Abstract
Inappropriate activation of PI3K signaling has been implicated strongly in human cancer. Although studies on the role of PI3K signaling in breast tumorigenesis and progression have focused most intensively on PI3Kα, a role for PI3Kβ has begun to emerge. The PI3Kβ isoform is unique among class IA PI3K enzymes in that it is activated by both receptor tyrosine kinases and G-protein-coupled receptors (GPCR). In previous work, we identified a mutation that specifically abolished PI3Kβ binding to Gβγ (p110526KK-DD). Expression of this mutant in p110β-silenced breast cancer cells inhibits multiple steps of the metastatic cascade in vitro and in vivo and causes a cell autonomous defect in invadopodial matrix degradation. Our results identify a novel link between GPCR and PI3Kβ in mediating metastasis, suggesting that disruption of this link might offer a novel therapeutic target to prevent the development of metastatic disease.
Keywords: GPCR, PI3Kβ, breast cancer, invasion, metastasis
Introduction
Metastasis is a highly complex process that requires coordinated, bidirectional interactions between tumor cells and their microenvironment (1). These interactions are often mediated by chemokines and their receptors, many of which belong to the G-protein-coupled receptor (GPCR) family of receptors (2). In tumors, local production of chemokines plays an important role in attracting leukocytes and other inflammatory cells (3). Similarly, tumor cells express receptors that respond to chemokines secreted by stromal cells, tumor-associated macrophages, tumor-infiltrating leukocytes, as well as chemokines secreted by adjacent tumor cells or in an autocrine manner (2). Numerous chemokines have been shown to promote cellular behaviors associated with breast tumor metastasis, including cell migration, increased expression and activity of matrix metalloproteinases (MMPs), increased heparanase activity, and invasion (4–12). Expression of chemokine receptors such as CXCR4 and CCR7 in tumor cells has also been linked to the dissemination of breast cancer cells to secondary organ sites that express high levels of their respective ligands (12,13). In addition to chemokine receptors, several other types of GPCRs, including protease-activated receptor-1 (PAR1), lysophosphatidic acid (LPA), and prostaglandin (EP) receptors, have been implicated in breast cancer metastasis (14–18).
Ligand-stimulated GPCRs signal through both activated Gα and Gβγ subunits (19). Amongst the downstream effectors of Gβγ are the Class I PI 3-kinases, PI3Kβ and PI3Kγ (20–22). Gβγ activates these PI3K isoforms by directly binding to the p110β and p110γ catalytic subunits (23,24). Given the high frequency of PIK3CA mutations in breast cancer patients (25), studies on breast cancer tumorigenesis and progression have focused most intensively on p110α (26), which is regulated primarily by RTKs. However, a role for the GPCR-coupled PI3Ks has begun to emerge. For example, loss of p110γ activity reduces tumor growth and myeloid cell recruitment in the MMTV-PyMT breast cancer model (27). Similarly, p110β kinase-dead transgenic mice are protected from tumor progression in an ErbB2-driven mammary cancer model (28). Moreover, clinical data show that p110β expression correlates with poor prognosis and distant metastasis in breast cancer patients (29). A recent study also shows an important role for p110β in the resistance of tumors to inhibitors of p110α (30).
The p110β isoform is unique among class IA PI3Ks as it can be activated both by receptor tyrosine kinases (RTKs), through p85 SH2 domain binding to activated receptors, and by GPCRs (31). We previously defined the region of p110β that binds to Gβγ and identified a mutation that abolishes this interaction (p110β526KK-DD) (23). This mutant p110β is activated normally by binding to tyrosine phosphorylated peptides but is specifically defective for activation by GPCRs. Using p110β526KK-DD, we now demonstrate a previously unidentified role for GPCR signaling to p110β in tumor cell-macrophage paracrine interactions and in invadopodia function, which impacts multiple steps of the metastatic process. Our data suggest that disruption of the Gβγ-p110β interaction might constitute a novel pharmacologic target for the prevention of breast cancer metastasis.
Materials and Methods
Cell culture and stable cell lines
The human breast cancer cell lines MDA-MB-231, BT-549, and T47D were obtained from American Type Culture Collection (ATCC) and maintained as recommended. Bone marrow-derived macrophages (BMMs) were isolated from tibias and femurs of C57BL/6 mice as previously described (32) and grown in Complete BMM Medium (αMEM (Invitrogen) containing 15% FBS, 120 ng/ml recombinant human CSF-1, 0.02 mg/ml asparagine, 2 mM glutamine and 100 U/ml penicillin and 100 µg/ml streptomycin). Bac1.2F5 macrophages were grown in αMEM (Invitrogen) containing 10% FBS, 36 ng/ml recombinant human CSF-1, 0.02 mg/ml asparagine, and 2 mM glutamine. 3B-11 endothelial cells were obtained from ATCC and grown in DMEM containing 10% FBS. All media were supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin
MDA-MB-231, BT-549, and T47D cells were infected with lentivirus expressing shRNA against human p110β (target sequence: 5'GCGGGAGAGTAGAATATGTTT3') and selected using 5 µg/ml puromycin (Sigma). Stable knockdown cell lines were confirmed by western blot for endogenous p110β and then transfected with cDNA expressing myc-tagged murine wild type p110β, p110βKK-DD or p110βK-R using Lipofectamine 3000 (Life Technologies). Stable transfectants were selected using 800 µg/ml Geneticin (Enzo Life Sciences), and expression of exogenous p110β was verified by western blotting with antibodies against p110β and the myc epitope.
Antibodies and Reagents
The rabbit p110β antibody was produced in-house. Rabbit anti-p110α, phospho-AKT (Thr308), total AKT, and phospho-ERK1/2 (Thr202/Tyr204) were purchased from Cell Signaling Technology. The pan ERK antibody was purchased from BD Biosciences. Lysophosphatidic acid (LPA) and epidermal growth factor (EGF) were purchased from Sigma and Millipore, respectively. SDF-1 and IL8 were purchased from R&D Systems. TGX-221 was purchased from Echelon.
Western Blotting
For western blot experiments, MDA-MB-231, BT-549, or T47D cells expressing wild type or mutant p110β grown in 6-well plates were starved for 18 h in serum-free media supplemented with 0.1% BSA and then stimulated for 5 minutes at 37 °C with 5 nM EGF or 10 µM LPA. Cells were lysed in 2X Laemmli sample buffer with phosphatase inhibitor cocktails (Sigma and Calbiochem) and boiled for 5 minutes. Cell lysates were separated by SDS-PAGE and blots were visualized using ECL (Thermo Scientific) and quantitated by densitometry.
Transwell migration and invasion assays
For migration assays, MDA-MB-231 cells expressing wild type or mutant p110β were starved overnight in serum-free DMEM containing 0.1% BSA. 3×104 cells were plated in starvation media in the upper wells of transwell chambers (8 µm pore; BD Biosciences). For macrophage-stimulated migration, 1×106 bone marrow-derived macrophages (BMMs) were plated in the lower chamber in Complete BMM Medium and incubated overnight. The media was changed to serum-free starvation media supplemented with 120 ng/ml CSF-1 before plating the tumor cells in the transwells. For LPA-stimulated migration, starvation media without or with 10 µM LPA was placed in the lower chamber. MDA-MB-231 cells were allowed to migrate for 8 h.
