Abstract
The objective of this systematic review is to identify and evaluate cost estimates reported in scientific literature regarding practices to prevent infection among residents and staff of long-term care facilities. Included papers represent diverse study designs and low methodological transparency.
INTRODUCTION
Healthcare-associated infections (HAI) represent a significant cause of morbidity and mortality for the 2.5 million Americans residing in long-term care facilities (LTCFs) (Strausbaugh & Joseph, 2000). According to the U.S. Department of Health and Human Services, LTCFs are institutions that provide healthcare programs and services outside of an acute care hospital and encompass both skilled nursing facilities and nursing homes (U.S. Department of Health and Human Services, 2013). Within U.S. nursing homes alone, an estimated 1.6 to 3.8 million HAI occur annually. These HAI cost $38–$137 million for antimicrobial therapy and $637 million to $2 billion for hospitalizations due to infections each year (Castle, Wagner, Ferguson, & Handler, 2012). The Centers for Disease Control and Prevention (CDC) recognize that reducing HAI is a priority that extends to all LTCFs (Siegel, Rhinehart, Jackson, Chiarello, & The Healthcare Infection Control Practices Advisory Committee, 2007), considering that most HAI can be prevented through appropriate infection control and prevention practices (Srinivasan, Craig, & Cardo, 2012).
Long-term care demographic and industry trends challenge provision of effective care and infection prevention. The average long-term care resident today is older, with higher case acuity and complexity than two decades ago (Mor, Caswell, Littlehale, Niemi, & Fogel, 2009), and is thereby more vulnerable to infection (Siegel et al., 2007). However, infection control and prevention efforts must compete for resources with other care priorities as most LTCFs face increasing budget constraints following Medicare’s shift to a prospective payment system though the Balanced Budget Act of 1997 (Bowblis, 2011). Given lower, bundled reimbursement rates, there is a need for evidence-based efficiency improvements, and more specifically, a need to weigh benefits and costs of infection-control activities in non-acute settings (Freixas, Salles, & Garcia, 2009).
While the financial burden of some HAI have been characterized in this setting (Capitano & Nicolau, 2003; Jamshed, Woods, Desai, Dhanani, & Taler, 2011), there are a limited number of papers that address the costs of efforts to prevent HAI. Analyzing the balance between relative costs as well as benefits of infection prevention activities is particularly important to this residential population among which efforts to reduce infection may have detrimental effects with respect to resident psychological health (Siegel et al., 2007) and quality of life (Armstrong-Evans et al., 1999).
A systematic review of literature to weigh benefits, costs, and harms of clinical practices in LTCF and thereby inform decisions of infection prevention coordinators in this setting would be useful (Mullen & Ramirez, 2006). To our knowledge, no such systematic review has been previously published.
Objective
The objective of this systematic review is to identify and evaluate cost estimates reported in the scientific literature of structure and processes intended to prevent infection among residents and staff of LTCFs.
Theoretical Framework
The Epidemiologic Triad of Disease informed identification of activities intended to prevent infectious disease. This theoretical framework outlines the concepts of host (long-term care resident or staff), agent (pathogen) and environment and their relationships to each other to perpetuate infectious disease transmission (Clark, 1954). Activities intended to reduce host susceptibility, agent presence (or virulence) or environment severity were therefore considered an infection prevention practice.
METHODS
The authors followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher, Liberati, Tetzlaff, Altman, & The PRISMA Group, 2010) to conduct this systematic review.
Eligibility Criteria
Inclusion and exclusion criteria were developed based on the following core elements: publication type and date, setting, conditions, study subjects, and perspectives. Inclusion criteria for this review were as follows: 1) original research published in a peer-reviewed, scientific journal in the English language within the past 25 years (1989–2013); 2) setting was long-term care; 3) the research focus was infection prevention or controlling outbreaks; 4) study subjects were either residents of or healthcare workers in the LTCFs; and, 5) the study included an analysis from institutional, societal or public health perspectives to identify cost estimates most relevant to LTCFs.
Excluded studies met the following criteria: 1) the study was an editorial, correspondence, commentary, letter, or proceeding paper; 2) the study setting was any other than LTCFs, such as acute-care hospitals; and, 3) the study perspective was exclusive to another setting such as a hospital.
Search Strategy
With the help of university librarians, the first author, C.C.C., conducted scientific literature searches in June-July 2013 within the following online databases: PubMed, Ovid Medline, Scopus, EBSCO Cumulative Index of Nursing and Allied Health Literature (CINAHL), and Cochrane. To maximize results, searches included both keyword and medical subject headings (MeSH), where applicable, and combined terms describing the intended setting (“nursing home”, “nursing facility”, “skilled nursing facility”, “long-term care”, “aged home”, “extended care”) with “infection” or “cross-infection” and “cost” or “economic”.
Study Selection
One reviewer (C.C.C.) performed an initial screen of the titles and abstracts of the search results according to the inclusion and exclusion criteria. When the title and abstract appeared to meet the inclusion criteria, or this information was not enough to determine whether the study met the inclusion criteria (i.e., publication had no abstract) the full text was obtained and reviewed. All the authors discussed eligibility of publications that were likely or borderline for inclusion. Final inclusions were determined by consensus, and the reasons for exclusion were recorded.
Data Abstraction
Two reviewers, Y.J.C and C.C.C., each abstracted data from eligible papers and confirmed the accuracy of each other’s work in congruence with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Data elements and operational definitions that comprise the recent and relevant 24-item CHEERS checklist (e.g., target population, comparators, health outcomes) are described elsewhere (Husereau et al., 2013). Disagreements were resolved by discussion between the two reviewers and, if necessary, consultation with a third reviewer.
