Abstract
Autonomic nervous system activity is an important component of human emotion. Mental processes influence bodily physiology, which in turn feeds back to influence thoughts and feelings. Afferent cardiovascular signals from arterial baroreceptors in the carotid sinuses are processed within the brain and contribute to this two-way communication with the body. These carotid baroreceptors can be stimulated non-invasively by externally applying focal negative pressure bilaterally to the neck. In an experiment combining functional neuroimaging (fMRI) with carotid stimulation in healthy participants, we tested the hypothesis that manipulating afferent cardiovascular signals alters the central processing of emotional information (fearful and neutral facial expressions). Carotid stimulation, compared with sham stimulation, broadly attenuated activity across cortical and brainstem regions. Modulation of emotional processing was apparent as a significant expression-by-stimulation interaction within left amygdala, where responses during appraisal of fearful faces were selectively reduced by carotid stimulation. Moreover, activity reductions within insula, amygdala, and hippocampus correlated with the degree of stimulation-evoked change in the explicit emotional ratings of fearful faces. Across participants, individual differences in autonomic state (heart rate variability, a proxy measure of autonomic balance toward parasympathetic activity) predicted the extent to which carotid stimulation influenced neural (amygdala) responses during appraisal and subjective rating of fearful faces. Together our results provide mechanistic insight into the visceral component of emotion by identifying the neural substrates mediating cardiovascular influences on the processing of fear signals, potentially implicating central baroreflex mechanisms for anxiolytic treatment targets.
INTRODUCTION
Influential theories emphasize the contribution of bodily arousal to emotion and affective feelings (James, 1894; Damasio, 1994; Barrett et al, 2007; Dolan, 2002). Even without a specific mapping of bodily state to emotional type (eg Cannon, 1927), the cognitive interpretation of a change in bodily arousal can shape the emotional experience (Schachter and Singer, 1962; Barrett, Lindquist and Gendron, 2007). Improved anatomical and functional description of bidirectional interactions between body and brain has advanced our understanding of emotional mechanisms (Craig, 2002; Critchley et al, 2002, 2004, Critchley, 2005a, 2005b; Harrison et al, 2009) and, for some emotions, there is good evidence of specific coupling with autonomically mediated changes in peripheral physiology (Ekman et al, 1983; Harrison et al, 2006).
There is particular interest in the relationship between parasympathetic control of the heart and emotion. Here, heart rate variability (HRV) (derived from electrocardiography) indexes the parasympathetic regulation of the heart via the vagus nerve and reflects the degree to which the cardiac activity can be modulated to meet changing situational and emotional demands (Thayer and Lane, 2000; Thayer and Brosschot, 2005). Moreover, it is closely linked to individual differences in emotional responding, and capacity for self-regulation (see Friedman, 2007; Thayer et al, 2009). Increased HRV is associated with adaptive emotional responses to threat (Thayer and Lane, 2000; Thayer et al, 2009) and increased sensitivity to the emotions of others (Quintana et al, 2012).
The human amygdala mediates interaction between the body and the brain during affective processing. The amygdala supports the perception of fear signals and threat (Zald, 2003; Phelps and LeDoux, 2005), and its activity correlates with the emotional intensity rating of affective pictures (Phan et al, 2004), including facial expressions (Hamann and Mao, 2002). Outputs from the amygdala innervate hypothalamic and brainstem autonomic circuits to trigger autonomic arousal responses to emotional challenges, particularly threats (LeDoux, 2000). Amygdala-induced autonomic arousal is expressed as increased sympathetic activity and/or decreased HRV (Critchley et al, 2005b; Gianaros et al, 2012). The amygdala is also sensitive to feedback from the periphery regarding state of bodily arousal (Critchley et al, 2002).
Carotid baroreceptors signal the state of cardiovascular arousal to the brain: when arterial blood pressure rises, baroreceptors drive afferent neuronal firing to evoke a reflex-mediated increase in parasympathetic activity and a decrease in sympathetic activity (Sagawa, 1983; reviewed in Fadel et al, 2003). These natural fluctuations in baroreceptor activity (with cardiac cycle) can influence different cognitive functions. Cardiac cycle has modulatory effects on simple reaction time in different sensory modalities (Edwards et al, 2008), somatosensory thresholds (Wilkinson et al, 2013), on memory for words (Garfinkel et al, 2013), and processing of fear (Garfinkel et al, 2014). Although the literature argues for an inhibitory effect of systole, recent data (Garfinkel et al, 2014) show an enhancing effect on fear processing, and others report reduction of somatosensory thresholds (eg, Edwards et al, 2008; Wilkinson et al, 2013). Speculatively, these discrepancies suggest two competing mechanisms that may reflect different roles and afferent channels (cranial nerves IX and X, respectively) of carotid and cardiac baroreceptors. In fact, diastolic activation of low pressure cardiac baroreceptors during cardiac filling is known to inhibit neurons within the locus coeruleus (Morilak et al, 1986; Jacobs et al, 1991).