For invasion assays, transwell chambers (8 µm pore; BD Biosciences) were coated with 300 µg/ml growth factor-reduced Matrigel (BD Biosciences) for 2 h at 37°C. MDA-MB-231, BT-549, or T47D cells were starved in serum-free media containing 0.1% BSA for 6 h. For ligand-stimulated invasion, starvation media without or with 100 ng/ml SDF-1, 100 ng/ml IL8, or 5 nM EGF was placed in the lower chamber. For macrophage-stimulated invasion, 1×106 bone marrow-derived macrophages (BMMs) were plated in the lower chamber in Complete BMM Medium and incubated overnight. The media was changed to serum-free starvation media supplemented with 120 ng/ml CSF-1 before plating the tumor cells in the transwells. 100 nM TGX-221 was included as indicated. Tumor cells were allowed to invade for 24 h.
After 8 h for migration assays and 24 h for invasion assays, cells on the upper surface of the membrane were removed with a cotton swab. Cells on the lower surface of the membrane were fixed with 4% paraformaldehyde in PBS for 10 min, washed twice with PBS, and stained with DAPI. Five random fields at 10X magnification from at least two transwells per condition were imaged, and the cells were counted using ImageJ.
3D collagen invasion assay
3D in vitro invasion assays were performed and quantified as described (33). Briefly, Bac1.2F5 macrophages and MDA-MB-231 cells expressing wild type or mutant p110β were labeled with CellTracker Red CMPTX and CellTracker Green CMFDA, respectively. 80,000 tumor cells and were plated on MatTek dishes without or with 200,000 Bac1.2F5 macrophages and grown in BAC1.2F5 medium for 16 hrs. Cells were overlaid with 5.8 mg/ml type I collagen, incubated for 24 h and fixed. Invasion into the collagen gel was quantified by laser scanning confocal microscopy detection of the fluorescent signals from the red and green CellTracker dyes.
Extravasation-transendothelial migration (eTEM) assay
Transwell chambers (8 µm pore; BD Biosciences) were coated with 300 µg/ml growth factor-reduced Matrigel (BD Biosciences) for 2 h at 37°C. 2×104 3B-11 endothelial cells were plated on the Matrigel layer and incubated for 48 h at 37°C to allow the formation of a tight monolayer, as indicated by resistance measurement. 104 BMMs were plated on the underside of the membrane and allowed to attach for 30 minutes. MDA-MB-231 or BT-549 cells expressing wild type or mutant p110β were labeled with CellTracker Green CMFDA dye (Invitrogen) in serum-free medium for 30 minutes at 37°C. 2×104 tumor cells were plated on top of the endothelial cell monolayer in the upper chamber of the transwells and allowed to migrate for 36 h at 37°C. After removing non-migrated cells with a cotton swab, cells on the lower surface of the membrane were fixed with 4% paraformaldehyde for 10 min and washed twice with PBS. Six random fields at 20X magnification from duplicate or triplicate wells for each condition were imaged using a fluorescent microscope.
Experimental metastasis
4×105 MDA-MB-231 cells stably expressing wild type or mutant p110β were injected intravenously into the lateral tail vein of SCID mice, respectively. After 6 weeks the mice were sacrificed. Lungs were collected, fixed in 10% neutral buffered formalin and embedded in paraffin followed by serial sectioning. Lung sections were stained with Hematoxylin and Eosin (H&E) and scanned. The tumor nodules were quantified by thresholding the images using ImageJ software to determine the number of nodules per lung section as well as the size of individual nodules, expressed in arbitrary units.
Xenografts and tumor cell blood burden
2×106 MDA-MB-231 cells stably expressing wild type or mutant p110β were injected into the right fourth mammary fat pad of 6 to 8-week old SCID mice. Tumors were measured three times per week, and tumor mass was calculated using the formula tumor mass (g) = 0.1 × length in mm × (0.1 × width in mm)2. Mice were sacrificed when the tumor mass reached approximately 1 gram.
Gelatin degradation
MDA-MB-231 cells expressing wild type or mutant p110β were plated on glass coverslips coated with Oregon Green 488-conjugated gelatin (Molecular Probes) as previously described (34). Briefly, coverslips were treated with 50 µg/ml poly-l-lysine for 10 minutes at room temperature followed by 0.5% glutaraldehyde for 10 minutes at room temperature. The treated coverslips were then coated with 200 µg/ml gelatin for 15 minutes at room temperature, treated with 0.1 M glycine for 10 minutes, and extensively washed with PBS. 4×104 tumor cells in DMEM containing 10% FBS were plated on the coverslips and incubated for 18 h. Cells were then fixed, stained with rhodamine phalloidin, and immunostained for cortactin. At least 10 fields per condition were imaged at 60X magnification as described above. To quantify matrix degradation, experiments were performed in triplicate with a minimum of 120 cells per condition examined. Cells with at least one degraded spot were counted as positive for gelatin degradation. The area of degradation per field was measured by thresholding the images using ImageJ software to determine the total area in the field that lacks fluorescence. The total area was then divided by the number of degrading cells in the field.
Statistical analysis
Quantitative data are expressed as the mean ± SEM from three independent experiments. Statistical analysis was performed using ANOVA followed by Holm-Sidak post-hoc testing to correct for multiple comparisons. A p value less than 0.05 was considered statistically significant.
Results
Expression of GPCR-uncoupled or kinase dead PI3Kβ inhibits GPCR signaling in breast cancer cells
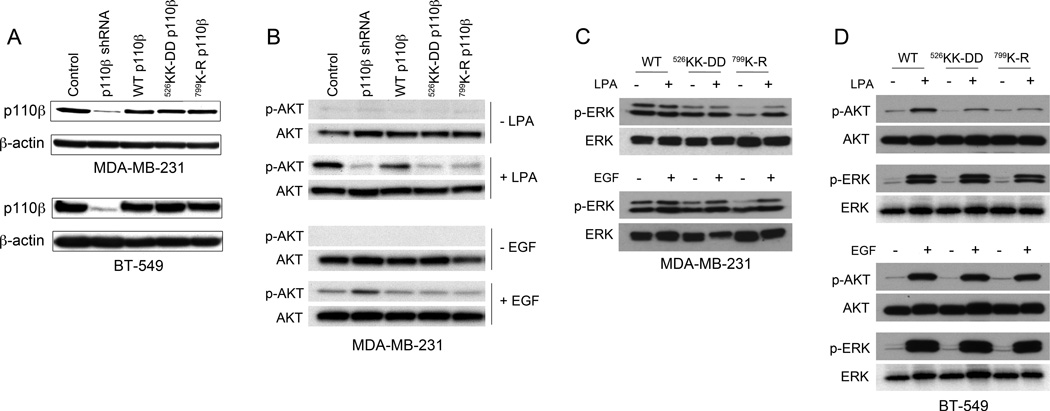
We tested the role of PI3Kβ in two triple negative breast cancer cell lines. MDA-MB-231 cells express mutant Ras and Raf, but PI3Ks and PTEN are wild type. In contrast, BT-549 express a truncated catalytically inactive mutant of PTEN (35). We generated stable MDA-MB-231 and BT-549 human breast cancer cell lines in which endogenous p110β was knocked down by infecting cells with a lentivirus expressing a short hairpin RNA (shRNA) targeting p110β. The knockdown cells were then transfected with cDNA constructs encoding the murine wild type p110β or two previously described mutants that abolish its binding to Gβγ (mutation of residues 526KK to DD; p110βKK-DD) or its lipid kinase activity (mutation of residue 799K to R; p110βK-R) (23,28). The exogenous murine p110β was expressed at levels that were comparable to that of endogenous p110β in control cells (Fig. 1A). Although a myc blot showed a slightly higher expression level for the p110β mutants as compared to wild type p110β (Fig. S1A), expression of p110α was not affected in either cell line (Fig. S1B).