Summary measures such as cost perspective, data source(s) and measurement time horizon were compiled. Cost estimates reported in non-U.S. currencies were converted to United States Dollars (USD) using the Economic Research Federal Reserve Bank of St. Louis FRED II foreign exchange rates for January 1st of the study publication year (or year of cost estimate collection, if stated) (Federal Reserve Bank of St. Louis, 2013). The authors also standardized cost estimates into 2013 USD values using Bureau of Labor and Statistics Consumer Price Index calculator (Bureau of Labor Statistics).
Quality Appraisal
Two reviewers (C.C.C., Y.J.C.) then independently assessed the quality of included studies using the Quality of Health Economic Studies (QHES) instrument. QHES is a validated, pilot-tested tool, which includes 16 quality indicators with binary outcomes that can be weighted, then summed to a score ranging from 0 (lowest quality) to 100 (highest quality). QHES, while directed towards health economic analyses, can guide evaluation of bias in multiple study designs (Langer, 2012).
The reviewers agreed to a number of interpretations of QHES items before assessing the quality score of included papers. For example, if the cost estimate was not the primary outcome, the objective statement did not need to mention cost evaluation to achieve a high quality score. When the QHES quality items regarding model components and justification were not relevant, studies were evaluated with regards to study calculations. When studies did not include subgroup evaluation, the item regarding subgroup pre-specification was not relevant and the reviewers evaluated whether study authors had assessed the need for subgroups (i.e., any discussion of population heterogeneity).
The reviewers used the weighting system to calculate quality scores created by the QHES authors. The QHES quality score accounts for an item’s contribution to the perceived overall quality of the paper. This included multiplying the outcome of each item (i.e., 1 or 0) by the weighting of each item recommended by the QHES authors (Husereau et al., 2013) before summing the outcomes of each item to generate the overall quality score. All disagreements regarding quality assessments were resolved by discussion between the two reviewers and, if necessary, consultation with a third reviewer.
RESULTS
Results of Study Selection
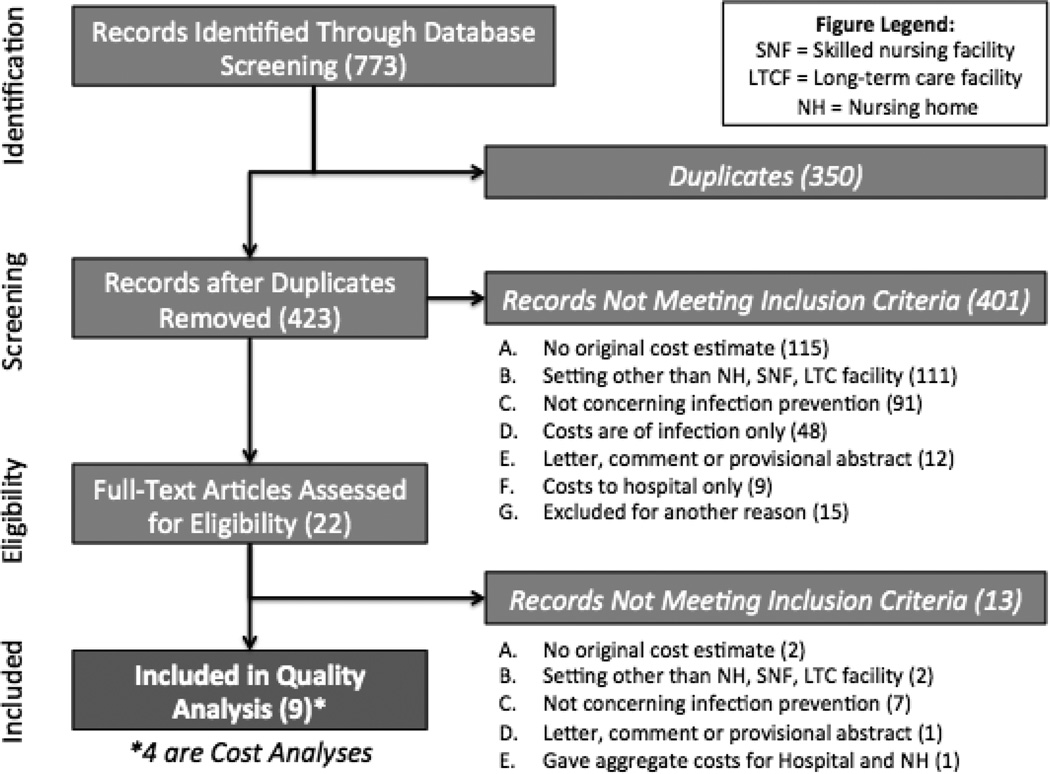
In total, 773 studies were identified by initial database search. After duplicates were removed (n = 350), 423 studies were eligible for screening by title and abstract; of these, 22 studies were identified for full-text assessment. Figure 1 shows the study selection process leading to the inclusion of 9 studies for the systematic review.
Figure 1.
Flow diagram of search and sorting results. Boxes on the left represent stages of evaluation of the papers returned through the original electronic database searches. The boxes on the right show how many articles were excluded by the primary reason for exclusion.