Artificial mechanical stimulation of baroreceptor activity also demonstrates interaction between autonomic and cognitive/emotional processes (Calcagnini et al, 2010; Basile et al, 2013a, 2013b). A non-invasive, automated neck suction device enables carotid stimulation (CS) to be implemented concurrently with functional magnetic resonance neuroimaging (fMRI): neck suction stimulates carotid baroreceptors by increasing transmural pressure within the carotid sinus (Cooper and Hainsworth; 2009). This evokes an enhanced parasympathetic cardiovascular drive via the baroreflex. Previous studies from our group show that the same CS procedure modulates activity within brain areas including amygdala and insula in brain at rest, when engaged in a cognitive task, or during emotional processing (Basile et al, 2013a, 2013b).
Motivated by recent observations regarding the influence of cardiac cycle on fear processing (Garfinkel et al, 2014), the aim of the present study was to investigate the effect of a direct parasympathetic perturbation by CS on neural and subjective responses to the appraisal of fearful (compared with neutral) facial expressions. Further, we test whether individual traits in basal vagal tone (expressed as resting HRV) predict the degree to which this perturbation of viscerosensory autonomic function influences fear processing. On the basis of extant literature, we hypothesized a priori specific involvement of amygdala, insula, periaqueductal grey (PAG) as regions of interest supporting interaction between fear processing and our experimental physiological manipulation.
MATERIALS AND METHODS
Participants
Twenty-one right-handed volunteers (12 females/9 males; mean age=27.3 years; SD=2.6; range, 23–32) with no neurologic, psychiatric disorders, and other major clinical conditions, underwent detailed autonomic examination, including electrocardiogram, assessment of arterial pressure, and respiratory frequency during the Valsalva maneuver and orthostatic challenge, indicating no sign of autonomic dysfunction. The study was approved by the Santa Lucia Foundation ethical committee. Written informed consent was obtained from all participants.
HRV Evaluation
HRV analysis of normal interbeat intervals was used to index autonomic balance biased toward parasympathetic activity (Malik and Camm, 1995; Heart rate variability, 1996). The evaluation of the autonomic system consisted in the Valsalva maneuver performed consecutively three times, with a 2-min rest, followed by a 10-min orthostatic and supine electrocardiography recording. The results obtained from 3-min recordings (extracted randomly) did not differ from the results obtained from 10-min recordings, allowing us to estimate the HRV on the basis of short time recordings. Two datasets, corresponding to three stable consecutive minutes of supine and orthostatic recordings, were analyzed using an autoregressive model (Baselli et al, 1987; Howorka et al, 2010).
Normal sinus to normal sinus (N-N) interbeat intervals were extracted to derive: (i) time domain measures (variance of N-N intervals) and (ii) frequency domain measures (power spectral density measures indexing distributed variance of N-N intervals as a function of frequency).
Within the time domain, we calculated the standard deviation of N-N intervals (SDNN), the square root of the mean of the squares of the differences between adjacent N-N intervals (rMSSD), the number of differences between successive N-N intervals greater than 50 ms (NN50), and the percentage of differences between adjacent N-N intervals that are >50 ms (pNN50).
The total power densities and the main power densities within high-frequency (HF) (0.15–0.4 Hz), low-frequency (LF) (0.04–0.15 Hz), and very low frequency (VLF) (0.003–0.04 Hz) bands were quantified in absolute values of power (ms2).
Paradigms and Procedure
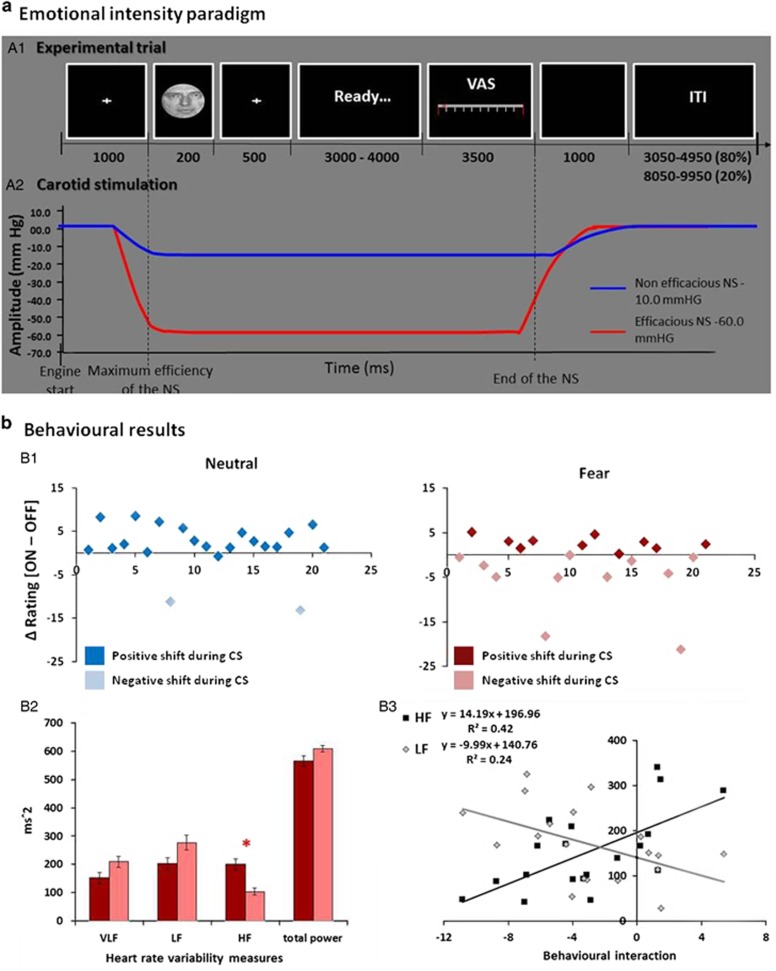
An event-related design, including two runs with 80 trials (total number of trials=160), randomly administered over 19.5 min of fMRI acquisition was used in this experiment (total experiment duration approximately 40 min; Figure 1a). Within each run, 40 fearful and 40 neutral faces were randomly presented. The trials lasted on average 9700 ms each (range 9200–10200 ms), followed by a variable inter-trial interval, lasting on average 4 s (range 3050–4950 ms) in 80% of the trials, and 9 s (range 8050–9950 ms) in 20% of the trials.