Figure 1. Stable replacement of endogenous p110β with mutant p110β inhibits PI3K but not MAPK signaling pathways in breast cancer cells.
A) Representative immunoblots showing expression of p110β and β-actin in MDA-MB-231 (upper panel) and BT-549 (lower panel) cells in which endogenous p110β was stably knocked down, followed by stable transfection with wild type p110β, p110βKK-DD, or p110βK-RB) Representative immunoblots showing pThr308-AKT and total AKT levels in stable MDA-MB-231 cell lines stimulated with LPA (upper panels) or EGF (lower panels). C) Representative immunoblots showing pThr202/Tyr204-ERK1/2 and total ERK levels in stable MDA-MB-231 cell lines stimulated with LPA (upper panel) or EGF (lower panel). D) Representative immunoblots showing pThr308-AKT/total AKT and pThr202/Tyr204-ERK1/2/total ERK levels in stable BT-549 cells stimulated with LPA (upper panels) or EGF (lower panels). Data are representative of at least two independent experiments for each condition.
We previously showed that the p110βKK-DD mutant is defective for Gβγ binding, but exhibits comparable interactions with tyrosine phosphopeptides, Rab5 (23) and Rac1 (data not shown) as wild type p110β. To validate that the mutations in the p110β Gβγ binding site disrupt p110β signaling in breast cancer cells, we measured AKT phosphorylation following stimulation with lysophosphatidic acid (LPA), a GPCR ligand, or epidermal growth factor (EGF), an RTK ligand (Fig. 1B–D, with quantitation in Fig. S2). LPA-stimulated MDA-MB-231 and BT-549 cell lines expressing p110βKK-DD or p110βK-R all showed markedly reduced phosphorylation of AKT as compared to cells expressing wild type p110β (Fig. 1B and D; upper panels). However, when stimulated with EGF, cells expressing mutant or wild type p110β showed similar levels of AKT phosphorylation (Fig. 1 B and D; lower panels).
To analyze effects on the mitogen-activated protein kinase (MAPK) pathway, we measured ERK1/2 phosphorylation following stimulation with LPA or EGF. MDA-MB-231 cells expressing wild type p110β showed high levels of basal ERK1/2 phosphorylation, which was not further stimulated by LPA (Fig. 1C; upper panel) or EGF (Fig. 1C; lower panel). This is consistent with the expression of activated forms of KRAS and BRAF in this cell line (35). Interestingly, quiescent MDA-MB-231 cells expressing p110βKK-DD or p110βK-R showed lower levels of ERK1/2 phosphorylation; the reason for this decrease is not clear. However, LPA and EGF-stimulated ERK1/2 phosphorylation in MDA-MB-231 cells expressing p110βKK-DD or p110βK-R was similar to that in cells expressing wild type p110β (Fig. 1B). BT-549 cells expressing wild type or mutant p110β showed similar levels of basal as well as LPA-stimulated and EGF-stimulated phosphorylation of ERK1/2 (Fig. 1D; upper and lower panels, respectively), indicating that the MAPK signaling pathway is intact in these cell lines.
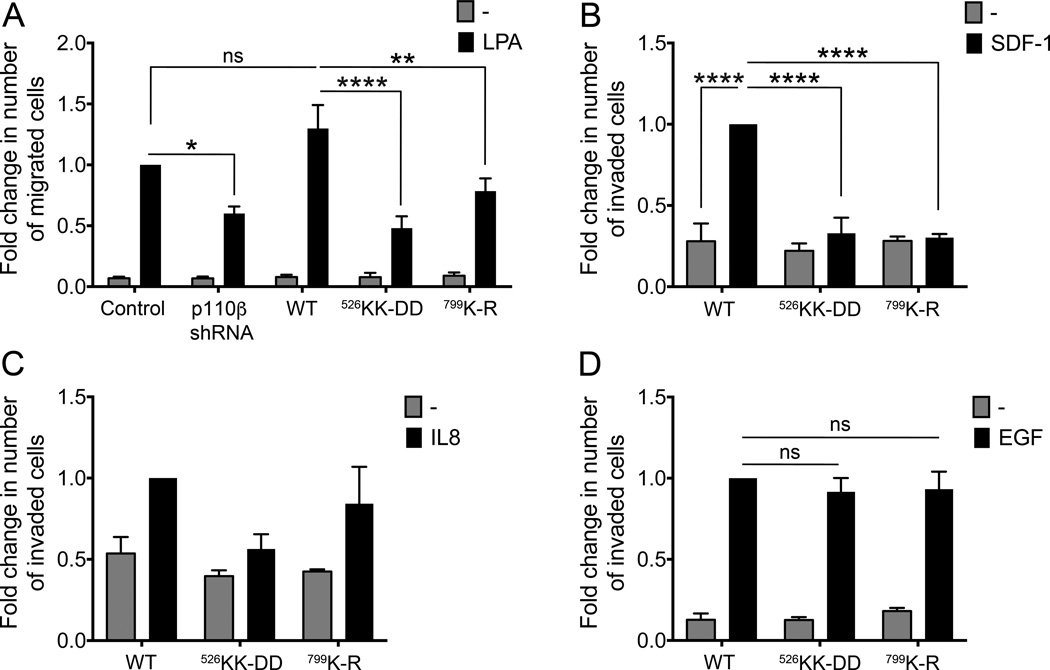
To investigate the role of p110β in breast cancer cell chemotaxis, we assayed the migration of the MDA-MB-231 cell lines towards LPA in a transwell assay (Fig. 2). p110β knockdown cells showed decreased cell migration in response to LPA. This was rescued by expression of wild type p110β but not p110βKK-DD or p110βK-R; migration of cells expressing either mutant was similar to that seen with p110β knockdown cells. We also measured invasion in response to SDF-1 and IL8, which have been implicated in paracrine signaling between breast tumor cells and macrophages (36,37). Migration to SDF-1 was almost completely suppressed in cells expressing mutant p110β (Fig. 2B). IL-8 caused a trend toward increased migration in cells expressing wild type p110β and p110βK-R but not in cells expressing p110βKK-DD (Fig. 2C). As expected, migration toward the RTK ligand EGF was unaffected by expression of mutant p110β (Fig. 2D). Taken together, these data demonstrate that expression of p110βKK-DD or p110βK-R in breast cancer cells specifically inhibits GPCR regulation of AKT signaling and chemotaxis.