Of the 22 papers that underwent full text review, the primary reason for exclusion was that the intervention of interest was not sufficiently focused on infection prevention. Papers that required extensive discussion but were ultimately excluded for this reason were an evaluation of a skin tear treatment that may prevent infection by closing open wounds, but was not antibacterial (Milne & Corbett, 2005), a comparison of infection surveillance techniques that could theoretically reduce infections as a result of interventions following surveillance (Church et al., 2002), but not directly from the surveillance techniques under consideration, and a comparison of enteral feeding techniques to reduce bacterial contamination of feeding bags, which could theoretically reduce GI infection, though this was not an outcome of the study (Moffitt, Gohman, Sass, & Faucher, 1997). Another study was also excluded after careful deliberation as it addressed an intervention in both an acute care and a LTCF setting, but then appeared to provide cost of the intervention to the hospital rather than that of the LTCF (Brooks et al., 1992). Others were excluded after it was determined that the cost estimates were not original research (i.e., they had previously been published, n = 1), found to be a published correspondence on further review (n = 2) or determined that the study’s intervention and cost measurements took place in a setting that did not meet inclusion criteria (n = 2).
Included Study Characteristics
Included studies employed observational designs (Armstrong-Evans et al., 1999; de Beer, Miller, Tremblay, & Monette, 2006; Dorr, Horn, & Smout, 2005; Larson et al., 1992) and interventional designs (Duffy et al., 1995; Jaqua-Stewart et al., 1999; Robinson & Rosher, 2002). A minority of the studies’ samples specifically included residents of nursing homes (n = 3) (Dorr et al., 2005; Duffy et al., 1995; Robinson & Rosher, 2002), as opposed to skilled nursing facilities. Of the 9 studies included, approximately half were published in the 1990s (n = 5) (Armstrong-Evans et al., 1999; Duffy et al., 1995; Jaqua-Stewart et al., 1999; Larson et al., 1992; Marchand, Tousignant, & Chang, 1999). Most studies were performed in either the United States (n = 5) (Dorr et al., 2005; Duffy et al., 1995; Jaqua-Stewart et al., 1999; Larson et al., 1992; Robinson & Rosher, 2002) or Canada (n = 3) (Armstrong-Evans et al., 1999; de Beer et al., 2006; Marchand et al., 1999), and one study was completed using cohort data from Hong Kong (You, Wong, Ip, Lee, & Ho, 2009). Table 1 summarizes characteristics of the eligible studies, including objective, design, study population, setting, time horizon, outcome measures, results and QHES quality score.
Table 1.
Summary of Included Studies
| First Author |
Year | Objective | Sample/ Setting (Country) |
Study Design (Time Horizon) |
Health Outcomes |
Infection Prevention Resources |
Cost Estimate |
QHE S Scor e |
|---|---|---|---|---|---|---|---|---|
|
Armstron g-Evans (Armstrong-Evans et al., 1999) |
1999 | To describe the investigation and control of transmission of vancomycin-resistant enterococci (VRE) in a residential LTC setting |
5 residents of 1 LTCF (Canada) |
Prospective cohort (13 months) |
Resident VRE colonization |
Disposable supplies; cleaning and disinfection; reusable gowns; personal care caddies; staff time; formal education; screening |
Total cost of intervention: $11,379 |
49 |
|
De Beer (de Beer et al., 2006) |
2006 | To describe [a Scabies] outbreak and determine whether the effectiveness of the treatment protocol justified its future use |
387 residents and 700 staff of 1 LTCF (Canada) |
Retrospecti ve cohort (2 months) |
Incident cases of scabies |
Topical permethrin; overtime and additional salary costs; security guard salary; disposable gowns and gloves; cleaning supplies; laundry |
Total costs of outbreak minus treatment: $144,977 |
23 |
|
Dorr (Dorr et al., 2005) |
2005 | To examine potential cost savings from decreased adverse resident outcomes versus additional wages of nurses when nursing homes have adequate staffing |
1376 residents of 82 NHs (United States) |
Retrospecti ve cohort (1 year) |
UTI rate | RN wages and benefits; Hospitalizati on |
Cost savings per 100 NH residents per year: $40,724.83 |
61 |
|
Duffy (Duffy et al., 1995) |
1995 | To compare the safety and cost of clean versus sterile intermittent bladder catheterization in male nursing home residents |
80 male veterans in 3 VA NH with need for catheterization (United States) |
Randomize d clinical trial (125 days), with cost- effectivenes s analysis |
UTI; Pressure ulcer; Hospitalization rates |
Nurse time and supplies for catheterizati on |
Incremental cost of sterile vs. clean catheterizati on per 1 catheterizati on: $3.23 |
94 |
|
Jaqua- Stewart (Jaqua-Stewart et al., 1999) |
1999 | To decrease MRSA colonization and infection rates and prevent the introduction of additional colonized patients into a closed nursing home environment |
42 residents in 1 extended care unit of VA hospital (United States) |
Quasi- experiment al with cost- benefit analysis (3 years) |
MRSA colonization rate; MRSA infection rate; difference in colonization and infection rates pre-and post intervention |
PPE; medications ; nursing and environmen tal manageme nt services; laboratory costs; miscellaneo us items; |
Total cost of intervention: $32,242.24 |
22 |
|
Larson (Larson et al., 1992) |
1992 | To examine the prevalence of C. difficile and MRSA resident on hands of nursing staff of a 233-bed long-term care facility during a 24-hour period, as well as the prevalence of C. difficile and MRSA carriage of patient in the wad with expected high rates of C. difficile and MRSA carriage and in the adjacent wards |