Figure 1.
(a) Emotional intensity paradigm. (A1) Each trial started with a fixation cross, followed by the presentation of the visual expression (neutral or fearful, 200 ms), and a second fixation cross, a variable ‘ready' interval, and a VAS task in which the subject had to label faces' emotional intensity by button pressing on a scale ranging from 0—no emotional intensity—to 100—extreme emotional intensity. A variable ITI was introduced at the end of the trial. (A2) The efficacious (ON) and non-efficacious (OFF) stimulation was randomly delivered to each participant. The neck suction engine started 500 ms after the onset on the pre-face fixation cross, and reached the set value (−10 or −60 mm HG) at the onset of the face. Accordingly, each stimulation offsets at the end of the VAS rating event. (b) Behavioral results. (B1) Effect of CS during fearful and neutral intensity rating documented on a subject level. The y axis represents Δ rating [CS ON—OFF] for neutral (left) and fearful (right) faces. Positive values indicate increased ratings during the CS ON condition. For neutral faces (left graph), the majority of participants has a positive shift during CS ON (ie, increased perceived emotional intensity). In the case of fearful faces, half of the participants exhibited a positive shift and half of the participants exhibited a negative shift (rating of fearful faces lower during CS ON condition). (B2) Participants with a negative shift in fearful rating reported lower baseline parasympathetic activity, expressed by lower HF spectral power. (B3) HRV parameters—HF and LF—correlated with the behavioral interaction between CS and emotional rating.
During the fMRI, each participant was presented with emotional and neutral faces from the Ekman set (Ekman and Friesen, 1974). First, a fixation cross appeared for 1 s, followed by brief presentation of a face stimulus (200 ms; the 200 ms period was selected for because it would allow comparison with planned studies on natural baroreceptor activation within the cardiac cycle). A total of 80 fearful and 80 neutral faces were randomly presented over two runs. A post-stimulus fixation cross was then presented for 500 ms, followed by a blank screen with a 'ready' message (3–4 s). A visual analogue scale (VAS) was then presented for 3500 ms for the participant to rate emotional intensity of the face (in this study, emotional intensity refers to the intensity of emotional experience that the facial expression evokes in the participant. In the case of neutral faces, based on previous studies, we assume that an inexpressive face is not necessarily an emotionally neutral stimulus and could, therefore, trigger an emotional experience in the individual (Phillips et al, 1997)) on a scale ranging from 0—no emotional intensity—to 100—extreme emotional intensity (see Figure 1). There was a variable inter-trial interval lasting on average 4000 ms (range 3050–4950 ms) for 80% of the trials and 9000 ms (range 8050–9950 ms) for 20% of the trials.
CS Delivery
Using a laboratory-built device for MRI, neck suction was delivered through two individual cuffs within a neck collar (Calcagnini et al, 2010; Basile et al, 2013a, 2013b). The pressure was set by controlling the aspiration level of a vacuum source (placed in the MRI control room) by a PC. The actual pressure within each cuff of the neck collar was continuously and independently monitored. Specific placement of neck suction cuffs was tailored for each participant, using carotid angiograms acquired earlier to localize points of carotid artery bifurcation. CS was delivered in pulses of variable duration, ranging from 7200 to 8200 ms. In order to assure an efficient influence of the ANS perturbation on the emotional elaboration of the faces, the CS was time-locked to the onset of the face stimulus and offset of the VAS. Periods of efficacious (−60 mm Hg pressure) and non-efficacious (−10 mm Hg pressure) CS were randomly delivered. Active pulses were always followed by an inter-trial interval of a variable duration (Figure 1a, panel A), during which CS was not delivered and participants were not engaged in any active task. This reduced the likelihood of baroreceptor response accommodation.
