Abstract
Objective
To examine the extent to which nicotine dependence alters endogenous opioid regulation of the hypothalamic-pituitary-adrenocortical (HPA) axis functions. Endogenous opiates play an important role in regulating mood, pain, and drug reward. They also regulate the HPA functions. Previous work has demonstrated an abnormal HPA response to psychological stress among dependent smokers.
Methods
Smokers and nonsmokers (total n = 48 participants) completed two sessions during which a placebo or 50 mg of naltrexone was administered, using a double-blind design. Blood and saliva samples, cardiovascular and mood measures were obtained during a resting absorption period, after exposure to two noxious stimuli, and during an extended recovery period. Thermal pain threshold and tolerance were assessed in both sessions. Participants also rated pain during a 90-second cold pressor test.
Results
Opioid blockade increased adrenocorticotropin, plasma cortisol, and salivary cortisol levels; these increases were enhanced by exposure to the noxious stimuli. These responses were blunted in smokers relative to nonsmokers. Smokers tended to report less pain than nonsmokers, and women reported more pain during both pain procedures, although sex differences in pain were significant only among nonsmokers.
Conclusions
We conclude that nicotine dependence is associated with attenuated opioid modulation of the HPA. This dysregulation may play a role in the previously observed blunted responses to stress among dependent smokers.
Keywords: smoking, endogenous opioids, cortisol, adrenocorticotropin, stress, pain, naltrexone
INTRODUCTION
The hypothalamic-pituitary-adrenocortical (HPA) axis is regulated by the endogenous opioid system (1–3). Previous research has documented altered HPA functions among dependent cigarette smokers (4). For example, smokers exhibit a smaller cortisol response to acute stress when compared with nonsmokers (4–6), and this blunted cortisol response predicts difficulties during smoking abstinence (7). We examined the extent to which the endogenous opioid system is altered by nicotine dependence, to evaluate a potential mechanism for the dysregulated HPA response to stress in smokers (8).
Several studies have evaluated the functional status of the hypothalamic-opioid tone, using opioid receptor blockade (9–11). Removing the opioidergic inhibitory inputs to neurons that release corticotrophin-releasing factor (CRF) in the hypothalamus, using opioid receptor blockade medications, increases plasma adrenocorticotropin (ACTH) and cortisol release in nonsmokers (12–14). This opioid inhibitory effect may be altered by prolonged nicotine exposure, and studies in nicotine-dependent individuals support this possibility (15).
Among nonsmokers, women exhibit less pain tolerance than men do (16–21). The extent to which chronic smoking influences sex differences in pain sensitivity and opioid modulation of pain has not been directly investigated. One recent study assessed pain sensitivity in smokers and nonsmokers and found attenuated pain perception in smokers (22). Furthermore, women smokers exhibited lower pain sensitivity than their nonsmoking counterparts, suggesting that sex differences in pain regulatory mechanisms may be altered by chronic smoking (22).
This study was designed to examine differences between smokers and nonsmokers in opiate modulation of HPA hormone production and pain sensitivity. Two assessment sessions were conducted post administration of placebo or naltrexone. During these sessions, blood and saliva samples were collected repeatedly before and after naltrexone or placebo administration for measurements of ACTH and cortisol before and after completing two pain-induction procedures.
MATERIALS AND METHODS
Participants
Smokers and nonsmokers were recruited from the community by posters and newspaper advertisements. Participants had to meet the following criteria: 1) no regular use of prescribed or over-the-counter medications except contraceptives; 2) no current or prior treatment for hypertension, renal or hepatic disease, current or history of cardiac or other chronic diseases (e.g., coronary heart disease, diabetes, neurological disorders, thyroid disorder, respiratory disorders); 3) no current or history of major psychiatric disorders (e.g., depression, schizophrenia, alcohol and drug abuse); 4) no current opiate dependence, recent daily opiate use, or use of any narcotic medication within 3 days before the study; 5) no pregnancy; and 6) weight within ±30% of Metropolitan Life Insurance norms. Smokers were included if they had smoked at least 10 cigarettes per day for the past 2 years and were not interested in cessation at the time of the study. Nonsmokers were included if they had never smoked over the last 5 years or if they had smoked <100 cigarettes over their lifetime.
Participants meeting the inclusion criteria were scheduled for a medical examination to confirm health status and absence of contraindications to administration of naltrexone. To minimize any withdrawal effects, smokers were asked to maintain their normal smoking patterns and were asked to smoke one cigarette of their preferred brand 30 minutes before each laboratory session. However, they were deprived from smoking during the laboratory session (approximately 4 hours). Participants signed a consent form approved by the Institutional Review Board of the University of Minnesota and received a monetary incentive for participation (US $20 per hour).
Apparatus and Measures
Cold Pressor Test (CPT)
CPT has been established as a reliable and valid method in pain measurement (23–25). Pain was assessed during and after CPT, using a visual, numerical rating scale with a range from 0 (not at all painful) to 100 (extremely painful); additional labels on 25 (somewhat painful), 50 (moderately painful), and 75 (very painful) were used to rate pain intensity during and after the CPT. The CPT apparatus consisted of a one-gallon container that was filled with an ice-water slurry (temperature range = 0–4°C). Participants were asked to rate their pain at 15-second intervals throughout the 90-second exposure to the CPT and the 90-second recovery period. We also evaluated participants’ global pain experience, using the short form of the McGill Pain Questionnaire (MPQ) (26), which includes sensory and affective subscales. MPQ instructions asked participants to recall the most intense pain they felt during the study.
Thermal Stimuli
Thermal pain stimuli were delivered to the skin of the left volar forearm, using a computer-controlled 2 cm2 Peltier contact thermode affixed in place with a Velcro strap. Temperature was monitored by a contactor-contained thermistor (Medoc TSA 2001, Minneapolis, Minnesota). The thermode was returned to the adapting temperature (32°C) between trials by active cooling at a rate of 10°C/second. Thermal pain threshold and tolerance were assessed, using an ascending method of limits with a staircase ramp of 1°C/second (27).