207 residents and 84 staff of 1 LTCF (United States) |
Prospective cohort (6 months) |
Chronic hand carriage of C. difficile and MRSA among healthcare workers; C. difficile and MRSA colonization rates among residents |
Gloves | Mean cost of gloves per month in LTC: $2,253.89; Mean cost of gloves per month SN unit: $376.20 |
73 |
|
Marchand (Marchand et al., 1999) |
1999 | To determine if the more interventionist approach of screening with the tuberculin test and chemoprophylaxis for high- risk positive reactors to control tuberculosis in long- term care facilities is cost- effective when compared to the case-finding and treatment approach |
Newly admitted residents to 1 LTCF (Canada) |
Cost-utility decision- analysis model (15 years) |
Life-year (LY); quality-adjusted life-year (QALY); annual Tb cases; Tb-related deaths |
Staff time; screening supplies |
$4,734.21 per LY; $3,782.41 per QALY |
83 |
|
Robinson (Robinson & Rosher, 2002) |
2002 | To determine the effect of a specific program on the level of hydration and the prevention of conditions associated with dehydration |
51 residents in 1 NH (United States) |
Quasi- experiment al (9 weeks) |
Total body water; Intracellular body water; Extracellular body water; delirium; UTI; respiratory infections; falls; skin breakdown; constipation |
Staff time; beverages; cups |
Total cost of intervention: $210.31 |
25 |
|
You (You et al., 2009) |
2009 | To compare cost and QALYs gained by influenza vaccination alone and in combination with pneumococcal vaccination in elderly people living in long-term care from a Hong Kong public health provider's perspective |
1016 hypothetical elderly LTCF cohort (Hong Kong) |
Cost- effectivenes s analysis with Markov model (5 years) |
Quality-adjusted life years (QALYs); death |
Cost of vaccine, RN time for administrati on; RN treatment of vaccination side effects |
Total cost of intervention: $202.48; Incremental cost per QALY gained for influenza and pneumonia vaccines vs. influenza vaccine alone: $544.30 |
83 |
Note.
QHES quality assessment score range is 0 (worst) to 100 (best);
LTCF = Long-Term Care Facility; NH = Nursing home; VA = Veteran’s Administration; UTI= urinary tract infection; VRE= vancomycin-resistant enterococci; Tb = Tuberculosis; PPE = personal protective equipment.
Data Abstraction Results
Most studies reviewed infection prevention interventions for a specific disease, such as vancomycin-resistant enterococcus (VRE) (n = 1) (Armstrong-Evans et al., 1999), methicillin-resistant Staphylococcus aureus (MRSA) influenza (n = 2) (Jaqua-Stewart et al., 1999; Larson et al., 1992), or tuberculosis (n = 1) (Marchand et al., 1999). In two studies, the interventions were aimed at preventing urinary tract infections, as well as other diseases and conditions (Dorr et al., 2005; Robinson & Rosher, 2002). Given the diversity of infection prevention activities addressed, as well as the variety in study designs, outcome synthesis was not possible.
Cost estimates most often included additional staff time for an intervention (n = 6) (Armstrong-Evans et al., 1999; de Beer et al., 2006; Duffy et al., 1995; Marchand et al., 1999; Robinson & Rosher, 2002; You et al., 2009), increased use of disposable items such as gowns and gloves and incremental use of cleaning supplies (n = 6) (Armstrong-Evans et al., 1999; de Beer et al., 2006; Duffy et al., 1995; Jaqua-Stewart et al., 1999; Larson et al., 1992; Robinson & Rosher, 2002). These expenditures were often the market price per unit multiplied by the number of units used. Four papers included a statistical analysis, and the same four displayed differences in cost and outcomes between two alternative infection prevention practices (Dorr et al., 2005; Duffy et al., 1995; Marchand et al., 1999; You et al., 2009). Only three studies provided sensitivity analyses of cost estimates (Dorr et al., 2005; Marchand et al., 1999; You et al., 2009).
Three publications specifically reported the perspective of the cost estimate including societal (Dorr et al., 2005), healthcare system (Marchand et al., 1999), and public health care provider (You et al., 2009). Dorr et al. (2005) also included a sub-analysis from an institutional perspective. Four of the eligible papers included a cost analysis: one cost-utility (Marchand et al., 1999), one cost-benefit (Jaqua-Stewart et al., 1999), and two cost-effectiveness (Duffy et al., 1995; You et al., 2009). In the majority of studies, the investigators indicated the specific abstraction method through which the primary outcome measure and costs were determined (n = 6) (Armstrong-Evans et al., 1999; Dorr et al., 2005; Duffy et al., 1995; Larson et al., 1992; Marchand et al., 1999; You et al., 2009). Two papers included a model of cost estimates (Marchand et al., 1999; You et al., 2009). None of the included studies’ authors performed a subgroup analysis. Only four papers contained discussion of the homogeneity of the study population (de Beer et al., 2006; Dorr et al., 2005; Duffy et al., 1995; Robinson & Rosher, 2002), likely due to the fact that most included a single LTCF (n = 5) (Armstrong-Evans et al., 1999; de Beer et al., 2006; Jaqua-Stewart et al., 1999; Larson et al., 1992; Robinson & Rosher, 2002). As most papers did not discuss homogeneity of study population characteristics, this limited the ability to determine generalizability of the study and potential sampling bias.
While some authors discussed limitations of their respective study (Armstrong-Evans et al., 1999; Dorr et al., 2005; You et al., 2009), only Larson et al. (1992) included discussion of the magnitude and direction of potential biases on the cost estimates (Larson et al., 1992).