Physiological Signal Acquisition and Analysis
Electrocardiography, pulse oximetry, and respiration were recorded during fMRI (Biopac Systems Ins, CA). N-N interbeat intervals were extracted from the electrocardiography. Cardiovascular response to CS was assessed as the percentage difference between the average N-N interval during the neck suction and the average N-N interval during the preceding inter trial interval. No motion artifacts were detected when the CS was applied using the two-cuff device. Physiological monitoring and CS delivery did not induce an increase in radio frequency noise.
fMRI Acquisition and Preprocessing
Neuroimaging data were acquired using a head-only 3.0 T MR scanner (Siemens Magnetom Allegra, Siemens Medical Solutions, Erlangen, Germany). Functional brain images optimized for blood oxygenation level-dependent contrast were collected using an echo-planar T2*-weighted sequence (TR=2.08 s, 32 axial slices, slice thickness=2.5 mm, gap=1.3 mm). Data were processed using MATLAB 7.0 (MathWork, Natick, MA) and SPM8 (Statistical Parametrical Mapping, http://www.fil.ion.ucl.ac.uk). In both experiments, the first four volumes were discharged to allow for T1 equilibration effects
EPI images were realigned to the first image and normalized to a standard echoplanar image template. Normalized functional scans were smoothed with a Gaussian kernel of 8-mm (full-width half maximum).
Data Analysis
A repeated measures 2 × 2 ANOVA was conducted to test the effect of CS on emotional processing. CS (efficacious-ON, non-efficacious-OFF) × emotion (fear, neutral) were entered as within-participant variables. A high-pass filtering to 1/128 Hz was applied to remove low-frequency noise
The stimulation-by-emotion interaction effect on ratings was then explored using post hoc t-tests Bonferroni-corrected for multiple comparisons. Further, we performed a median-split analysis to explore for differences in a physiological measure in the group of participants, based on their performance on the behavioral task.
First-level analyses estimating contrasts of interest for each participant were followed by second-level mixed-effect analyses for statistical inference at the group level (Friston et al, 2002).
The first-level multiple regression model included eight conditions, four corresponding to the face event and four corresponding to the VAS task, in both cases reflecting a combination of emotion (fear, neutral) and CS (ON, OFF), which were modeled and convolved with a canonical hemodynamic response function. The four face event conditions were modeled as miniblocks, time-locked at the onset of the face with a duration of 200 ms, whereas the four VAS conditions were time-locked at the onset of the VAS rating event with a duration of 3500 ms. All predictors were convolved with the SPM8 hemodynamic response function, and realignment parameters were included as covariates of no interest.
At the group level, two different analyses were carried out. In a first analysis, the four conditions resulting from the emotion × CS combination (of both the face event and the VAS period) were modeled within a 2 × 2 within-participant ANOVA, to test for the main effect of the stimulation (ON<OFF; ON>OFF) across emotional conditions, and for the interactions between the two factors [(fearON<OFF) -(neutralON<OFF)]. A correlational analysis was then carried out between the difference between fearful [ON—OFF] and neutral ratings [ON—OFF] and brain activity during the VAS task. The statistical threshold was set to P<0.05, FWE corrected at the voxel level for whole brain.
Moreover, for the region-of-interest analyses, anatomical masks were constructed using the anatomical toolbox in SPM (Tzourio-Mazoyer et al, 2002) for bilateral insula. A sphere (6 mm radius) was applied on the region of the PAG, based on MNI coordinates [0 −28 −9] and a 10 mm radius sphere on the amygdala based on coordinates [32 0 −24] from Garfinkel et al, (2014). Statistical threshold was set to P<0.05—FWE-corrected at cluster level (cluster size defined using uncorrected voxel-level threshold P<0.005).
Next, we tested whether the areas showing an effect of CS over emotional appraisal were associated with basal HRV measures. We considered a sphere (10 mm radius) centred on peak voxel within the region-of-interest analyses described above. The association between contrast estimates from our region-of-interests and HRV measures were evaluated using Pearson correlations.
RESULTS
As expected, all subjects (but one) had an increase of R-R intervals during the efficacious stimulation (see Supplementary Figure S1 for the individual response to efficacious CS). Thus, efficacious CS engendered a significant increase in the interbeat (R-R) interval indicating autonomic perturbation (average increase in R-R interval: 19.2 ms; pre vs during CS, t(20)=8.4, P<0.001). Non-efficacious CS resulted in a non-significant increase of 6.0 ms in (pre vs during CS, t<1).
Behavioural Results
We observed a significant main effect of emotion, reflecting greater intensity ratings for fear faces relative to neutral faces (F(1, 18)=334.9, P<0.001), and no main effect of CS (F(1, 20) <1) on intensity ratings. Importantly, we observed a significant CS-by-emotion interaction (F(1, 20)=14.8, P<0.001).