In the threshold assessment, participants were instructed to press a button when the thermal stimulus first felt painful. For the tolerance assessment, subjects were instructed to press the button when the pain became intolerable. The assessment was repeated four times at each skin site and the average of the last three trials was calculated to determine thermal pain threshold and tolerance. To avoid sensitization or habituation, the position of the thermode was moved approximately 1 cm after each trial (but still remained on the ventral forearm). The maximum temperature allowed was 50°C, and if participants failed to report pain threshold or pain tolerance before reaching the maximum, a value of 50°C was entered for that variable.
Participants also rated their pain to a series of thermal stimuli. There were five thermal intensities: 45°, 46°, 47°, 48°, and 49°C; and after each stimulus, the participant rated intensity of pain, using the visual, numerical rating scale. Each stimulus was presented four times in random order, and the responses were averaged for each temperature. Participants also completed the MPQ after the completion of the thermal pain test.
Hormonal Measures
During each session, blood and saliva samples were collected with one sample before drug or placebo administration and seven samples thereafter. Blood samples were assayed for ACTH and cortisol. Saliva samples were assayed for cortisol. Blood collection was accomplished, using a 20-gauge intravenous Teflon catheter inserted in a left forearm vein. The catheter was fitted with a rubber infusion plug through which samples were drawn. Sterile saline for injection was used to keep the system patent between sampling. Each sample was collected in an 8-mL ethylenediaminetetraacetic acid (EDTA) Vacutainer tube. At the end of the session, samples were centrifuged and stored at −70°C. ACTH was assessed using RIA (Nicols Institute, Bad Nauheim, Germany), with a lower sensitivity of 1 pg/ml. Plasma cortisol was assayed, using EIA (DSL, Sinsheim, Germany), with a lower sensitivity of 0.1 μg/dL. Inter- and intra-assay coefficients of variance for these assays were <10%. Saliva samples were collected, using cotton dental rolls held in the mouth until saturated and collected into a plastic tube (Salivette tubes, Sarstedt, Rommelsdorf, Germany). Salivary cortisol assays were conducted, using a time-resolved immunoassay with fluorometric end point detection. The assay has a minimum sensitivity of 0.5 nmol/L (28).
Cardiovascular Measures
Systolic and diastolic blood pressures (BP) and heart rate were measured, using an oscillometric monitor (Dinamap, Critikon, Tampa, Florida).
Mood Reports
Participants completed mood state ratings before each blood sampling. Ratings covered two factors—positive affect and distress—adopted from scales that were previously used successfully in similar laboratory protocols and have satisfactory psychometric properties (29–31). Each item references a 7-point scale anchored by the end points, “Not at All” and “Very Strong.” Items that cover positive affect include ratings of how cheerful, content, calm/relaxed, happy, in control, and interested the participant felt. Distress items include ratings of how irritable, anxious/tense, sad/depressed, angry, confused, and impatient the participant felt.
At the end of each laboratory session, participants also completed a form that contained commonly reported side effects of naltrexone, including nausea, vomiting, agitation, anxiety, lightheadedness, and dizziness. This form has been used previously in clinical and laboratory studies of naltrexone (32).
Procedures
Participants attended a screening session and completed a series of questionnaires. Smokers answered questions about age when they first started to smoke and about their current daily smoking rate. They completed the Fagerström Test of Nicotine Dependence (FTND) (33) to assess the level of nicotine dependence. Participants also completed the State-Trait Anxiety Inventory (Trait-Form) (34) and the 10-item version of the Perceived Stress Scale (35).
Those participants who met the study criteria were scheduled for two sessions, each lasting for approximately 4 hours, separated by a minimum of 72 hours. A pregnancy test was administered during the screening and at the beginning of each laboratory session. To control for circadian rhythm effects, testing sessions started at approximately 12 PM. Women were tested during the follicular phase of their menstrual cycle (i.e., within 3–13 days after the onset of menses). Before each session, participants were asked to abstain from alcohol or analgesic medication for 24 hours before each session and narcotic medication for 3 days before their participation, and they were asked to consume a light meal 1 hour before each session.
At the beginning of each session, women were tested for pregnancy. Then, an IV catheter was inserted, a BP cuff was attached, and participants were asked to sit for 30 minutes at the time predrug baseline BP measurements were obtained at 5-minute intervals. This process was followed by completing a brief questionnaire about mood states, drawing the initial blood sample, and collecting the first saliva sample. Participants then ingested a capsule containing either 50 mg of naltrexone or placebo. The experimenter was blinded to the order of placebo/naltrexone administration. Naltrexone reaches peak plasma concentrations within 1 hour of administration, and its mean elimination half-life is 4 hours. Therefore, the drug administration was followed by a 60-minute rest period to allow peak plasma concentration of naltrexone to be achieved. During this period, participants could read neutral-topic magazines or rest quietly. Cardiovascular measures were obtained every 10 minutes, and blood and saliva samples were collected every 20 minutes post administration of naltrexone or placebo.
The two pain induction procedures were then administered, separated by a 20-minute rest period. After the second pain test (CPT or thermal pain), participants rested for 60 minutes. During this period, cardiovascular parameters were measured every 10 minutes, and blood and saliva samples were collected every 30 minutes.
Dependent Variables and Data Analyses
The primary variables were plasma and salivary cortisol, ACTH concentrations, pain, and cardiovascular and mood state measures. Hormonal levels before drug administration were compared, using a 2 (Smoking Status) × 2 (Sex) × 2 (Drug Condition: placebo, naltrexone) analysis of variance (ANOVA). Subsequent analyses covaried for the predrug levels of each hormonal measure obtained on the placebo day and used a 2 (Smoking Status) × 2 (Sex) × 2 (Drug Condition) × 7 (Samples obtained after ingestion of the drug capsule) multivariate repeated-measure analysis of covariance (MANCOVA), using Smoking Status and Sex as between-subject factors, and Drug Condition as a within-subject variable.
Thermal pain threshold and tolerance as well as pain reports were analyzed using a 2 (Smoking Status: smokers, nonsmokers) × 2 (Sex) × 2 (Drug Condition) ANOVAs. Resting cardiovascular data were analyzed using a 2 (Smoking Status) × 2 (Sex) × 2 (Drug Condition) × 2 (Period: predrug baseline, postdrug rest) ANOVAs. Cardiovascular responses to the CPT were calculated by subtracting baseline predrug values from values obtained during the CPT. These change scores were analyzed, using a 2 (Smoking Status) × 2 (Sex) × 2 (Drug Condition) ANOVA.