Figure 2. Gβγ binding to p110β is required for MDA-MB-231 migration in response to GPCR ligands but not EGF.
A) LPA-stimulated transwell migration of MDA-MB-231 p110β knockdown cells without or with stable rescue by wild type or mutant p110β. Results are expressed as the fold change in the number of migrated cells, relative to values obtained with LPA-stimulated parental cells. B-D) Transwell Matrigel invasion assay of stable MDA-MB-231 cell lines towards (B) SDF-1, (C) IL8, or (D) EGF. Results are expressed as the fold change in the number of invaded cells, relative to values obtained with ligand-stimulated cells expressing wild type p110β. Data represent the mean ± SEM from three independent experiments. In C, the p value for WT versus WT with IL8 is 0.135, and that for WT IL8 versus p110βKK-DD IL8 is 0.156. *: p<0.05; **: p<0.01; ***: p<0.001
Gβγ signaling to p110β is required for macrophage-stimulated breast cancer cell invasion
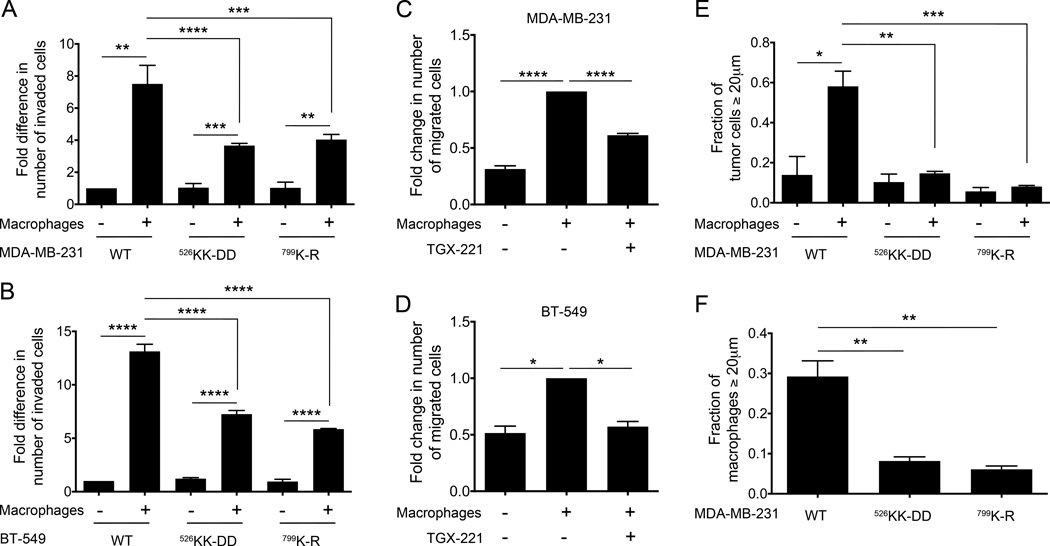
Macrophages are obligate partners of breast cancer cells during invasion (38). We therefore assayed cell invasion using a modified transwell invasion assay, in which tumor cell invasion through Matrigel is measured in the absence or presence of macrophages plated in the lower well, in the absence of any added chemoattractant. As compared to wells where macrophages were absent, the presence of macrophages in the lower well increased the invasion of MDA-MB-231 and BT-549 cells expressing wild type p110β by 7.5- and 13-fold, respectively (Fig. 3A and B). However, macrophage-stimulated invasion was reduced by approximately 50% in cells expressing p110βKK-DD or p110βK-R (Fig. 3A and B). Macrophage-stimulated invasion was also inhibited by the p110β-specific kinase inhibitor TGX-221 (Fig. 3C and 3D). We next evaluated the MDA-MB-231 cell lines in an assay that directly measures macrophage-dependent invasion into a 3D collagen gel (33). For tumor cells expressing wild type p110β, the addition of macrophages caused a 4-fold increase in the number of tumor cells that invaded the collagen gel (from 14% to 58%) (Fig. 3E). However, macrophage-stimulated invasion was completely abolished in MDA-MB-231 cells expressing either p110βKK-DD or p110βK-R (Fig. 3E). These data demonstrate that GPCR signaling to PI3Kβ in tumor cells is critical for macrophage-dependent tumor cell invasion.
Figure 3. Gβγ binding to p110β is required for macrophage-stimulated breast cancer cell invasion.
Macrophage-stimulated invasion of (A) MDA-MB-231 or (B) BT-549 cells is expressed as the fold change in the number of invaded cells, relative to values obtained with unstimulated cells expressing wild type p110β. Macrophage-stimulated invasion of parental (C) MDA-MB-231 or (D) BT-549 cells without or with treatment with TGX-221 is expressed as the fold change in the number of invaded cells, relative to values in the absence of macrophages and TGX-221. E) Macrophage-stimulated invasion of MDA-MB-231 cells in a 3D in vitro collagen invasion assay is expressed as the fraction of cells that invaded ≥ 20 µm. F) Invasion of macrophages, co-plated with MDA-MB-231 cells in the 3D invasion assay is expressed as the number of cells that invaded ≥ 20 µm. Data represent the mean ± SEM from three (A, B, C, E, and F) or two (D) independent experiments. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001
Interestingly, macrophage invasion into the collagen gel was also reduced when they were co-cultured with MDA-MB-231 cells expressing p110βKK-DD or p110βK-R as compared to wild type p110β (Fig. 3F). To test whether this was due to a reduction in secreted factors by the tumor cells, we repeated the Boyden invasion experiment, but instead measured the ability of macrophages in the upper chamber to invade through a Matrigel layer in response to tumor cells in the lower chamber. We saw no differences in migration towards tumor cells expressing wild type versus mutant p110β (data not shown), suggesting that tumor cell signaling to macrophages was unaffected by mutation of p110β.