For neutral faces, the efficacious CS induced a positive shift (ie, higher) emotional intensity ratings; efficacious-ON = 33.79, SD = 15.66 vs non-efficacious-OFF = 32.07, SD = 18.99). This positive shift occurred in almost all participants (Figure 1b, panel A). Planned contrasts for each emotion type revealed a trend in the effect of CS on appraisal of neutral expressions (18/20 positive events, two-tailed sign test P<0.01). Across the group, there was no overall significant difference for fearful expressions (efficacious-ON=66.73, SD=20.30 vs non-efficacious-OFF 68.45, SD=14.67; t(20)=1.2, P=0.1). However, during the appraisal of fearful stimuli, 10 participants exhibited a positive shift and 11 participants exhibited a negative shift.
We therefore performed a median-split of participants to compare HRV measures of the participants exhibiting a positive shift and those exhibiting a negative shift. Participants with a positive shift in fearful rating manifest higher basal vagal parasympathetic tone compared with those with a negative shift, expressed as higher HF spectral power and rMSSD, NN50, and pNN50 HRV time-domain indices (Figure 1b, panel B). Therefore, when controlling for the basal vagal activity of each participant, a significant difference emerged for fear ratings when comparing ON vs OFF conditions (66.73 vs 68.46, F(1, 19)=4.96, P<0.05).
Relation to HRV Parameters
HRV parameters correlated with the magnitude of the behavioral expression of the stimulation-by-emotion interaction on intensity ratings ([fearON-OFF] - [neutralON-OFF]) (Figure 1b, panel C). A positive correlation was evident between the behavioral interaction and time domain measures of parasympathetic response: NN50 (r=0.48, P<0.02), pNN50 (r=0.50, P<0.03). Similarly, this behavioral interaction negatively correlated with LF (r=−0.49, P<0.03) and VLF spectrum (r=−.55, P<0.01), yet positively correlated with HF spectrum (r=−.65, P<0.02). In all the cases, the interaction was driven by a correlation with the simple effect of CS on perceived intensity of fear stimuli (fear ON-OFF; pNN50 r=0.49; HF r=0.48, VLF r=−0.57, LF r=−0.47; all P<0.05), whereas no correlation was evident with the effect of CS on neutral ratings (neutral ON–OFF). This result extends the findings of the median-split analysis to indicate that the individual basal vagal activity differently affected the modulation of emotional ratings by CS: Individuals with higher basal HRV exhibited a positive shift in the rating of fearful faces during the ON condition. Notably, the same influence was not evident during the neutral rating condition. In order to examine whether the carotid effect was confined among participants with high or low HRV, a second median-split control analysis was conducted, splitting the group in ‘high' and ‘low' HRV participants. The difference between ON and OFF in rating of fearful expressions was coherent with the first median split analysis, that is, the group of ‘high HRV' group reported a positive shift in fearful rating during CS ON, whereas the ‘low HRV' group reported a negative shift, although both effects were not significant (P<0.1 one-tailed t test) (see Supplementary Material for a graphic representation, Supplementary Figure S2).
Neuroimaging Results
Main effect of efficacious CS
A whole-brain neuroimaging analysis was conducted to test the effect of CS during the appraisal of facial expressions (ie, VAS rating period), although an exploratory analysis has also been conducted to test the effect of CS on perception of facial expressions (ie, face presentation event, see Figure 1).
Consistent with our knowledge concerning the delay in effects of maximum baroreceptor stimulation with this method from our previous studies (eg, Basile et al, 2013a, 2013b), we anticipated effects to emerge after the face stimuli were presented. The analysis focused on the VAS event revealed a significant main effect of CS during the rating of face stimuli, irrespective of emotion for stimulation ON<OFF (see Table 1) manifest as a distributed decrease in brain activity during efficacious stimulation in the following areas: bilateral hippocampus, bilateral amygdala, left thalamus, and temporal fusiform areas (see Figure 2 and Table 1 for the complete list of brain areas). The region-of-interest analysis revealed a significant effect also in bilateral insula. No positive effects of efficacious CS on brain activity (ON>OFF) were evident.
Table 1. Brain Activation Underlying Main Effect of Carotid Stimulation, Collapsed Over Specific Emotion Type.