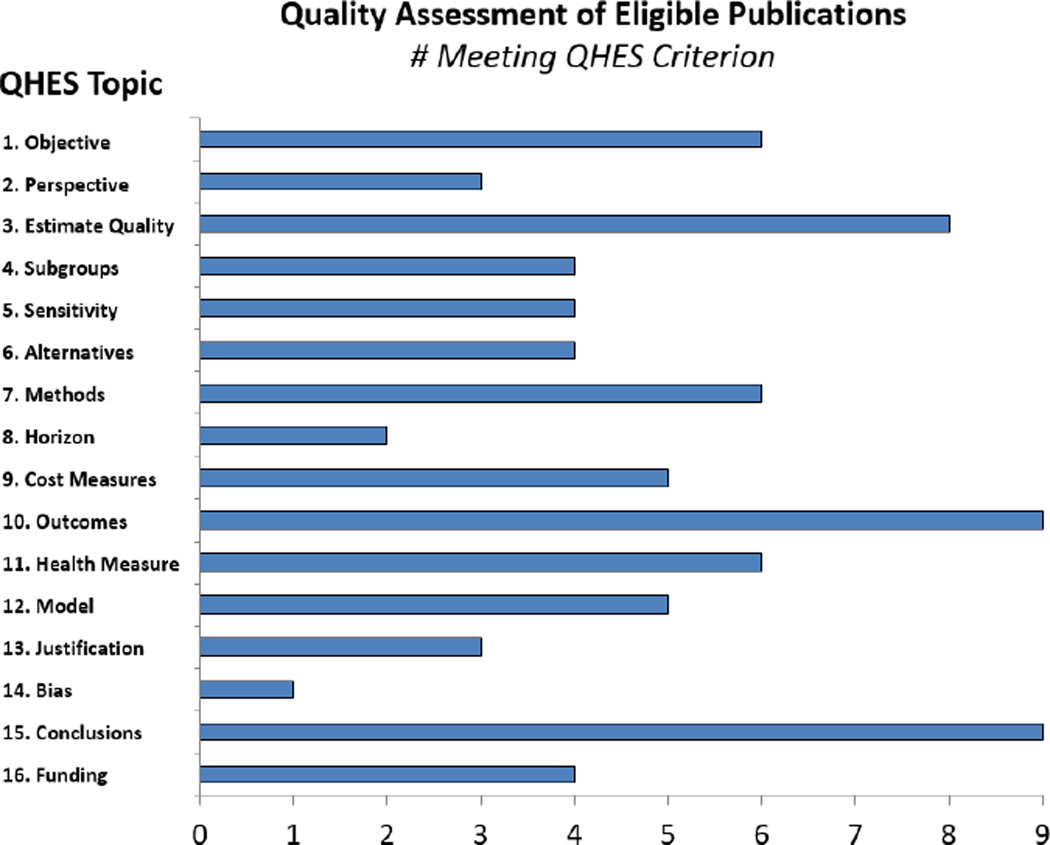
Results of Quality Assessment
The QHES scores ranged from 22 to 94, with an average of 56.6. Figure 2 shows the QHES score of each study and summarizes the results of quality assessment using the QHES instrument. In this figure, the length of the bar indicates the percentage of the studies achieving high quality regarding each item.
Figure 2.
Summary of quality assessment using the Quality of Health Economic Studies (QHES) Instrument. The Y axis corresponds to each of the items on the QHES instrument and the x-axis represents the number of articles included in the review meeting the QHES criteria on each item.
Sixty-seven percent of the studies included a high-quality, specific objectives statement, which had a stated measurable outcome of interest, setting or population, and intervention, if applicable (Armstrong-Evans et al., 1999; Dorr et al., 2005; Duffy et al., 1995; Larson et al., 1992; Marchand et al., 1999; You et al., 2009). Most study designs appeared to be the most appropriate method of determining relevant health outcomes and cost (n = 8) (Armstrong-Evans et al., 1999; de Beer et al., 2006; Dorr et al., 2005; Duffy et al., 1995; Jaqua-Stewart et al., 1999; Larson et al., 1992; Marchand et al., 1999; You et al., 2009). However, time horizon was not clearly appropriate to effectively assess health outcomes in a number of publications (Armstrong-Evans et al., 1999; de Beer et al., 2006; Duffy et al., 1995; Jaqua-Stewart et al., 1999; Robinson & Rosher, 2002). For example, a study of an intervention to eradicate a scabies outbreak from a single facility had not eradicated scabies from the institution by the end of the study as treatment failures occurred later that year (de Beer et al., 2006). Measures of health outcomes were sufficiently validated and/or justified in six of the nine publications (Armstrong-Evans et al., 1999; Dorr et al., 2005; Larson et al., 1992; Marchand et al., 1999; Robinson & Rosher, 2002; You et al., 2009). All but four papers received lower quality scores for both a lack of statistical analysis and lack of comparison to alternatives (n = 5) (Armstrong-Evans et al., 1999; de Beer et al., 2006; Jaqua-Stewart et al., 1999; Larson et al., 1992; Robinson & Rosher, 2002).
Cost estimate measurements and calculations were often unclear and the source and calculation of the estimates was not stated (n = 4) (de Beer et al., 2006; Duffy et al., 1995; Jaqua-Stewart et al., 1999; Robinson & Rosher, 2002). As noted above, most papers did not explicitly state the perspective from which the cost estimates were measured (n = 6), which is especially critical to evaluate whether all relevant costs were included and measured appropriately. Furthermore, only two papers avoided bias by stating a clearly appropriate time horizon or discount rate (n = 2) (Dorr et al., 2005; Larson et al., 1992). One paper that collected data over three years did not mention how these costs were discounted, if at all (Jaqua-Stewart et al., 1999) and those that used a discount rate did not provide justification for that specific rate (n = 2) (Marchand et al., 1999; You et al., 2009).