Gβγ signaling to p110β is required for breast cancer cell extravasation
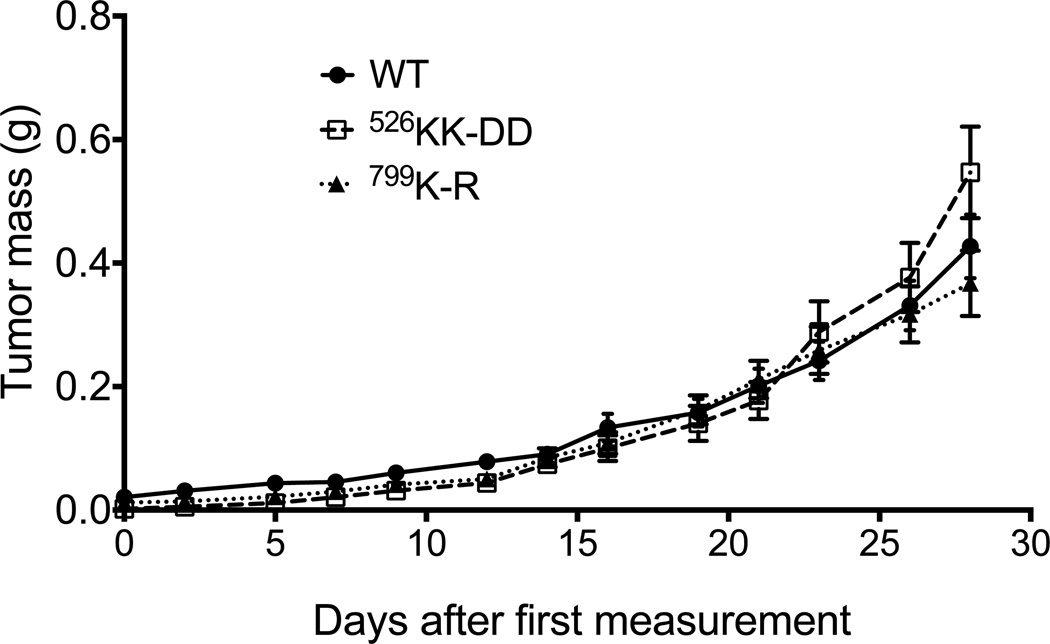
To examine the contribution of GPCR signaling to p110β during tumor growth, we injected MDA-MB-231 cells expressing wild type p110β, p110βKK-DD, or p110βK-R into the mammary fat pad of SCID mice. Xenograft tumors derived from all three cell lines showed similar growth rates (Fig. 4), which is consistent with in vitro assays in which the cells exhibit similar proliferation rates under complete and low serum conditions (Fig. S3A and S3B).
Figure 4. Expression of GPCR-uncoupled or kinase-dead p110β in MDA-MB-231 cells has no effect on tumor growth.
Growth of MDA-MB-231 tumors expressing wild type or mutant p110β in SCID mice. Data represent the mean ± SEM from 10, 13, and 12 mice for wild type p110β, p110βKK-DD, or p110βK-R tumors, respectively.
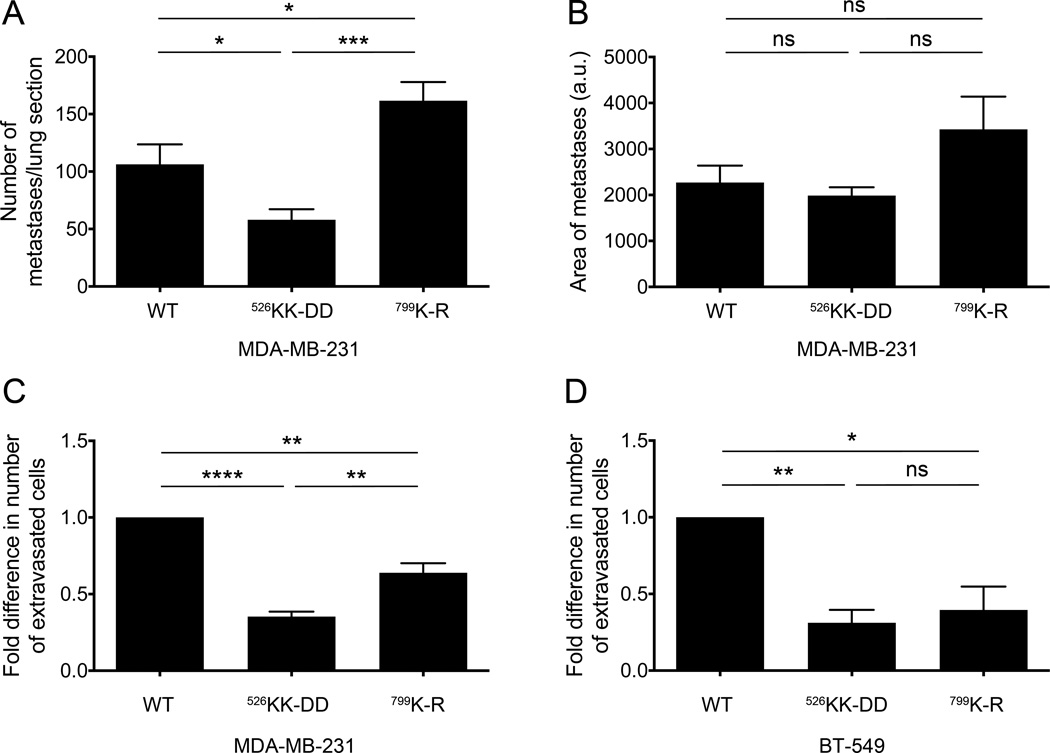
To investigate extravasation in vivo, the MDA-MB-231 cell lines were injected into the lateral tail vein of SCID mice. There was a 50% reduction in the number of lung metastases in mice injected with p110βKK-DD-expressing cells as compared to mice injected with cells expressing wild type p110β. In contrast, the number of lung metastases in mice injected with p110βK-R-expressing cells was actually greater than observed for mice injected with wild type p110β cells (Fig. 5A). The difference in the number of lung metastases observed for p110βKK-DD-expressing cells was not due to alterations in cell proliferation, since the size of the metastatic lesions was similar for all three cell lines (Fig. 5B).
Figure 5. Gβγ binding to p110β is important for breast cancer cell extravasation in vitro and in vivo.
A) The number of metastases per lung section from SCID mice injected with MDA-MB-231 cells expressing wild type or mutant p110β into the lateral tail vein. B) Average size of lung metastases. The data represent the mean ± SEM from 4, 6, and 5 mice for wild type p110β, p110βKK-DD, or p110βK-R, respectively. Macrophage-stimulated transmigration of (C) MDA-MB-231 or (D) BT-549 cells expressing wild type or mutant p110β in the eTEM assay is expressed as the fold change in the number of transmigrated cells relative to values obtained from cells expressing wild type p110β. The data represent the mean ± SEM from three independent experiments. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001; ns: not significant.
At metastatic sites, a discrete population of macrophages has been shown to promote tumor cell extravasation and seeding (39). To directly measure tumor cell extravasation, we used an in vitro transendothelial migration assay in which macrophages plated on the lower face of the transwell filter stimulate tumor cell invasion through an endothelial cell layer plated on the upper face of the filter (40). Consistent with the in vivo metastasis assay, MDA-MB-231 and BT-549 cells expressing p110βKK-DD showed 65% and 75% reductions, respectively, in the number of transmigrated cells as compared to cells expressing wild type p110β (Fig. 5C and 5D). BT-549 cells expressing p110βK-R showed a similar reduction in transmigration (Fig. 5D), whereas MDA-MB-231 cells expressing p110βK-R showed only a 35% reduction (Fig. 5C). Taken together, these data demonstrate a requirement for GPCR signaling to PI3Kβ in breast tumor extravasation.