|
ON<OFF | ||||
|---|---|---|---|---|
| Brain region |
Cluster |
Voxel |
||
| k | P FWE | Z | MNI xyz | |
| Temporal pole R | 31860 | 0.000a | 5.63 | 34 14 −40 |
| Temporal pole L | 5.19 | −42 14 −32 | ||
| Superior frontal gyrus R | 5.09 | 22 20 52 | ||
| Superior frontal gyrus L | 4.89 | −20 22 48 | ||
| Hippocampus L | 5.08 | −24 −12 −20 | ||
| Hippocampus R | 4.78 | 26 −10 −22 | ||
| Postcentral gyrus/precuneus R | 5.02 | 6 −36 58 | ||
| Postcentral gyrus L | 4.44 | −44 −12 28 | ||
| Parahippocampal gyrus R | 4.79 | 36 −32 −14 | ||
| Amygdala R | 4.78 | 26 4 −24 | ||
| Amygdala L | 4.65 | −28 −4 −24 | ||
| Middle temporal gyrus R | 4.72 | 52 −2 −24 | ||
| Temporal fusiform area L | 4.54 | −34 −40 −12 | ||
| Superior parietal lobule/postcentral gyrus R | 4.54 | 32 −38 46 | ||
| Thalamus L | 4.52 | −16 −22 18 | ||
| Cerebellum, L VI | 561 | 0.009a | 3.84 | −8 −64 −18 |
| Insula L | 9 | 0.02b,c | 3.56 | −32 −26 16 |
| Insula R | 12 | 0.02b,c | 3.41 | 44 −6 −18 |
| Brainstem, midbrain | 231 | 0.001b | 4.33 | −2 −16 −18 |
FWE whole brain cluster level.
FWE ROI.
FWE whole brain peak level.
Figure 2.
(a) Main effect of CS (averaged across emotional condition). Notably, brain activity was attenuated during CS in bilateral hippocampus, amygdala, and insula (see Table 1 for the complete list of brain areas). (b) (B1) An effect of the CS × emotional condition interaction emerged in the activity of left amygdala. (B2) The interaction was mainly driven by the attenuation of activity in left amygdala during the rating of fearful faces.
Regarding face perception, the results are marginal for the purpose of the current study, and are presented as Supplementary Material. Overall, a significant main effect was evident during face perception, this manifested both as increased neural activity in the lateral occipital cortex bilaterally and decreased activity in bilateral temporal pole (see Supplementary Figure S3 and Supplementary Table S1). Moreover, increased activity was present during the perception of fearful over neutral faces irrespective of CS, in left occipital fusiform gyrus, left anterior cingulated gyrus, and right postcentral gyrus.
Interaction between CS and emotional rating condition
We next tested for interaction between CS (ON, OFF) and emotion ([fearON<OFF] vs [neutral ON>OFF]) to investigate the specific effect of CS on emotional appraisal. Conversely, the activity within left amygdala showed a significant CS-by-emotion interaction (T=2.88, 19 voxel, P<0.05 FWE-corrected for small-volume, Figure 2b, panel A) during the appraisal of facial expressions (ie, the VAS period). Analysis of the mean activation from left amygdala showed that the interaction (F(1,20)=7.42, P<0.01) was particularly driven by attenuation of amygdala reactivity during CS when appraising the intensity of fear stimuli (fearON vs fearOFF t(20)=4.52, P<0.001) as no difference was evident when comparing neutralON vs neutralOFF activation in left amygdala (t(20)<1) (Figure 2b, panel B).
Consistent with our expectations, we did not observe any significant CS-by-emotion interaction during the perception of faces.
Correlations with emotional ratings
We tested for regions whose activity correlated directly with the behavioural shift induced by CS during fearful and neutral rating. Overall, a positive correlation was evident between changes in the rating of fearful faces [fearful ON – OFF] and the relative attenuation of brain activation by CS. A correlation was evident within bilateral thalamus, hippocampus, cerebellum, middle temporal gyrus, putamen and globus pallidus, frontal orbital cortex, bilateral amygdala and bilateral insula, and PAG (see Figure 3a and Table 2 for the complete list of brain areas).
Figure 3.
(a) Graph reporting brain areas significantly correlating with the behavioral shift in fearful rating during CS efficacious stimulation. The activation in left amygdala and PAG correlated with HRV measures, with individuals with higher LF and lower HF indexes reporting stronger attenuation during CS efficacious stimulation. (b) The correlation between the shift in fearful rating and the neural activation in left amygdala during the appraisal of fearful faces, in individuals having higher and lower HF. Overall, Individuals exhibiting a negative shift in fearful rating had the strongest deactivation of the amygdala and lower parasympathetic tone. (c) The behavioral shift in neutral rating during CS efficacious stimulation correlated with the activity in PAG area.
Table 2. Brain Areas That Positively Correlated With a Rating (On-Off) of Fearful Faces.