Analysis of mood data (both the positive affect and distress) was conducted, using a 2 (Smoking Status) × 2 (Sex) × 2 (Drug Condition) × 8 (Periods) MANOVA. We used Wilkes’ Lambda correction to test period effect and to correct for repeated measures in all applicable analyses (36,37).
RESULTS
Participant Characteristics
Thirty-four men and twenty women (27 smokers and 27 nonsmokers) were eligible to participate. Recruitment for this study took place between 2003 and 2005. A total of 51 participants (25 smokers and 26 nonsmokers) completed at least one session, and 48 participants (23 smokers and 25 nonsmokers) completed both sessions. Due to technical difficulties, plasma hormones were incomplete for nine participants (four smokers and five nonsmokers). However, these participants had complete saliva cortisol samples. Due to missing data, variations exist in degrees of freedom for the reported variables.
Participants’ characteristics are included in Table 1. Smokers and nonsmokers did not differ in age, body mass index (BMI), or years of education (F < 1.4). These measures also did not differ between men and women, with the exception of BMI, which was greater in men than women (F(1,50) = 4.09; p < .05). Smokers reported consuming more daily caffeine-containing drinks than nonsmokers (F(1,50) = 15.21; p < .001). They also reported greater trait anxiety than nonsmokers (F(1,50) = 9.25; p < .01), but groups did not differ in perceived stress (F < 1). Caffeine consumption and anxiety did not correlate with cortisol or ACTH during either the placebo or naltrexone condition (r < .23; p > .16).
TABLE 1.
Subject Characteristics (Mean ± Standard Error of the Mean)
| Subject Characteristics | Nonsmokers (n = 25)
|
Smokers (n = 23)
|
||
|---|---|---|---|---|
| Women (n = 11) | Men (n = 14) | Women (n = 7) | Men (n = 16) | |
| Age (years) | 21.5 ± 1.9 | 21.1 ± 1.7 | 22.3 ± 2.3 | 24.7 ± 1.6 |
| Height (m) | 1.66 ± 0.02 | 1.74 ± 0.02 | 1.68 ± 0.03 | 1.82 ± 0.02 |
| Weight (kg) | 64.3 ± 3.1 | 77.3 ± 2.7 | 67.4 ± 3.7 | 82.8 ± 2.4 |
| BMI (kg/m2)a | 23.2 ± 0.84 | 25.5 ± 0.75 | 23.8 ± 1.03 | 25.0 ± 0.69 |
| Education (years) | 14.5 ± 0.6 | 14.9 ± 0.5 | 14.9 ± 0.7 | 14.3 ± 0.5 |
| Caffeine use (serving)b | 0.7 ± 0.5 | 1.2 ± 0.4 | 3.3 ± 0.6 | 2.4 ± 0.4 |
| STAI-traitb | 35.3 ± 2.2 | 33.4 ± 2.0 | 42.3 ± 2.7 | 39.9 ± 1.8 |
| Perceived stress scale | 16.2 ± 1.4 | 17.1 ± 1.2 | 20.12 ± 1.7 | 18.1 ± 1.1 |
| Smoking rate (cigs/day)c | — | — | 12.6 ± 2.2 | 17.6 ± 1.4 |
| Duration at this rate (years) | — | — | 4.9 ± 2.0 | 6.3 ± 1.3 |
| Age first smoked (years) | — | — | 16.5 ± 1.0 | 15.8 ± 0.7 |
| FTND | — | — | 3.4 ± 0.7 | 4.7 ± 0.4 |
| Resting systolic BP | 103 ± 3.0 | 115 ± 2.8 | 105 ± 3.9 | 118 ± 2.5 |
| Resting diastolic BP | 62 ± 2.1 | 62 ± 1.9 | 65 ± 2.8 | 61 ± 1.7 |
| Resting heart rate | 67 ± 2.3 | 60 ± 2.1 | 66 ± 3.0 | 64 ± 1.5 |
BMI = body mass index; STAI = State-Trait Anxiety Inventory (Trait); FTND = Fagerström Test of Nicotine Dependence; BP = blood pressure.
Men > women, p < .05.
Smokers > nonsmokers, p < .01.
Men > women, p < .10.
When comparing smoking men and women, no gender difference was found in age of smoking initiation or duration at the current rate (F < 1). There were only trends of higher rates of cigarette smoking and higher scores on the measure of nicotine dependence, FTND, in men when compared with women (F = 3.6; p > .07) (Table 1).
Hormonal Responses to Opioid Blockade
Predrug Concentrations
Analyses focusing on hormonal levels before the administration of naltrexone or placebo showed no differences between the two drug conditions in any of these hormonal measures (F < 1). There was no difference between smokers and nonsmokers or between men and women in salivary cortisol concentration (F < 1.8; p > .11). However, smokers had lower levels of plasma cortisol concentrations than nonsmokers, and women had lower ACTH concentrations than men (F(1,37) > 5.8; p < .05). We then used the first sample as a covariate in all subsequent analyses of the hormonal measures.
Adrenocorticotropin
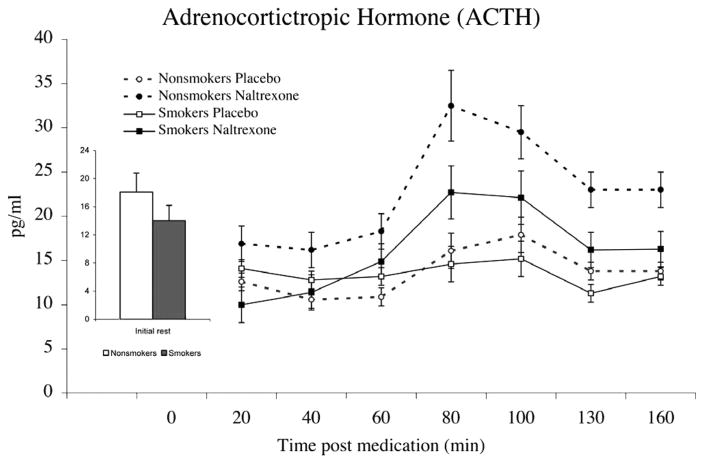
Naltrexone-related increases in ACTH were smaller among smokers relative to nonsmokers, as evidenced by Smoking Status × Drug Condition interaction (F(1,31) = 5.66; p< .05) (Figure 1). In both groups, ACTH demonstrated significant increases post naltrexone administration (F(1,31) = 9.35; p < .01), and this increase was progressive across time, as evidenced by a Period main effect (F(6,26) = 6.10; p < .001). No gender differences were found in ACTH responses to naltrexone (F < 2.6; p > .11). To further test the differences between smokers and nonsmokers in effects of endogenous opioid blockade, levels of hormones obtained in the placebo conditions were subtracted from corresponding values obtained in the naltrexone condition, and subsequent analyses were covaried for premedication levels. Main effect of smoking status on ACTH was found, with smokers exhibiting less ACTH elevation in response to naltrexone than nonsmokers (F(1,32) = 5.82; p = .02).