Gβγ signaling to p110β is required for invadopodia-mediated gelatin degradation in MDA-MB-231 cells
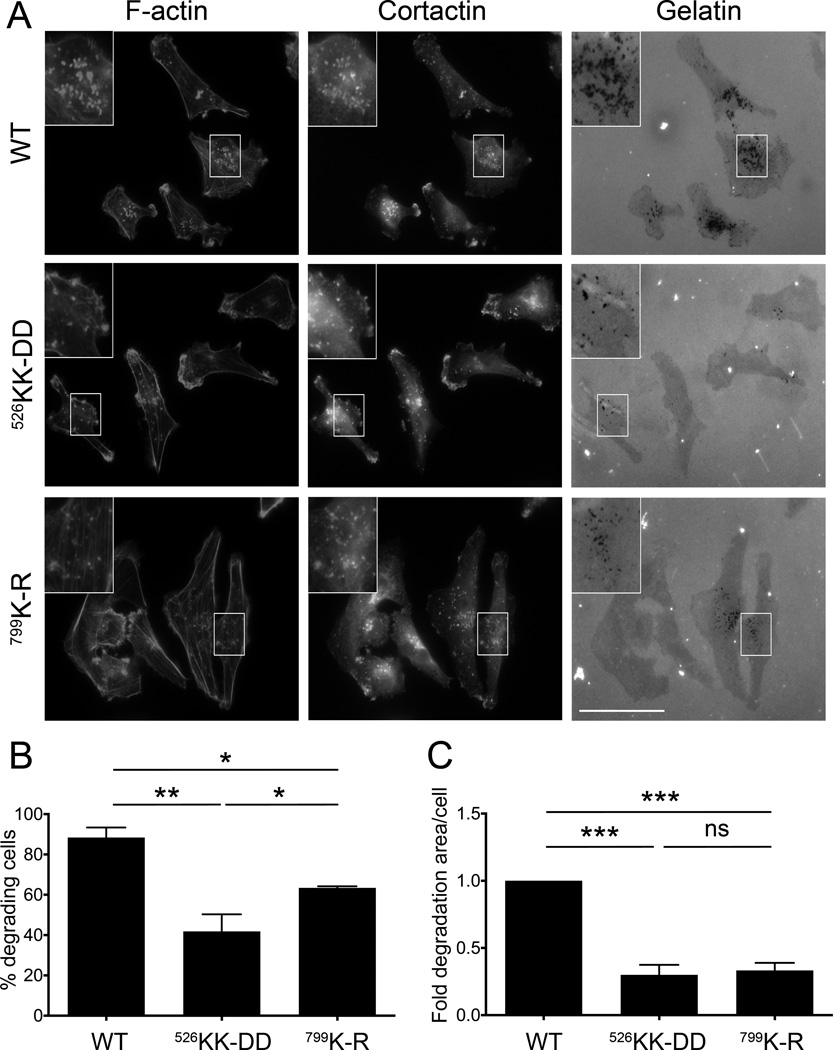
The ability of tumor cells to degrade the extracellular matrix (ECM) is crucial for invasion and metastasis. To degrade the ECM, tumor cells form specialized structures called invadopodia, which are actin-rich protrusions that allow for the local secretion of matrix metalloproteinases and matrix degradation (41). To investigate the role of GPCR signaling to PI3Kβ in the formation and activity of invadopodia, MDA-MB-231 cells expressing wild type or mutant p110β were plated on fluorescent gelatin and assayed for matrix degradation. Degradative invadopodia were identified by the appearance of black foci in the fluorescent gelatin that co-localized with the invadopodial markers F-actin and cortactin (41) (Fig. 6A). Whereas approximately 90% of cells expressing wild type p110β degraded the gelatin, the expression of p110βKK-DD or p110βK-R significantly reduced matrix degradation by 40% and 60%, respectively (Fig. 6B). Moreover, the total degradative area was markedly suppressed in cells expressing p110βKK-DD and p110βK-R as compared to cells expressing wild type p110β (Fig. 6C).
Figure 6. Gβγ binding to p110β is required for gelatin degradation.
A) Representative micrographs of MDA-MB-231 cells expressing wild type or mutant p110β, plated on Oregon Green 488-conjugated gelatin. Cells were stained with rhodamine phalloidin and cortactin to identify invadopodia. B) The percentage of cells with degradative invadopodia. C) The average area of gelatin degradation per cell. Values were normalized to those obtained from cells expressing wild type p110β. Data represent the mean ± SEM from three independent experiments, with a total of 146, 138, and 120 cells for wild type p110β, p110βKK-DD, or p110βK-R, respectively. **: p<0.01; ***: p<0.001.
ECM degradation is a component of all the macrophage-stimulated metastasis assays used in this study. We therefore considered whether the reduction in matrix degradation in cells expressing mutant p110β could account for the observed decreases in invasion and extravasation, independent of the effects on GPCR-mediated paracrine signaling from macrophages to tumor cells. We used a simplified chemotaxis assay, in which macrophages promote the migration of tumor cells through a transwell filter in the absence of Matrigel or other ECM. We observed that macrophages stimulated the migration of tumor cells expressing wild type PI3Kβ. However, macrophage-mediated tumor cell migration was markedly reduced in cells expressing mutant PI3Kβ (Fig. S4). These data suggest that the loss of GPCR-mediated paracrine signaling from macrophages to tumor cells contributes to the defects in metastasis observed cells expressing p110βKK-DD.
To test the role of GPCR signaling to p110β in the context of mutant PIK3CA, we knocked down p110β in T47D cells (Fig. S5A), which express the oncogenic H1047R mutant of p110α (35). Although we were not able to restore p110β expression to the levels observed in parental cells (Fig. S5A and S5B), cells expressing wild type p110β showed enhanced macrophage-stimulated invasion as compared to cells expressing p110βKK-DD and p110βK-R (Fig. S5C).
Discussion
A large number of reports implicate GPCR signaling in cancer initiation, progression, and metastasis (42). Specifically, signaling through the Gâã subunit has been shown to play a critical role in breast cancer tumorigenesis and metastasis (43,44). The PI3K signaling pathway, which is one the major pathways activated downstream of GPCRs, is also one the most commonly disrupted pathways in cancer (45,46). Two Class I PI3K isoforms are activated downstream of GPCRs, p110â and p110ã. However, defining the role of GPCR signaling to p110â has been challenging since it is also activated by RTKs. Our recent identification a p110â mutant that is deficient for Gâã binding and specifically abrogates its activation by GPCRs, but not RTKs (23) has allowed us to specifically test the role of Gâã binding to the p110â PI3K in breast cancer invasion and metastasis.
The ability of cancer cells to disseminate depends on signals from cells in the tumor microenvironment, including tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), myeloid derived suppressor cells (MDSCs), regulatory T cells (Treg), and cancer-associated fibroblasts (CAFs) (47). A paracrine signaling loop involving secretion of EGF and CSF-1 from breast cancer cells and tumor-associated macrophages, respectively, has been shown to be crucial for breast cancer cell invasion and intravasation (33,48). However, tumor infiltrating cells secrete a variety of chemokines and other GPCR ligands that signal to tumor cells and promote tumor progression and metastasis (49). Consequently, we reasoned that Gâã-mediated activation of p110â in breast cancer cells might play a role in breast cancer metastasis by transducing pro-metastatic signals from GPCR ligands produced by the tumor microenvironment.
