDAC which is preserved in implantable models (Fig. 5). However, if a therapy is being evaluated for potential translation to the clinic, we recommend that findings observed in implantable models also be validated in a genetically engineered mouse model. Thus, it is becoming more common to conduct initial mechanistic studies in implantable models of PDAC with validation of findings and correlatives in a genetic model such as the KPC model.
Conclusions
Translation of laboratory findings into the clinic is benefited by a preclinical cancer model that can reliably inform the efficacy and biology of therapies. The KPC model and other genetic models of PDAC are valuable resources for studying immunotherapy and the immunobiology of PDAC given their ability to reproduce salient features of disease progression seen in humans including the immune reaction. PDAC that arises in the KPC model reproduces a dense desmoplastic reaction infiltrated by myeloid cells with rare penetrance by effector T cells. Malignant cells in KPC mice can metastasize to similar organs seen in human disease and therefore, this model offers an opportunity to also study the immunobiology and its diversity in lesions that are frequently difficult to assess in patients. Overall, the immune reaction to PDAC as revealed by genetic models of this disease appears primarily supportive of tumor development and progression. The KPC model can be used to dissect immune mechanisms of action and to evaluate the efficacy of immunotherapies designed to modulate the immune microenvironment of PDAC and to harness immune effectors. Multiple variants of the KPC model including high-throughput implantable models can be incorporated to establish a pipeline approach to drug development. Thus, the KPC model and similar genetic models, despite lacking a high mutation burden and the potential for abundant neoantigens, represent practical and reproducible systems for the discovery and evaluation of immuno-oncology therapeutic agents.
Acknowledgments
NIH-funded author: Yes. This work was supported by a National Institutes of Health grant K08 CA138907 (G.L.B.), by a National Institutes of Health grant F30 CA196106 (J.W.L.), by a Molecular Biology and Molecular Pathology and Imaging Cores of the Penn Center for the Molecular Studies in Digestive and Liver Diseases grant (P30 DK050306), by the 2015 Pancreatic Cancer Action Network-AACR Career Development Award, supported by an anonymous foundation, Grant Number 15-20-25-BEAT, and by the Damon Runyon Cancer Research Foundation grant DRR-15-12 for which G.L.B. is the Nadia’s Gift Foundation Innovator of the Damon Runyon-Rachleff Innovation Award.
We thank Santiago Lombo Luque for technical assistance. This work was supported by a National Institutes of Health grant K08 CA138907 (G.L.B.); by a National Institutes of Health grant F30 CA196106 (J.W.L.); by a Molecular Biology and Molecular Pathology and Imaging Cores of the Penn Center for the Molecular Studies in Digestive and Liver Diseases grant (P30 DK050306); by the 2015 Pancreatic Cancer Action Network-AACR Career Development Award, supported by an anonymous foundation, Grant Number 15-20-25-BEAT; and by the Damon Runyon Cancer Research Foundation grant DRR-15-12 for which G.L.B. is the Nadia’s Gift Foundation Innovator of the Damon Runyon-Rachleff Innovation Award.
Footnotes
Conflict of interest statement: The authors have no competing financial interests.
References
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. The New England journal of medicine. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C, Pederzoli P, Falconi M. Resectable pancreatic cancer: who really benefits from resection? Ann SurgOncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science (New York, NY. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21:687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Paterson Y. IFN-gamma can promote tumor evasion of the immune system in vivo by down-regulating cellular levels of an endogenous tumor antigen. J Immunol. 2000;165:5502–5508. doi: 10.4049/jimmunol.165.10.5502. [DOI] [PubMed] [Google Scholar]
- Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O’Dwyer P. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, Vonderheide RH. Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages. Gastroenterology. 2015;149:201–210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boj SF, Hwang CI, Baker LA, Chio, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Ohlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, BorelRinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DZ, Ma Y, Ji B, Wang H, Deng D, Liu Y, Abbruzzese JL, Liu YJ, Logsdon CD, Hwu P. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17:7015–7023. doi: 10.1158/1078-0432.CCR-11-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin EK, Scarlett CJ. A historical perspective of pancreatic cancer mouse models. Semin Cell Dev Biol. 2014;27:96–105. doi: 10.1016/j.semcdb.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature cell biology. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCant BT, Principe DR, Guerra C, Pasca di Magliano M, Grippo PJ. Utilizing past and present mouse systems to engineer more relevant pancreatic cancer models. Front Physiol. 2014;5:464. doi: 10.3389/fphys.2014.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- Ding Y, Cravero JD, Adrian K, Grippo P. Modeling pancreatic cancer in vivo: from xenograft and carcinogen-induced systems to genetically engineered mice. Pancreas. 2010;39:283–292. doi: 10.1097/MPA.0b013e3181c15619. [DOI] [PubMed] [Google Scholar]