Figure 1.
Mean adrenocorticotropic hormone (ACTH) concentrations after treatment with placebo or naltrexone. Due to similar changes in men and women, data were collapsed across gender. Bar graph depicts predrug concentrations averaged across the 2 days. Pain tests were administered between minutes 80 and 100 after drug administration. Opioid blockade-related increases in ACTH were smaller among smokers relative to nonsmokers (p < .01). Line bars indicate standard error of the mean.
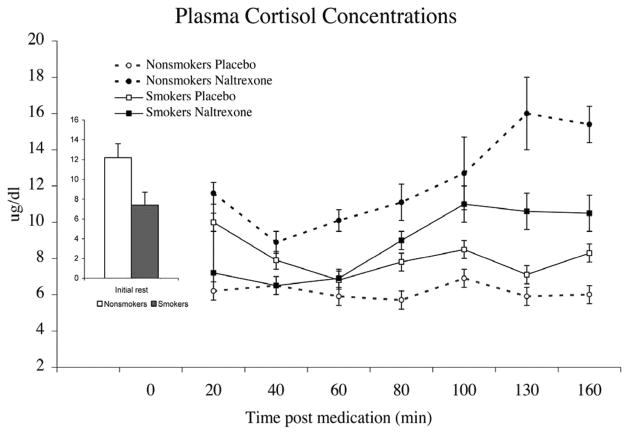
Plasma Cortisol
Naltrexone-related increases in plasma cortisol were blunted among smokers relative to nonsmokers, as shown by a significant Smoking Status × Drug Condition interaction (F(1,32) = 8.96; p < .01) (Figure 2). Other significant results were the progressive increases after naltrexone, as evidenced by a significant Period × Drug Condition interaction (F(6,27) = 4.68; p < .05). No gender differences were found in plasma cortisol responses to opioid blockade (F < 1). Analyses using naltrexone-related changes showed that smokers exhibited lower plasma cortisol response than nonsmokers (F(1,32) = 7.94; p = .01).
Figure 2.
Mean plasma cortisol concentrations obtained after treatment with placebo or naltrexone. Data were collapsed across gender. Bar graph depicts predrug concentrations averaged across the 2 days. Pain tests were administered between minutes 80 and 100 after drug administration. Opioid blockade-related increases in plasma cortisol were smaller among smokers relative to nonsmokers (p < .01). Line bars indicate standard error of the mean.
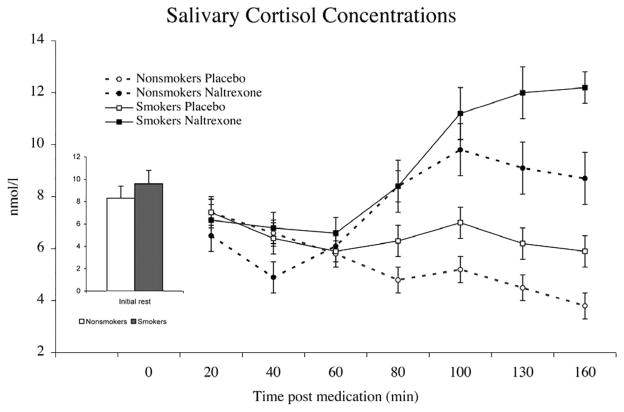
Salivary Cortisol
Although no differences between smokers and nonsmokers were found in salivary cortisol after opioid blockade (F< 1.7), this measure showed significant increases in the naltrexone condition relative to placebo (F(1,41) = 18.9; p < .0001) (Figure 3) and across time after the ingestion of naltrexone (significant Period × Drug Condition interaction (F(6,36) = 5.22; p < .001). There was a trend of greater increases in salivary cortisol in women than in men in the blockade condition (F(1,41) = 3.72; p < .06).
Figure 3.
Mean salivary cortisol concentrations obtained after treatment with placebo or naltrexone. Bar graph depicts predrug concentrations averaged across the 2 days. Pain tests were administered between minutes 80 and 100 after drug administration. Opioid blockade was associated with increased salivary cortisol in smokers and nonsmokers (p < .01). Line bars indicate standard error of the mean.
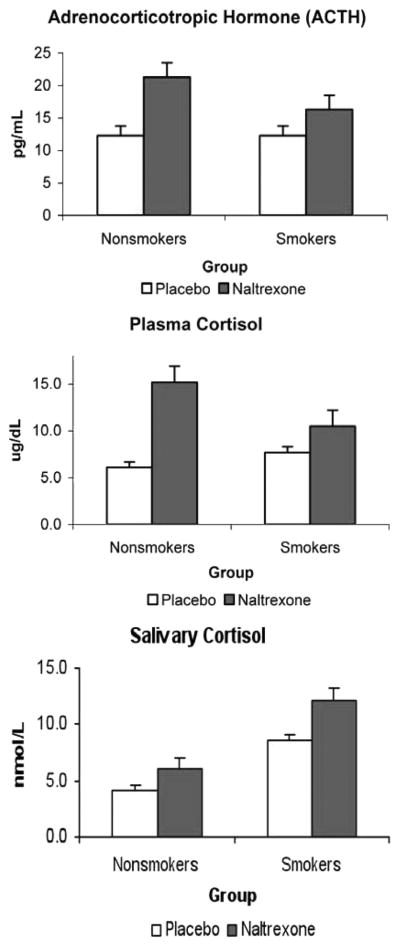
Hormonal Responses to Pain Challenges
To specifically assess the effects of the acute pain, we averaged the two samples after the pain challenges and compared them across the two conditions (placebo and naltrexone) in smokers and nonsmokers. Smokers had greater salivary cortisol concentrations after the pain challenges in both conditions (F(1,42) = 7.3; p < .01). ACTH, plasma cortisol, and salivary cortisol levels significantly increased in response to the opioid blockade (F(1,34) > 10.1; p < .001) (Figure 4). Similar to the overall analysis, these blockade-related increases were attenuated in smokers relative to nonsmokers, especially for plasma cortisol (F(1,34) = 5. 1; p < .05).