Transparency regarding primary and/or cost outcomes and stated conclusions were directly justified by study findings improved the quality of all included papers. However, most papers received a lower quality score for lacking a discussion of study limitations (n = 6) (Armstrong-Evans et al., 1999; de Beer et al., 2006; Duffy et al., 1995; Jaqua-Stewart et al., 1999; Larson et al., 1992; Robinson & Rosher, 2002).
DISCUSSION
Of the studies deemed eligible for inclusion, most were low to moderate quality given lack of information regarding study methods, especially the cost measurement perspective, data collection time horizon, model or calculation justification and anticipated bias magnitude and direction. However, many included studies received higher QHES quality scores due to transparent justification for conclusions, explicitly stated primary and secondary outcomes and having collected data from the best available source(s).
It is possible that many poor scores on the quality assessment items might indicate lack of publication transparency rather than methodology sophistication or accuracy. For example, stating the perspective from which costs were determined can substantially improve the readers’ ability to determine generalizability and cost estimate applicability. Future cost and cost analysis studies regarding infection prevention in LTCFs may improve on the current body of work by ensuring that the manuscript addresses all items in the CHEERS, QHES or a similar health economic publication checklists and we encourage authors, reviewers and editors to use these developed checklists.
The small volume of publications regarding the cost of infection prevention in LTCFs identified in the scientific literature cover a wide variety of interventions. Given the diversity of study designs in the papers, health outcomes and cost measures, further generation of evidence would be required to meaningfully aggregate and compare results of these studies. Our findings are similar to those of Stone, Braccia and Larson (2005). These authors reviewed economic analyses related to HAI in multiple settings. While their results demonstrated an increasing quality of cost analyses over time, the existing body of literature did not offer specific public policy or practice implications at that time (Stone, Braccia, & Larson, 2005). In our review, most of the authors derived cost estimates from a single LTCF. The high heterogeneity of populations and service specialization of LTCFs limits the external validity of these studies (U.S. Department of Health and Human Services, 2013). Furthermore, it is unlikely that study methodology and calculation of cost estimates could be repeated given the information provided in these publications. Therefore, as in Stone et al. (2005), we also cannot recommend specific infection prevention practices based on cost estimates or cost-effectiveness based on these data.
Implications for policy, practice and education
The rising costs of infection in LCTFs without attention to prevention may result in three different scenarios. First, LTCFs may be able to secure new funding to cover the rising costs of infection. However, this is likely to continue increasing the costs of treatment and is ultimately unsustainable (Orszag & Ellis, 2007a). Second, in the absence of sufficient funding, LTCF executives might shift resources from other budget items to cover increased costs of infections, which may be detrimental to other areas of care. Last, LTCF exceed their budgets, possibly resulting in facility closures. Given these scenarios, investments in infection prevention is much more acceptable. Considering that an estimated 380,000 LTCF residents die of infection annually (Centers for Disease Control and Prevention, 2013) and that the nursing home population alone is expected to grow from 1.5 million today to 5.3 million by 2030 (Strausbaugh & Joseph, 2000), it is important to invest in infection prevention activities to reduce morbidity, mortality in this vulnerable population as well as reduce costs to LTCFs and the already overburdened U.S. healthcare system.
Cost-effectiveness research is needed to inform nurse executives’ decisions on how best to prevent infections, which should avoid adverse events among residents and curb program costs (Orszag & Ellis, 2007b). In acute care, multifaceted infection prevention programs have been found to be cost-saving (Dick et al., In Press) and while there are as of yet, no well developed economic models of infection prevention in LTCFs, it is likely that they will also be found to be cost-saving. However, where multiple alternative processes exist to prevent infection, deciding between alternative structures, practices or products requires not only knowledge of the relative intervention effectiveness but also the cost trade-off for that level of effectiveness (Frick, 2013).
Therefore, nurse executives should consider costs as well as health outcomes when generating new policy regarding procedures or products related to infection prevention. In doing so, administrators should cautiously evaluate the recommendations of published studies containing a cost estimation based on the quality of the estimate in addition to assessing applicability of the results to their own facility and resident population. Unfortunately, the authors have no knowledge of additional sources of cost estimates for infection prevention activities in LTCFs at this time beyond publications reported in this review. As demonstrated in this review, those who wish to evaluate others’ cost estimates or establish one tailored to their own facility may wish to pay particular attention to the time horizon in which both health and cost outcomes occur, costs from the perspectives of multiple stakeholders (i.e., what costs exist to facility, staff, patients and payers) and the additional time required from staff. For example, while many of the included studies measured additional staff time required for the intervention in this review, none included the resources required for educational in-services regarding the intervention. This may be a key consideration depending on available resources and need for staff compliance with the new policy.
To prepare future nurse leaders to meet the challenges of evaluating costs of infection prevention (and other quality improvement activities), nurse educators should include economic evaluations in curricula, if these factors are not already included. Indeed, understanding healthcare financing, business principles and how they influence clinical outcomes and cost factors is an American Association of Colleges of Nursing essential element in baccalaureate education (American Association of Colleges of Nursing).