| Correlation with fear rating | ||||
|---|---|---|---|---|
| Brain region |
Cluster |
Voxel |
||
| k | P FWE | Z | MNI xyz | |
| Thalamus R | 9182 | 0.00a | 4.89 | 22 −34 −2 |
| Thalamus L | 4.02 | −10 −18 12 | ||
| Temporal occipital fusiform R | 4.64 | 22 −52 −16 | ||
| Temporal occipital fusiform L | 3.89 | −36 −56 −14 | ||
| Hippocampus L | 4.51 | −24 −24 −6 | ||
| Putamen | 4.21 | −32 −4 8 | ||
| Cerebellum | 4.07 | −38 −50 −30 | ||
| Middle temporal pole | 3.99 | −56 6 −26 | ||
| Parahippocampal gyrus, posterior L | 3.84 | −28 −36 −16 | ||
| Globus pallidum L | 3.75 | −14 −4 −4 | ||
| Inferior temporal gyrus L | 3.72 | 56 −56 −14 | ||
| Frontal orbital cortex | 3.45 | −38 30 −12 | ||
| Brain-stem | 3.42 | 10−20 −22 | ||
| Postcentral gyrus L | 867 | 0.03a | 4.48 | −44 −38 60 |
| Superior parietal lobule | 2.69 | −22 −46 56 | ||
| Precentral gyrus R | 718 | 0.06a | 4.19 | 46 −4 32 |
| Amygdala L | 39 | 0.003b,c | 3.92 | −36 4 −16 |
| Insula L | 24 | 0.02b,c | 3.47 | −44 −12 6 |
| Insula R | 12 | 0.01b,c | 3.62 | 32 −26−12 |
| PAG | 171 | 0.01b,c | 3.37 | 12 −30 −6 |
FWE whole brain peak level.
FWE whole brain cluster level.
FWE ROI.
We extracted the mean activation from amygdala, insula, and PAG in order to perform additional analyses. A significant correlation was obtained between HRV parameters and amygdala activation during fear rating. Basal LF, HF, and RMSSD parameters correlated with activity in left amygdala (LF r=−0.55, P<0.015; HF r=0.47, P<0.05; RMSSD r=0.45, P=0.05) and PAG (LF r=−0.48, P<0.05; HF r=0.52, P<0.05, Figure 2a). Analysis of the mean activation from left amygdala showed that the individuals who had a negative shift in the rating of fearful faces reported a stronger attenuation in the activity of amygdala and lower basal vagal activity (Figure 3b).
With neutral faces, a negative correlation was evident between the change in neutral rating [ON—OFF] and in the attenuated activation of PAG during active CS (T=3.46, 7 voxel, P<0.037 FWE-corrected for small-volume, Figure 3c).
DISCUSSION
The aim of this study was to explore how afferent cardiovascular information contributes to the processing of emotion stimuli, notably the fear signals of others. Using stimulation of carotid baroreceptors to perturb parasympathetic drive, we tested for hypothesized effects on the neural processing and appraisal of fearful (relative to neutral) facial expressions. In addition, we explored how individual differences in autonomic state (particularly the basal vagal parasympathetic tone) predicted the influence of CS on emotional processing.
Regarding the effect of CS during the appraisal of facial emotions, the observed brain activity is consistent with earlier observations that activation of arterial baroreceptors may inhibit sensory processing and cortical excitability (Koriath et al, 1987).
Here, efficacious CS resulted in attenuated activation across cortical and subcortical brain areas during the facial appraisal, including insula, amygdala, hippocampus, thalamus, and brainstem. Many of these brain centers are implicated in autonomic regulation (Critchley, 2005a; Kimmerly et al, 2005). In a previous study, our group used the same methodology to describe the modulation by CS of activity within regions, including insula, amygdala, and PAG, both at rest and when participants were engaged in a cognitive task (Basile et al, 2013a), or during processing of sad, happy, angry, and neutral faces (Basile et al, 2013b).
Conversely, the effect of the CS during early events of the trial (ie, face presentation) affected mainly occipital and temporal areas, consistent with the expected delay in effects of maximum baroreceptor stimulation with this method on our areas of interest (ie, insula, amygdaala, PAG).
A noteworthy aspect of the present study is that CS elicited a differential effect on neural activity during the appraisal of fearful and neutral faces. Activity within amygdala was affected by CS exclusively during the appraisal of fearful faces. In addition, the attenuation of left amygdala activity during the appraisal of fearful expressions predicted the behavioral consequences, as reflected in the magnitude of the behavioral interaction. Thus, our results extend mechanistic understanding of the role of the amygdala in the perception and processing of threat, by highlighting the integration of viscerosensory signaling and autonomic control with the neural and behavioral sensitivity to the fear signals of other people (Zald, 2003; Phelps and LeDoux, 2005; Garfinkel et al, 2014).
These insights were further endorsed by the observation that at the level of individual differences, with individuals with low basal parasympathetic activity (across HRV-related measures) manifesting a decreased perception of fear during the CS. The degree of change in the behavioral ratings evoked by CS correlated with activity across a set of related regions including insula, amygdala, hippocampus, and orbitofrontal cortex. We were particularly interested in the putative role of vagal parasympathetic tone as an index of adaptive emotional regulatory capacity (Friedman, 2007). Low vagal activity is associated with anticipatory anxiety and hypervigilance toward potential threat (Thayer et al, 2009), and interestingly those individuals who exhibited a negative shift in fearful ratings during the CS manifest the greatest attenuation of the amygdala activity and had lower parasympathetic tone. We showed this effect also in a direct correlation between HRV measures and attenuation of amygdala and PAG activity when appraising fear faces. These findings strongly endorse the perspective that the degree to which viscerosensory afferent information impacts upon both neural reactivity and emotional processing is highly dependent on the integrity of parasympathetic autonomic control and individual characteristics in basal vagal cardiovascular regulation. Lower vagal activity has been linked to reduced prefrontal inhibitory control over the amygdala (Thayer et al, 2009), and is observed in patients with generalized anxiety disorders, panic disorder, and even children of patients with panic disorder (see Friedman, 2007 for a review; Srinivasan et al, 2002). Here, we show that individuals with lower HRV were more responsive towards the CS, by reporting a stronger deactivation of amygdala and lower rating of fearful stimuli, according to the literature reporting that higher parasympathetic tone is associated with reduced sensitivity to fear perception (Thayer et al, 2009).