Figure 4.
Mean adrenocorticotropic hormone (ACTH) and cortisol concentrations after pain induction procedures after treatment with placebo or naltrexone. ACTH and cortisol showed significant increases in response to opioid blockade during this period (p < .01), although responses tended to be attenuated in smokers. Salivary cortisol levels after the pain challenges in both conditions were higher in smokers than in nonsmokers (p < .05).
Thermal Pain Measures
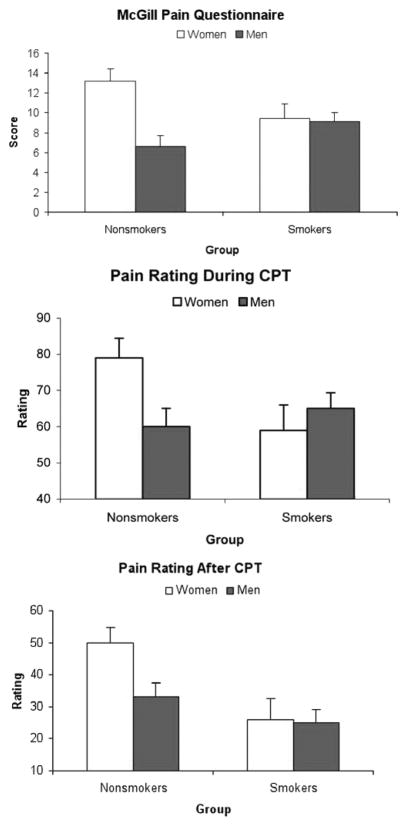
Men had a higher thermal pain threshold and tolerance than women (F(1,44) > 4.12; p < .05). MPQ total scores showed a significant Sex × Smoking Status interaction (F(1,44) = 4.04; p < .05) (Figure 5; upper). This was due to significant gender differences in nonsmokers (F(1,23) = 18.22; p < .001), but not in smokers (F < 1).
Figure 5.
Mean total scores of the McGill Pain Questionnaire (MPQ) obtained after the thermal pain (upper). Mean pain ratings during (middle) and after (lower) the cold pressor test (CPT). Line bars indicate standard error of the mean. Nonsmoking women reported greater pain than nonsmoking men (p < .01), but no such difference was found among smokers (F < 1). Line bars indicate standard error of the mean.
Pain ratings increased with the increase in heat intensity (F(4,38) = 60.4; p < .0001). Smokers reported less pain on all thermal stimuli than nonsmokers (F(1,41) = 7.25; p < .01), but there was a significant Smoking Status × Sex interaction (F(1,41) = 8.66; p < .01). This was due to significant gender differences in nonsmokers (F(1,21) = 14.34; p < .01), but not in smokers (F < 1). There was no effect of opioid blockade on pain ratings in any group (F < 1.8; p > .20).
Cold Pressor Pain Measures
Similar to thermal pain results, a significant Smoking Status × Sex interaction in pain ratings during the CPT was found (F(1,44) = 4.01; p < .05) (Figure 5; middle), and simple effect tests revealed that nonsmoking women reported greater pain than nonsmoking men (F(1,23) = 11.44; p < .01), but no such a difference was found among smokers (F < 1). Women reported greater pain than men on the MPQ after CPT (F(1,44) = 5.87; p < .05), but no effect of drug or smoking status was found (F < 1).
Cardiovascular Measures
Men showed higher resting systolic BP than women (F(1,44) = 18.90; p < .001), and women showed higher resting HR than men (F(1,44) = 9.39; p < .01). These differences remained significant after covarying for caffeine intake and trait anxiety for systolic BP (F(1,42) = 16.63; p < .01) and for HR (F(1,42) = 8.62; p < .01). Analysis of covariance also showed a significant Sex × Drug Condition interaction in HR (F(1,42) = 9.97; p < .01), indicating greater increases in HR in the naltrexone relative to placebo condition in women than in men. Analyses, using CPT reactivity scores, indicated greater diastolic BP responses to the CPT by smokers compared with nonsmokers (F(1,44) = 12.65; p < .001). This effect remained significant, albeit weaker, after controlling for caffeine intake and trait anxiety (F(1,42) = 6.25; p < .05). Systolic and diastolic BP responses to CPT were blunted in the naltrexone condition (F(1,44) > 5.6; p < .05), and this was particularly the case in women, as evidenced by Sex × Drug Condition interactions before (F(1,44) > 5.93; p <.05) and after controlling for caffeine intake and trait anxiety (F(1,42) = 5.71; p < .05).
Mood States
Distress was increased in all groups during the pain tests, as indicated by the Period main effect (F(7,38) = 6.15; p <.001), but there was no effect of the opioid blockade (F <1). Whereas nonsmoking women reported greater distress in both conditions than nonsmoking men, the opposite pattern was found among smokers, as evidenced by a significant Smoking Status × Sex interaction (F(1,44) = 5.82; p <.05). Positive affect was reduced following the pain procedures, as evidenced by a main effect of period (F(7,38) = 7.70; p <.001). No effect of naltrexone or differences between men and women were found on the positive affect scale (F <1). No differences were found between smokers and nonsmokers or between men and women in the side effect profile (F <2.8; p >.10).
DISCUSSION
The attenuated ACTH and plasma cortisol responses to naltrexone among smokers observed in this study suggest that endogenous opioid modulation of the HPA axis may be altered by nicotine dependence in both men and women. These results agree with previous research, indicating reduced HPA responses to stress after chronic exposure to nicotine (5,38). These altered HPA responses may intensify withdrawal symptoms and smoking urges, especially under stress (39), possibly due to reduced abilities to cope with negative affect and to priming for greater reinforcement drawn from smoking, although mechanistic preclinical work is still needed to define the underlying neuronal circuitry that mediate this vulnerability.