- Faca VM, Song KS, Wang H, Zhang Q, Krasnoselsky AL, Newcomb LF, Plentz RR, Gurumurthy S, Redston MS, Pitteri SJ, Pereira-Faca SR, Ireton RC, Katayama H, Glukhova V, Phanstiel D, Brenner DE, Anderson MA, Misek D, Scholler N, Urban ND, Barnett MJ, Edelstein C, Goodman GE, Thornquist MD, McIntosh MW, DePinho RA, Bardeesy N, Hanash SM. A mouse to human search for plasma proteome changes associated with pancreatic tumor development. PLoS medicine. 2008;5:e123. doi: 10.1371/journal.pmed.0050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl AcadSci U S A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, Hebrok M. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, NY. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Maelandsmo GM, Roman-Roman S, Seoane J, Trusolino L, Villanueva A. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer discovery. 2014;4:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J SurgPathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Hwang CI, Boj SF, Clevers H, Tuveson DA. Pre-clinical Models of Pancreatic Ductal Adenocarcinoma. J Pathol. 2015 doi: 10.1002/path.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science (New York, NY. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science (New York, NY. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, Rucki AA, Gunderson AJ, Coussens LM, Brockstedt DG, Dubensky TW, Jr, Hassan R, Armstrong TD, Jaffee EM. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology. 2014;146:1784–1794 e1786. doi: 10.1053/j.gastro.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Jr, Donehower RC, Jaffee EM, Laheru DA. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA., Jr PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine. 2015a;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T, Jr, Brockstedt DG, Jaffee EM. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J ClinOncol. 2015b;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algul H. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Lesokhin AM, Callahan MK, Postow MA, Wolchok JD. On being less tolerant: enhanced cancer immunosurveillance enabled by targeting checkpoints and agonists of T cell activation. Science translational medicine. 2015;7:280sr281. doi: 10.1126/scitranslmed.3010274. [DOI] [PubMed] [Google Scholar]
- Liou GY, Doppler H, Necela B, Edenfield B, Zhang L, Dawson DW, Storz P. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer discovery. 2015;5:52–63. doi: 10.1158/2159-8290.CD-14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A, Wang LC, Scholler J, Monslow J, Avery D, Newick K, O’Brien S, Evans RA, Bajor DL, Clendenin C, Durham AC, Buza EL, Vonderheide RH, June CH, Albelda SM, Pure E. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer research. 2015 doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KB, Beatty GL. Harnessing the antitumor potential of macrophages for cancer immunotherapy. Oncoimmunology. 2013;2:e26860. doi: 10.4161/onci.26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddipati R, Stanger BZ. Pancreatic Cancer Metastases Harbor Evidence of Polyclonality. Cancer discovery. 2015;5:1086–1097. doi: 10.1158/2159-8290.CD-15-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann O, Strate T, Schneider C, Yekebas EF, Izbicki JR. Surgery for advanced and metastatic pancreatic cancer--current state and perspectives. Anticancer Res. 2006;26:681–686. [PubMed] [Google Scholar]
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, Hundal J, Wendl MC, Demeter R, Wylie T, Allison JP, Smyth MJ, Old LJ, Mardis ER, Schreiber RD. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, Jaffee EM, Drake CG, Housseau F, Maitra A, Kolls JK, Sears CL, Pardoll DM, Leach SD. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer cell. 2014;25:621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer research. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine 2015 [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277–5287. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- Perez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What we have learned about pancreatic cancer from mouse models. Gastroenterology. 2012;142:1079–1092. doi: 10.1053/j.gastro.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rei M, Pennington DJ, Silva-Santos B. The emerging Protumor role of gammadelta T lymphocytes: implications for cancer immunotherapy. Cancer research. 2015;75:798–802. doi: 10.1158/0008-5472.CAN-14-3228. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Thege FI, Santana SM, Lannin TB, Saha TN, Tsai S, Maggs LR, Kochman ML, Ginsberg GG, Lieb JG, Chandrasekhara V, Drebin JA, Ahmad N, Yang YX, Kirby BJ, Stanger BZ. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146:647–651. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine. 2015a;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A. Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2015b;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Manz MG, Flavell RA. Development and function of human innate immune cells in a humanized mouse model. Nature biotechnology. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nature reviews. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Current opinion in immunology. 2015;33:101–111. doi: 10.1016/j.coi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Sastra SA, Olive KP. Quantification of murine pancreatic tumors by high-resolution ultrasound. Methods in molecular biology (Clifton, NJ. 2013;980:249–266. doi: 10.1007/978-1-62703-287-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J ClinOncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, NY. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (New York, NY. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, Thompson JD, Cheng JH, BouReslan H, Ho CC, Cao TC, Lee CV, Nannini MA, Fuh G, Carano RA, Koeppen H, Yu RX, Forrest WF, Plowman GD, Johnson L. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nature biotechnology. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- Soares KC, Foley K, Olino K, Leubner A, Mayo SC, Jain A, Jaffee E, Schulick RD, Yoshimura K, Edil B, Zheng L. A preclinical murine model of hepatic metastases. J Vis Exp. 2014;51677 doi: 10.3791/51677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky HI. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Schmitt TM, Hulbert A, Brockenbrough JS, Nguyen HN, Cuevas C, Dotson AM, Tan X, Hotes JL, Greenberg PD, Hingorani SR. T Cells Engineered against a Native Antigen Can Surmount Immunologic and Physical Barriers to Treat Pancreatic Ductal Adenocarcinoma. Cancer cell. 2015;28:638–652. doi: 10.1016/j.ccell.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, Laheru DA, Goggins M, Hruban RH, Jaffee EM. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grutzmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA, Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen CB, Olive KP. Genetically engineered mouse models of pancreatic cancer. Cancer J. 2012;18:502–510. doi: 10.1097/PPO.0b013e31827ab4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, Hostetter G, Shepard HM, Von Hoff DD, Han H. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]