In the current study, we established MDA-MB-231 (wild type PTEN and PIK3CA), and BT-549 (mutant PTEN) stable cell lines in which we knocked down endogenous p110β and replaced it with exogenous wild type or mutant p110â at levels comparable to the parental cell lines. The knockdown-replace approach is critical for studying the roles of specific PI3K isoforms in cellular processes, since all p110α, p110β, and p110δ compete for a common pool of p85 regulatory subunits (50). As a consequence deletion of a single p110 isoform can lead to alterations in signaling by the remaining isoforms. For example, deletion of p110â leads to an increase in RTK-stimulated phosphorylation of AKT through p110α (51). Using these cells lines, we showed that the Gâã-p110â interaction is required for LPA, but not EGF-stimulated AKT phosphorylation. We also showed that Gâã-mediated activation of p110â is required for migration of MDA-MB-231 cells towards LPA (52) in a transwell migration assay. Since the expression of mutant p110â only reduced cell migration by approximately 50%, it is likely p110ã, which is also expressed in these cells (53), contributes to GPCR-mediated migration.
In accordance with previous reports (33), we found that macrophages greatly increased the 3D invasion of breast cancer cells into Matrigel and collagen matrices. Remarkably, cells expressing Gâã-uncoupled or kinase-dead p110â showed a significant reduction in macrophage-stimulated invasion in the transwell assay and were completely unresponsive to the presence of macrophages in the 3D invasion assay. Interestingly, the invasion of macrophages themselves was also significantly reduced when they were co-cultured with breast tumor cells expressing mutant p110â in the 3D collagen invasion assay. This suggests that the ability of tumor cells to stimulate macrophage invasion also depends on the Gâã-p110â interaction within tumor cells. Notably, tumor cells expressing wild type or mutant p110â stimulated macrophage chemotaxis to similar extents, suggesting that signaling from tumor cells to macrophages is not affected by disruption of the Gâã-p110â interaction in tumor cells. However, recent studies have suggested that macrophage-stimulated tumor cell invasion is mediated, at least in part, by direct contact between the two cell types (54). Therefore, it is possible that contact-dependent signaling between tumor cells and macrophages requires GPCR signaling through PI3Kβ.
We also achieved partial rescue of p110β knockdown in T47D cells (PIK3CA mutant). Consistent with the data in MDA-MB-231 and BT549 cells, expression of mutant p110β inhibited macrophage-stimulated invasion.
p110β has been implicated in tumor growth in certain genetic backgrounds (e.g., PTEN loss or ERBB2 overexpression) (23,28,55). However, we observed that expression of mutant p110â has no effect on orthotopic MDA-MB-231 tumor growth in SCID mice. In contrast, GPCR signaling to p110â was critical for extravasation in both in vivo and in vitro assays.
Mutation of p110β has pronounced effects on matrix degradation, a process that is critical for all of the metastatic steps measured in this study. The reduction in degradative invadopodia occurs independently of macrophages and thus represents an intrinsic loss of function in tumor cells expressing mutant p110β. The molecular mechanisms by which Gβγ-p110β interactions promote the formation of these degradative structures will require further investigation. While alterations in invadopodia assembly or maturation likely contribute to defects in invasion and extravasation observed for p110βKK-DD-expressing tumor cells, our data also demonstrate a loss of paracrine signaling between macrophages and p110βKK-DD-tumor cells. It is therefore likely that both the loss of ECM degrading capacity and the loss of responsiveness to GPCR signaling from macrophages contribute to the observed defects in metastatic behavior.
p110â has been shown to have kinase-independent functions (55,56), and may act as a scaffold to integrate signaling by its binding partners Gβγ, Rac Rab5 and other proteins (23,57,58). A similar scaffolding function has been demonstrated for the other Gβγ-regulated PI3K, p110γ (59). Disruption of Gβγ-mediated targeting of PI3Kβ to cell membranes may inhibit both kinase-dependent and kinase-independent functions of p110β. It is therefore plausible that loss of Gβγ binding might have more severe effects on some cell behaviors than loss of kinase activity alone, which could account for the less pronounced effect of kinase-dead p110β in the transendothelial migration assay. However, it is surprising that expression of kinase-dead p110β inhibited transendothelial migration in vitro but slightly enhanced extravasation in SCID mice. In vivo, tumor cells are influenced by multiple stromal cell types (1), and are subjected to a more complex range of GPCR-dependent stimuli than occurs in vitro. Tumor cell responses to some of these stimuli might require p110β scaffolding functions that are intact in cells expressing kinase-dead p110β. Thus, a p110β kinase-dependent pathway that is rate limiting for transendothelial migration in vitro may be supplanted by a different pathway in vivo, one that does not require p110β activity but still requires Gβγ-mediated targeting of p110β. Alternatively, it is possible that loss of p110β kinase activity disrupts a negative feedback loop in vivo that is independent of Gβγ binding.
The finding that disruption of p110â binding to Gâã has a more deleterious effect on experimental tumor metastasis than disruption of kinase activity suggests that targeting the p110β-Gβγ binding interface might provide an effective alternative to small molecule inhibitors that compete for the ATP binding site on p110β. Disruption of p110β-Gβγ binding could constitute a novel therapeutic pharmacological approach to the treatment of metastasis in breast cancer patients.
Supplementary Material
Acknowledgments
This work was supported by NIH grants 1P01 CA100324 (J.M.B., A.R.B, J.C., B.K.), GM112524 (J.M.B., A.R.B.), the Janey Fund (B.K.), the Intravital Imaging, Cell Isolation and Fate Mapping Core of the Program Project in Motility and Invasion (1 P01 CA100324), and the Histotechnology and Comparative Pathology Core of the Einstein Cancer Center (P30-CA013330). We thank Drs. Jeffrey Segall and David Entenberg for helpful discussions.