The results also agree with and extend a previous smaller study that assessed the effects of naloxone on plasma cortisol concentrations in smokers and nonsmokers (15). In addition to the blunted cortisol response to opioid blockade, our ACTH results demonstrate that these effects are present higher in the HPA axis, possibly at the hypothalamic and limbic levels. Different from the previous study (15), smokers in our study smoked ad libitum until they arrived at the laboratory, although they were not allowed to smoke during the sessions, suggesting that the attenuated opioid regulation was independent of withdrawal.
We note that the hormonal increases in the naltrexone condition were pronounced during and after exposure to the acute pain challenges. Differences between smokers and non-smokers in ACTH and plasma cortisol were also evidenced during this period. In light of the fact that the effects of these challenges were small and insignificant on the placebo day and in the absence of significant group differences in these responses in both conditions, the results suggest that the changes observed in the naltrexone condition were primarily due to the effects of the opioid blockade. Nevertheless, this interpretation can only be confirmed by incorporating a rest day control condition that includes drug administration but no exposure to pain challenges.
Although smokers showed greater salivary cortisol concentrations than nonsmokers in response to pain challenges on both days, there was no group difference in the effects of naltrexone on this measure. This is in contrast to the ACTH and plasma cortisol findings. This possibly reflects different profiles of adrenocortical activities in the two methods of assessment. Plasma cortisol concentrations include the total cortisol levels in the circulation (both free and bound to cortisol-binding proteins). In humans, the percentage of cortisol that is bound to proteins is >90%. Cortisol in saliva represents the free fraction of cortisol; therefore, <10% of the total cortisol pool is reflected (40). It has therefore been recommended that, for careful assessment of adrenocortical functions in response to pharmacological or behavioral challenges, a simultaneous measurement of free (from saliva) and total (from plasma) cortisol levels be assessed (41). To that end, the current results may reflect significant variability in the levels of cortisol binding-globulin in smokers relative to nonsmokers (42), with smokers possibly having a reduced fraction of binding protein when compared with nonsmokers, although this hypothesis has not been directly tested.
The specific neurobiological mechanisms responsible for the blunted opioid effects on HPA are not fully known at this time, but are likely to involve multiple systems. The effects of opioid blockade on HPA activity is mediated by several central processes, including the direct blocking effects of β endorphin and enkephalins on CRF neurons (43) as well as the indirect effects of removing the opioid inhibitory inputs to adrenergic neurons within the locus coeruleus (44). The latter group of neurons project to the paraventricular nucleus, increasing norepinephrine and stimulating the production of CRF (45,46). Blunted responses to opioid blockade may therefore be caused by dysregulation in one or more of these systems.
Nicotine interacts with opioid receptors (47,48) and increases opiate peptides (49,50). It is therefore possible that chronic exposure to nicotine alters opioid receptor properties (e.g., density/and/or binding affinity) and may reduce synaptic opioids in multiple regions of the brain, including the paraventricular nucleus and the locus coeruleus. It is also possible that chronic exposure to nicotine produces a chronic state of activation that attenuates the sensitivity of the HPA axis to the regulatory effects of the opioid blockade (51). These long-term effects may contribute to blunted HPA reactivity previously observed among smokers (5).
We should note, however, that other procedural and individual difference factors may have also contributed to this blunted response to opioid blockade. Smokers smoked a cigarette before each laboratory session, and this may have dampened the effects of naltrexone. However, two components of the protocol challenge this possibility. First, HPA effects of smoking a single cigarette tend to be small and brief (15 to 30 minutes) (38,50), whereas in our protocol, group difference in response to naltrexone emerged approximately 2 hours after smoking. Second, smoking occurred in the placebo condition when baseline differences between the two groups were not significant, reducing the chances that acute smoking contributed to the observed blunted response to the opioid blockade in the smoking group.
Although pain sensitivity was not generally altered by opioid blockade, we found that gender differences in pain measures were consistently diminished among smokers. This finding was consistent across multiple pain measures. The mechanisms responsible for the lack of gender differences in smokers are not currently known. It is possible that female smokers exhibit reduced production of estradiol that contributes to the reduced pain sensitivity in this group (52). This possibility is supported by recent research, showing that female smokers have reduced estradiol production relative to nonsmoking women (22). Animal experiments have demonstrated that increased estrogen production leads to increased sensitivity to pain (53,54), and one study in humans showed that female smokers had reduced estrogen concentrations and also showed reduced thermal pain sensitivity relative to non-smoking females (55). It should be noted, however, that absence of gender differences in pain was independent of endogenous opioid function because this finding was observed in both placebo and naltrexone conditions.
We note that this study was limited by the inclusion of a young, relatively homogeneous group, the use of a single dose of naltrexone, and the process of testing women during the follicular phase of the menstrual cycle only, potentially limiting the generalizability of the obtained results. Nevertheless, the relatively large sample size, the well-controlled repeated-measure design of this study, and the important results obtained are strengths that justify further work addressing the interaction of the endogenous opioid system and HPA axis among smokers. Future work should closely examine the extent to which smoking-related alterations in the endogenous opioid system modulation of HPA are normalized by long-term abstinence and whether these alterations contribute to the maintenance of smoking behaviors and to relapse among smokers. This study examined the effects of a single dose of opioid blockade, and the use of multiple doses to examine these effects in smokers is still needed. It is also not clear whether the documented opioid-HPA alterations have resulted from ongoing tobacco dependence or were a risk factor predating nicotine dependence. The extent to which genetic factors contribute to this dysregulation is also not known. For example, evidence suggests that the minor allele of the mu-opioid receptor (A118G) contributes to differential HPA response to stress and opioid blockade (56,57).