Better understanding of economic analysis concepts, such as those included on the economic evaluation tools used in this study, may improve future nurse executives’ interpretation of the scientific literature and application to clinical practice. In this way, future studies may substantially contribute to clinical decision-making to reduce infection in LTCF.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Arlene Smaldone, PhD, CPNP, CDE, for her intellectual and editorial contributions to this manuscript, and Columbia University librarians at Medical Campus and Business School for their assistance in the design and execution of these searches.
Biographies
Catherine Cohen is a PhD student and a Training in Interdisciplinary Research to Prevention Infections (TIRI, T32 NR013454) pre-doctoral fellow. During her first year as a doctoral student, she was a research assistant on the Prevention of Nosocomial Infections & Cost Effectiveness in Nursing Homes (PNICE-NH, R01 NR013687) study. Her research interests include comparative and cost-effectiveness analyses of healthcare-associated infection prevention.
Yoon Jeong Choi is a PhD candidate at Columbia University and currently working on her dissertation. She graduated with both BSN and MSN from Seoul National University in South Korea. Her dissertation is focused on examining the association between out-of-pocket pharmacy costs and poor adherence to medication and the impact of Medicare Part D on improving medication adherence in elderly with diabetes.
Dr. Stone is the Centennial Professor of Health Policy at Columbia School of Nursing and director of the Center for Health Policy. Dr. Stone received her PhD from the University of Rochester and an MPH from Harvard University. Her research examines the impact of organizational factors on clinical patient safety/quality outcomes with a strong focus on healthcare-associated infections (HAIs) in the elderly.
Contributor Information
Catherine C. Cohen, Center for Interdisciplinary Research to Prevent Infections, Center for Health Policy, Columbia University School of Nursing, New York, NY.
Yoon Jeong Choi, Center for Health Policy, Columbia University School of Nursing, New York, NY.
Patricia W. Stone, Center for Health Policy, Columbia University School of Nursing, New York, NY.
REFERENCES
- American Association of Colleges of Nursing. Essentials Series. Washington, D.C: The essentials of baccalaureate education for professional nursing practice. [Google Scholar]
- Armstrong-Evans M, Litt M, McArthur MA, Willey B, Cann D, Liska S, McGeer A. Control of transmission of vancomycin-resistant enterococcus faecium in a long-term-care facility. Infection Control & Hospital Epidemiology. 1999;20(5):312–317. doi: 10.1086/501623. [DOI] [PubMed] [Google Scholar]
- Bowblis JR. Ownership conversion and closure in the nursing home industry. Health Economics. 2011;20(6):631–644. doi: 10.1002/hec.1618. [DOI] [PubMed] [Google Scholar]
- Brooks SE, Veal RO, Kramer M, Dore L, Schupf N, Adachi M. Reduction in the incidence of clostridium difficile-associated diarrhea in an acute care hospital and a skilled nursing facility following replacement of electronic thermometers with single-use disposables. Infection Control & Hospital Epidemiology. 1992;13(2):98–103. doi: 10.1086/646480. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. Cpi inflation calculator. [October 15 2013];2013 Retrieved, from http://www.bls.gov/data/inflation_calculator.htm.
- Capitano B, Nicolau DP. Evolving epidemiology and cost of resistance to antimicrobial agents in long-term care facilities. Journal of the American Medical Directors Association. 2003;4(3 Suppl):S90–99. doi: 10.1097/01.JAM.0000066029.00660.5A. [DOI] [PubMed] [Google Scholar]
- Castle N, Wagner L, Ferguson J, Handler S. Hand hygiene deficiency citations in nursing homes. Journal of Applied Gerontology. 2012 doi: 10.1177/0733464812449903. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National healthcare safety network (nhsn) tracking infections in long-term care facilities. [Retrieved July 16, 2014];2013 Jan 28; 2013, from http://www.cdc.gov/nhsn/LTC/
- Church DL, Davies HD, Mitton C, Semeniuk H, Logue M, Maxwell C, Donaldson C. Clinical and economic evaluation of rapid influenza a virus testing in nursing homes in calgary, canada. Clinical Infectious Diseases. 2002;34(6):790–795. doi: 10.1086/338960. [DOI] [PubMed] [Google Scholar]
- Clark EG. Natural history of syphilis and levels of prevention. British Journal of Venereal Diseases. 1954;30(4):191–197. doi: 10.1136/sti.30.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer G, Miller MA, Tremblay L, Monette J. An outbreak of scabies in a long-term care facility: The role of misdiagnosis and the costs associated with control. Infection Control & Hospital Epidemiology. 2006;27(5):517–518. doi: 10.1086/504365. [DOI] [PubMed] [Google Scholar]
- Dick A, Perencevich EN, Pogorzelska-Maziarz M, Zwanziger J, Larson EL, Stone PW. A decade of investment in infection prevention: A cost effectiveness analysis. American Journal of Infection Control. doi: 10.1016/j.ajic.2014.07.014. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr DA, Horn SD, Smout RJ. Cost analysis of nursing home registered nurse staffing times. Journal of the Americal Geriatrics Society. 2005;53(5):840–845. doi: 10.1111/j.1532-5415.2005.53267.x. [DOI] [PubMed] [Google Scholar]