Footnotes
Conflict of interest: The authors declare no conflict of interest
References
- 1.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F. Cancer and the chemokine network. Nature reviews Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 3.Palacios-Arreola MI, Nava-Castro KE, Castro JI, Garcia-Zepeda E, Carrero JC, Morales-Montor J. The role of chemokines in breast cancer pathology and its possible use as therapeutic targets. Journal of immunology research. 2014;2014:849720. doi: 10.1155/2014/849720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang WB, Jokar I, Zou A, Lambert D, Dendukuri P, Cheng N. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. The Journal of biological chemistry. 2012;287(43):36593–3608. doi: 10.1074/jbc.M112.365999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsigliante S, Vetrugno C, Muscella A. CCL20 induces migration and proliferation on breast epithelial cells. Journal of cellular physiology. 2013;228(9):1873–1883. doi: 10.1002/jcp.24349. [DOI] [PubMed] [Google Scholar]
- 6.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 7.Johnson-Holiday C, Singh R, Johnson E, Singh S, Stockard CR, Grizzle WE, et al. CCL25 mediates migration, invasion and matrix metalloproteinase expression by breast cancer cells in a CCR9-dependent fashion. International journal of oncology. 2011;38(5):1279–1285. doi: 10.3892/ijo.2011.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer cell. 2011;19(4):541–555. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu M, Berk R, Kosir MA. CXCL7-Mediated Stimulation of Lymphangiogenic Factors VEGF-C, VEGF-D in Human Breast Cancer Cells. Journal of oncology. 2010;2010:939407. doi: 10.1155/2010/939407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang H, Watkins G, Parr C, Douglas-Jones A, Mansel RE, Jiang WG. Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast cancer research : BCR. 2005;7(4):R402–R410. doi: 10.1186/bcr1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer cell. 2004;6(1):17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham HD, Shannon LA, Calloway PA, Fassold BC, Dunwiddie I, Vielhauer G, et al. Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Translational oncology. 2010;3(6):354–361. doi: 10.1593/tlo.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(25):9643–9648. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer cell. 2009;15(6):539–550. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reader J, Holt D, Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer metastasis reviews. 2011;30(3–4):449–463. doi: 10.1007/s10555-011-9303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang E, Boire A, Agarwal A, Nguyen N, O'Callaghan K, Tu P, et al. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer research. 2009;69(15):6223–6231. doi: 10.1158/0008-5472.CAN-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez NA, Correa E, Avila EP, Vela TA, Perez VM. PAR1 is selectively over expressed in high grade breast cancer patients: a cohort study. Journal of translational medicine. 2009;7:47. doi: 10.1186/1479-5876-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296(5573):1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 20.Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, et al. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. The Journal of biological chemistry. 1997;272(39):24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 21.Murga C, Fukuhara S, Gutkind JS. A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. The Journal of biological chemistry. 2000;275(16):12069–12073. doi: 10.1074/jbc.275.16.12069. [DOI] [PubMed] [Google Scholar]
- 22.Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nurnberg B. Gbetagamma stimulates phosphoinositide 3-kinase-gamma by direct interaction with two domains of the catalytic p110 subunit. The Journal of biological chemistry. 1998;273(12):7024–7029. doi: 10.1074/jbc.273.12.7024. [DOI] [PubMed] [Google Scholar]
- 23.Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, Khalil BD, et al. G protein-coupled receptor-mediated activation of p110beta by Gbetagamma is required for cellular transformation and invasiveness. Science signaling. 2012;5(253):ra89. doi: 10.1126/scisignal.2003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadas O, Dbouk HA, Shymanets A, Perisic O, Burke JE, Abi Saab WF, et al. Molecular determinants of PI3Kgamma-mediated activation downstream of G-protein-coupled receptors (GPCRs) Proceedings of the National Academy of Sciences of the United States of America. 2013;110(47):18862–18867. doi: 10.1073/pnas.1304801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 26.Zardavas D, Phillips WA, Loi S. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast cancer research : BCR. 2014;16(1):201. doi: 10.1186/bcr3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer cell. 2011;19(6):715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, et al. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Science signaling. 2008;1(36):ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho S, Milanezi F, Costa JL, Amendoeira I, Schmitt F. PIKing the right isoform: the emergent role of the p110beta subunit in breast cancer. Virchows Archiv : an international journal of pathology. 2010;456(3):235–243. doi: 10.1007/s00428-010-0881-0. [DOI] [PubMed] [Google Scholar]
- 30.Costa C, Ebi H, Martini M, Beausoleil SA, Faber AC, Jakubik CT, et al. Measurement of PIP3 levels reveals an unexpected role for p110beta in early adaptive responses to p110alpha-specific inhibitors in luminal breast cancer. Cancer cell. 2015;27(1):97–108. doi: 10.1016/j.ccell.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backer JM. The regulation of class IA PI 3-kinases by inter-subunit interactions. Current topics in microbiology and immunology. 2010;346:87–114. doi: 10.1007/82_2010_52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanley ER. Murine bone marrow-derived macrophages. Methods in molecular biology. 1997;75:301–304. doi: 10.1385/0-89603-441-0:301. [DOI] [PubMed] [Google Scholar]
- 33.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer research. 2005;65(12):5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 34.Starnes TW, Cortesio CL, Huttenlocher A. Imaging podosome dynamics and matrix degradation. Methods in molecular biology. 2011;769:111–136. doi: 10.1007/978-1-61779-207-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Molecular cancer research : MCR. 2007;5(2):195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 36.Patsialou A, Wang Y, Lin J, Whitney K, Goswami S, Kenny PA, et al. Selective gene-expression profiling of migratory tumor cells in vivo predicts clinical outcome in breast cancer patients. Breast cancer research : BCR. 2012;14(5):R139. doi: 10.1186/bcr3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boimel PJ, Smirnova T, Zhou ZN, Wyckoff J, Park H, Coniglio SJ, et al. Contribution of CXCL12 secretion to invasion of breast cancer cells. Breast cancer research : BCR. 2012;14(1):R23. doi: 10.1186/bcr3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PloS one. 2009;4(8):e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paz H, Pathak N, Yang J. Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene. 2014;33(33):4193–4202. doi: 10.1038/onc.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nature reviews Cancer. 2007;7(2):79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 43.Kirui JK, Xie Y, Wolff DW, Jiang H, Abel PW, Tu Y. Gbetagamma signaling promotes breast cancer cell migration and invasion. The Journal of pharmacology and experimental therapeutics. 2010;333(2):393–403. doi: 10.1124/jpet.109.164814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang X, Sun Z, Runne C, Madsen J, Domann F, Henry M, et al. A critical role of Gbetagamma in tumorigenesis and metastasis of breast cancer. The Journal of biological chemistry. 2011;286(15):13244–13254. doi: 10.1074/jbc.M110.206615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Hayre M, Degese MS, Gutkind JS. Novel insights into G protein and G protein-coupled receptor signaling in cancer. Current opinion in cell biology. 2014;27:126–135. doi: 10.1016/j.ceb.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15(2):73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer research. 2004;64(19):7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 49.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clinical & developmental immunology. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30(4):194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Utermark T, Rao T, Cheng H, Wang Q, Lee SH, Wang ZC, et al. The p110alpha and p110beta isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes & development. 2012;26(14):1573–1586. doi: 10.1101/gad.191973.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du J, Sun C, Hu Z, Yang Y, Zhu Y, Zheng D, et al. Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. PloS one. 2010;5(12):e15940. doi: 10.1371/journal.pone.0015940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brazzatti JA, Klingler-Hoffmann M, Haylock-Jacobs S, Harata-Lee Y, Niu M, Higgins MD, et al. Differential roles for the p101 and p84 regulatory subunits of PI3Kgamma in tumor growth and metastasis. Oncogene. 2012;31(18):2350–2361. doi: 10.1038/onc.2011.414. [DOI] [PubMed] [Google Scholar]
- 54.Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, et al. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2014;33(33):4203–4212. doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454(7205):776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dou Z, Pan JA, Dbouk HA, Ballou LM, DeLeon JL, Fan Y, et al. Class IA PI3K p110beta subunit promotes autophagy through Rab5 small GTPase in response to growth factor limitation. Molecular cell. 2013;50(1):29–42. doi: 10.1016/j.molcel.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell. 2013;153(5):1050–1063. doi: 10.1016/j.cell.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salamon RS, Dbouk HA, Collado D, Lopiccolo J, Bresnick AR, Backer JM. Identification of the Rab5 binding site in p110beta: assays for PI3Kbeta binding to Rab5. Methods Mol Biol. 2015;1298:271–281. doi: 10.1007/978-1-4939-2569-8_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costa C, Martin-Conte EL, Hirsch E. Phosphoinositide 3-kinase p110gamma in immunity. IUBMB Life. 2011;63(9):707–713. doi: 10.1002/iub.516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.