In summary, this study demonstrated blunted ACTH and cortisol responses to opioid blockade among smokers. This finding suggests alterations in the endogenous opioid system that may contribute to previously observed dysregulation of the stress response in this group. The diminished gender differences in pain perception among smokers are an indication that smoking-related pain alteration is more profound in women. In light of the interest in using naltrexone in the treatment of addictive disorders, our results point out the importance of considering tobacco use as a potential moderator of the effect of this medication. Interventions that produce their therapeutic effects by engaging the endogenous opioid system should take into consideration ongoing tobacco use or history of tobacco use.
Acknowledgments
We thank Clemens Kirschbaum, PhD, of Dresden University in Germany for help in assaying the hormonal samples and Deanna Ellestad, Angie Harju, and Amanda Moe for help with the data collection and management.
This study was supported, in part, by pilot Grant DA013333 from the Minnesota TTURC (M.A.), American Heart Association Grant-in-Aid (M.A.), and National Institute of Health Grants CA88272 and DA016351 (M.A.).
Glossary
- ACTH
adrenocorticotropin
- BP
blood pressure
- CO
cardiac output
- CPT
cold pressor test
- CRF
corticotrophin-releasing factor
- HPA
hypothalamic-pituitary-adrenocortical
- MPQ
McGill Pain Questionnaire
References
- 1.Buckingham JC, Cooper TA. Interrelationships of opioidergic and adrenergic mechanisms controlling the secretion of corticotrophin releasing factor in the rat. Neuroendocrinology. 1987;46:199–206. doi: 10.1159/000124820. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham JC. Stimulation and inhibition of corticotrophin releasing factor secretion by beta endorphin. Neuroendocrinology. 1986;42:148–52. doi: 10.1159/000124266. [DOI] [PubMed] [Google Scholar]
- 3.Feek CM, Marante DJ, Edwards CR. The hypothalamic-pituitary-adrenal axis. Clin Endocrinol Metab. 1983;12:597–618. doi: 10.1016/s0300-595x(83)80057-7. [DOI] [PubMed] [Google Scholar]
- 4.Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav. 1993;44:527–31. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- 5.al’Absi M, Wittmers LE, Erickson J, Hatsukami DK, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–10. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- 6.Roy MP, Steptoe A, Kirschbaum C. The association between smoking status and cardiovascular and cortisol stress responsivity in healthy young men. Int J Behav Med. 1994;1:264–83. doi: 10.1207/s15327558ijbm0103_6. [DOI] [PubMed] [Google Scholar]
- 7.al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–17. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- 8.Martin del Campo AF, Dowson JH, Herbert J, Paykel ES. Effects of naloxone on diurnal rhythms in mood and endocrine function: a dose-response study in man. Psychopharmacology (Berl) 1994;114:583–90. doi: 10.1007/BF02244988. [DOI] [PubMed] [Google Scholar]
- 9.Burnett FE, Scott LV, Weaver MG, Medbak SH, Dinan TG. The effect of naloxone on adrenocorticotropin and cortisol release: evidence for a reduced response in depression. J Affect Disord. 1999;53:263–8. doi: 10.1016/s0165-0327(98)00127-x. [DOI] [PubMed] [Google Scholar]
- 10.Schobel HP, Handwerker HO, Schmieder RE, Heusser K, Dominiak P, Luft FC. Effects of naloxone on hemodynamic and sympathetic nerve responses to pain in normotensive vs. borderline hypertensive men. J Auton Nerv Syst. 1998;69:49–55. doi: 10.1016/s0165-1838(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 11.McCubbin JA, Surwit RS, Williams RB, Nemeroff CB, McNeilly M. Altered pituitary hormone response to naloxone in hypertension development. Hypertens. 1989;14:636–44. doi: 10.1161/01.hyp.14.6.636. [DOI] [PubMed] [Google Scholar]
- 12.Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek MJ. Nalmefene causes greater hypothalamic-pituitary-adrenal axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcohol Clin Exp Res. 1998;22:1430–6. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- 13.Wand GS, Schumann H. Relationship between plasma adrenocorticotropin, hypothalamic opioid tone, and plasma leptin. J Clin Endocrinol Metab. 1998;83:2138–42. doi: 10.1210/jcem.83.6.4900. [DOI] [PubMed] [Google Scholar]
- 14.Delitala G, Trainer PJ, Oliva O, Fanciulli G, Grossman AB. Opioid peptide and alpha-adrenoceptor pathways in the regulation of the pituitary-adrenal axis in man. J Endocrinol. 1994;141:163–8. doi: 10.1677/joe.0.1410163. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan-Sarin S, Rosen MI, O’Malley SS. Naloxone challenge in smokers. Preliminary evidence of an opioid component in nicotine dependence. Arch Gen Psychiatry. 1999;56:663–8. doi: 10.1001/archpsyc.56.7.663. [DOI] [PubMed] [Google Scholar]
- 16.Feine J, Bushnell M, Miron D. Sex differences in the perception of noxious heat stimuli. Pain. 1991;44:255–62. doi: 10.1016/0304-3959(91)90094-E. [DOI] [PubMed] [Google Scholar]
- 17.Jensen R, Rasmussen B, Pedersen B. Cephalic muscle tenderness and pressure pain threshold in a general population. Pain. 1992;48:197–203. doi: 10.1016/0304-3959(92)90059-K. [DOI] [PubMed] [Google Scholar]
- 18.Fillingim R, Maixner W. The influence of resting blood pressure and gender on pain responses. Psychosom Med. 1996;58:326–32. doi: 10.1097/00006842-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 19.al’Absi M, Buchanan TW, Marrero A, Lovallo WR. Sex differences in pain perception and cardiovascular responses in persons with parental history for hypertension. Pain. 1999;83:331–8. doi: 10.1016/s0304-3959(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 20.Zatzic DF, Dimsdale JE. Cultural variations in response to painful stimuli. Psychosom Med. 1990;52:544–57. doi: 10.1097/00006842-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 21.al’Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- 22.Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–85. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Wolff BB. Methods of testing pain mechanisms in normal man. In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Churchill Livingstone; 1984. [Google Scholar]