- Duffy LM, Cleary J, Ahern S, Kuskowski MA, West M, Wheeler L, Mortimer JA. Clean intermittent catheterization: Safe, cost-effective bladder management for male residents of va nursing homes. Journal of the Americal Geriatrics Society. 1995;43(8):865–870. doi: 10.1111/j.1532-5415.1995.tb05528.x. [DOI] [PubMed] [Google Scholar]
- Federal Reserve Bank of St. Louis. Federal reserve economic data. [October 15th 2013];2013 Retrieved, from http://research.stlouisfed.org/fred2/ [Google Scholar]
- Freixas N, Salles M, Garcia L. Changes in nosocomial infection control: New challenges and responsibilities for the infection control nurse. Enfermedades Infecciosas y Microbiología Clínica. 2009;27(5):285–289. doi: 10.1016/j.eimc.2008.01.004. S0213-005X(09)00126-8 [pii] [DOI] [PubMed] [Google Scholar]
- Frick KD, Cohen CC, Stone PW. Analyzing economic outcomes in advanced practice nursing. In: Kleinpell RM, editor. Outcome assessment in advanced practice nursing. 3rd. New York: Springer Publishing Company; 2013. pp. 45–72. [Google Scholar]
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Loder E. Consolidated health economic evaluation reporting standards (cheers)--explanation and elaboration: A report of the ispor health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Jamshed N, Woods C, Desai S, Dhanani S, Taler G. Pneumonia in the long-term resident. Clinics in Geriatric Medicine. 2011;27(2):117–133. doi: 10.1016/j.cger.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Jaqua-Stewart MJ, Tjaden J, Humphreys DW, Bade P, Tille PM, Peterson KG, Salem AG. Reduction in methicillin-resistant staphylococcus aureus infection rate in a nursing home by aggressive containment strategies. South Dakota Journal of Medicine. 1999;52(7):241–247. [PubMed] [Google Scholar]
- Langer A. A framework for assessing health economic evaluation (hee) quality appraisal instruments. BMC Health Services Research. 2012;12:253–264. doi: 10.1186/1472-6963-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E, Bobo L, Bennett R, Murphy S, Seng ST, Choo JTE, Sisler J. Lack of care giver hand contamination with endemic bacterial pathogens in a nursing home. American Journal of Infection Control. 1992;20(1):11–15. doi: 10.1016/s0196-6553(05)80118-x. [DOI] [PubMed] [Google Scholar]
- Marchand R, Tousignant P, Chang H. Cost-effectiveness of screening compared to case-finding approaches to tuberculosis in long term care facilities for the elderly. International Journal of Epidemiology. 1999;28(3):563–570. doi: 10.1093/ije/28.3.563. [DOI] [PubMed] [Google Scholar]
- Milne CT, Corbett LQ. A new option in the treatment of skin tears for the institutionalized resident: Formulated 2-octylcyanoacrylate topical bandage. Geriatric Nursing. 2005;26(5):321–325. doi: 10.1016/j.gerinurse.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Moffitt SK, Gohman SM, Sass KM, Faucher KJ. Clinical and laboratory evaluation of a closed enteral feeding system under cyclic feeding conditions: A microbial and cost evaluation. Nutrition. 1997;13(7–8):622–628. doi: 10.1016/s0899-9007(97)83002-x. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. International Journal of Surgery. 2010;8(2010):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Mor V, Caswell C, Littlehale S, Niemi J, Fogel B. Changes in the quality of nursing homes in the us: A review and data update. American Health Care Association; 2009. pp. 1–24. [Google Scholar]
- Mullen PD, Ramirez G. The promise and pitfalls of systematic reviews. Annual Review of Public Health. 2006;27:81–102. doi: 10.1146/annurev.publhealth.27.021405.102239. [DOI] [PubMed] [Google Scholar]
- Orszag PR, Ellis P. Addressing rising health care costs--a view from the congressional budget office. N Engl J Med. 2007a;357(19):1885–1887. doi: 10.1056/NEJMp078191. [DOI] [PubMed] [Google Scholar]
- Orszag PR, Ellis P. The challenge of rising health care costs--a view from the congressional budget office. N Engl J Med. 2007b;357(18):1793–1795. doi: 10.1056/NEJMp078190. [DOI] [PubMed] [Google Scholar]
- Robinson SB, Rosher RB. Can a beverage cart help improve hydration? Geriatric Nursing. 2002;23(4):208–211. doi: 10.1067/mgn.2002.126967. [DOI] [PubMed] [Google Scholar]
- Siegel JD, Rhinehart E, Jackson M, Chiarello L The Healthcare Infection Control Practices Advisory Committee. Guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings. 2007:1–225. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, Craig M, Cardo D. The power of policy change, federal collaboration, and state coordination in healthcare-associated infection prevention. Clinical Infectious Diseases. 2012;55(3):426–431. doi: 10.1093/cid/cis407. [DOI] [PubMed] [Google Scholar]
- Stone PW, Braccia D, Larson E. Systematic review of economic analyses of health care-associated infections. American Journal of Infection Control. 2005;33(9):501–509. doi: 10.1016/j.ajic.2005.04.246. [DOI] [PubMed] [Google Scholar]
- Strausbaugh LJ, Joseph CL. The burden of infection in long-term care. Infection Control & Hospital Epidemiology. 2000;21(10):674–679. doi: 10.1086/501712. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Washington D.C: 2013. National action plan to prevent health care-associated infections: Road map to elimination. Retrieved from http://www.hhs.gov/ash/initiatives/hai/actionplan/index.html. [Google Scholar]
- You JHS, Wong WCW, Ip M, Lee NLS, Ho SC, et al. Cost-effectiveness analysis of influenza and pneumococcal vaccination for hong kong elderly in long-term care facilities. Journal of Epidemiology & Community Health. 2009;63(11):906–911. doi: 10.1136/jech.2008.081885. doi: http://dx.doi.org/10.1136/jech.2008.081885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.