- 24.Posner J, Telekes A, Crowley D, Phillipson R, Peck AW. Effects of an opiate on cold-induced pain and the CNS in healthy volunteers. Pain. 1985;23:73–82. doi: 10.1016/0304-3959(85)90232-5. [DOI] [PubMed] [Google Scholar]
- 25.Chen AC, Dworkin SF, Haug J, Gehrig J. Human pain responsivity in a tonic pain model: psychological determinants. Pain. 1989;37:143–60. doi: 10.1016/0304-3959(89)90126-7. [DOI] [PubMed] [Google Scholar]
- 26.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 27.Fillingim RB, Maixner W, Bunting S, Silva S. Resting blood pressure and thermal pain responses among females: effects on pain unpleasantness but not pain intensity. Int J Psychophysiol. 1998;30:313–8. doi: 10.1016/s0167-8760(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 28.Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–92. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- 29.al’Absi M, Lovallo WR, McKey B, Sung BH, Whitsett TL, Wilson MF. Hypothalamic-pituitary-adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosom Med. 1998;60:521–7. doi: 10.1097/00006842-199807000-00021. [DOI] [PubMed] [Google Scholar]
- 30.al’Absi M, Lovallo WR, McKey B, Pincomb G. Borderline hypertensives produce exaggerated adrenocortical responses to mental stress. Psychosom Med. 1994;56:245–50. doi: 10.1097/00006842-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg U, Frankenhaeuser M. Pituitary-adrenal and sympathetic-adrenal correlates of distress and effort. J Psychosom Res. 1980;24:125–30. doi: 10.1016/0022-3999(80)90033-1. [DOI] [PubMed] [Google Scholar]
- 32.King AC, Meyer PJ. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol Biochem Behav. 2000;66:563–72. doi: 10.1016/s0091-3057(00)00258-6. [DOI] [PubMed] [Google Scholar]
- 33.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 34.Spielberger CD, Gorsuch R, Lushene R. State-Trait Anxiety Manual. Palo Alto, CA: Consulting Psychological Press; 1983. [Google Scholar]
- 35.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 36.Vasey MA, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24:479–86. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 37.Jennings JR. Editorial policy on analyses of variance with repeated measures. Psychophysiology. 1987;24:474–5. [Google Scholar]
- 38.Gilbert DG, Meliska CJ, Plath LC. Noise stress does not modulate effects of smoking/nicotine on β-endorphin, cortisol, ACTH, glucose, and mood. Psychopharmacology. 1997;130:197–202. doi: 10.1007/s002130050229. [DOI] [PubMed] [Google Scholar]
- 39.Ussher M, West R, Evans P, Steptoe A, McEwen A, Clow A, Hucklebridge F. Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosom Med. 2006;68:299–306. doi: 10.1097/01.psy.0000204926.27215.a1. [DOI] [PubMed] [Google Scholar]
- 40.Kirschbaum C, Hallhammer D. Methodological aspects of salivary cortisol measurement. In: Kirschbaum C, Read GF, Hellhammer DH, editors. Assessment of Hormones and Drug in Saliva in Biobehavioral Research. Seattle: Hogrefe & Huber; 1992. [Google Scholar]
- 41.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 42.Dhillo WS, Kong WM, Le Roux CW, Alaghband-Zadeh J, Jones J, Carter G, Mendoza N, Meeran K, O’Shea D. Cortisol-binding globulin is important in the interpretation of dynamic tests of the hypothalamic-pituitary-adrenal axis. Eur J Endocrinol. 2002;146:231–5. doi: 10.1530/eje.0.1460231. [DOI] [PubMed] [Google Scholar]
- 43.Bujdoso E, Jaszberenyi M, Tomboly C, Toth G, Telegdy G. Effects of endomorphin-1 on open-field behavior and on the hypothalamic-pituitary-adrenal system. Endocrine. 2001;14:221–4. doi: 10.1385/ENDO:14:2:221. [DOI] [PubMed] [Google Scholar]
- 44.Valentino RJ, Van Bockstaele E. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl) 2001;158:331–42. doi: 10.1007/s002130000673. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham ETJ, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–67. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- 46.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 47.Wewers ME, Dhatt RK, Snively TA, Tejwani GA. The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res. 1999;822:107–13. doi: 10.1016/s0006-8993(99)01095-1. [DOI] [PubMed] [Google Scholar]
- 48.Aceto MD, Scates SM, Ji Z, Bowman ER. Nicotine’s opioid and anti-opioid interactions: proposed role in smoking behavior. Eur J Pharmacol. 1993;248:333–5. doi: 10.1016/0926-6917(93)90009-f. [DOI] [PubMed] [Google Scholar]
- 49.Pomerleau O. Endogenous opioids and Smoking: a review of progress and problems. Psychoneuroendocrinology. 1998;23:115–30. doi: 10.1016/s0306-4530(97)00074-7. [DOI] [PubMed] [Google Scholar]
- 50.Seyler LE, Jr, Fertig J, Pomerleau O, Hunt D, Parker K. The effects of smoking on ACTH and cortisol secretion. Life Sci. 1984;34:57–65. doi: 10.1016/0024-3205(84)90330-8. [DOI] [PubMed] [Google Scholar]
- 51.Lutfy K, Brown MC, Nerio N, Aimiuwu O, Tran B, Anghel A, Friedman TC. Repeated stress alters the ability of nicotine to activate the hypothalamic-pituitary-adrenal axis. J Neurochem. 2006;99:1321–7. doi: 10.1111/j.1471-4159.2006.04217.x. [DOI] [PubMed] [Google Scholar]
- 52.Baron JA, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol. 1990;162:502–14. doi: 10.1016/0002-9378(90)90420-c. [DOI] [PubMed] [Google Scholar]
- 53.Frye CA, Bock BC, Kanarek RB. Hormonal milieu affects tailflick latency in female rats and may be attenuated by access to sucrose. Physiol Behav. 1992;52:699–706. doi: 10.1016/0031-9384(92)90400-v. [DOI] [PubMed] [Google Scholar]
- 54.Ratka A, Simpkins JW. Effects of estradiol and progesterone on the sensitivity to pain and on morphine-induced antinociception in female rats. Horm Behav. 1991;25:217–28. doi: 10.1016/0018-506x(91)90052-j. [DOI] [PubMed] [Google Scholar]
- 55.Fillingim RB, Maixner W, Girdler SS, Light KC, Harris MB, Sheps DS, Mason GA. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom Med. 1997;59:512–20. doi: 10.1097/00006842-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–14. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 57.Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The Micro-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–11. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]