Individuals with higher HRV were, conversely, less responsive to CS, showing a less evident effect of the CS on amygdala and a weak positive shift in fearful rating. This raises the possibility that the relationship between parasympathetic tone and fear may not be linear (but instead follows an inverted U-shaped function), or implicates additional mechanisms such as enhanced interoceptive accuracy at lower heart rates (Pollatos et al, 2007). Future research is needed to fully delineate the mechanisms underlying the complex interplay between the basal HRV, stimulation of the parasympathetic tone, and the appraisal of emotions.
The influence of baroreceptor afferents on the processing of fearful stimuli was recently investigated by Garfinkel et al, (2014). Here, timing brief fear stimuli to systole, during natural baroreceptor firing, is associated with enhanced processing of fear and corresponding increased neural activity in regions including amygdala. There is moreover an attenuation of the processing of fearful stimuli during diastole. This latter observation may account for the apparent paradoxical findings with present study, where attenuated fear processing (albeit over longer time periods) occurred with the lengthening of inter-beat interval following artificial baroreceptor stimulation. Prolongation of diastole during CS may partly account for these apparently mixed findings, but there remains more to understand about the timing of baroreceptor influences on emotion and perception. Nevertheless, both studies highlight an important viscerosensory cardiovascular influence, specifically linked to the baroreflex, on the processing of threat signals and fear stimuli. Here, we quantify the neural and behavioral impact of baroreceptor firing on fear processing and extend previous results by noting the dependence of this effect on basal autonomic characteristics of individuals.
Overall, CS showed less pronounced effects on the ratings of neutral faces. PAG activity correlated with stimulation-induced change in the rating of neutral faces. The PAG region is previously implicated in the coupling of peripheral arousal state to emotional processing and expression of fear (Mobbs et al, 2009; Gray et al, 2012; Linnman et al, 2012). Consistently with these previous studies, our data show that a modulation of the neural activity in the PAG area caused by CS results in an increased evaluation of the emotional intensity in neutral faces.
Taken together, our results demonstrate how viscerosensory and autonomic perturbation affect appraisal of fearful and neutral faces at neural and subjective levels. Our study highlights the utility of integrating physiological and neuroimaging techniques to gain detailed insight into mechanisms underlying influential theories of emotion. Our device stimulated the carotid sinus baroreceptors and increased the heartbeat interval, affecting activation across distributed brain areas. Through the use of CS, we directly evaluated the influence of peripheral bodily arousal on emotional appraisal and further demonstrate the influence of individual basal vagal tone on these affective processes.
The current study not only provides neurophysiological insights on the interaction between body and brain, but can also potentially contribute to the understanding of pathophysiological conditions, not least the expression and maintenance of anxiety disorders (Garfinkel et al, 2014), and suggests that treatments for anxiety might target baroreflex-related central pathways.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
E. Makovac is supported by the grant RF09.150.1. HDC and SNG Aare supported by the ERC via and advanced grant ERC-2012-ADG_20120411 and by the Dr Mortimer and Dame Theresa Sackler Foundation via the Sackler Centre of Consciousness Science, University of Sussex
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Barrett LF, Lindquist K, Gendron M (2007). Language as context for the perception of emotion. Trends Cogn Sci 11: 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselli G, Cerutti S, Civardi S, Lombardi F, Malliani A, Merri M et al (1987). Heart rate variability signal processing: a quantitative approach as an aid to diagnosis in cardiovascular pathologies. Int J Biomed Comput 20: 51–70. [DOI] [PubMed] [Google Scholar]
- Basile B, Bassi A, Calcagnini G, Caltagirone C, Bozzali M (2013. b). Effect of parasympathetic stimulation on brain activity during emotional processing. Proceedings of the Human Brain Mapping annual meeting June 8-12, 2014, Hamburg, Germany.
- Basile B, Bassi A, Calcagnini G, Strano S, Caltagirone C, Macaluso E et al (2013. a). Direct stimulation of the autonomic nervous system modulates activity of the brain at rest and when engaged in a cognitive task. Hum Brain Mapp 34: 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnini G, Mattei E, Triventi M, Basile B, Bassi A, Bozzali M et al (2010). Investigation of the autonomic nervous system control of cardiovascular variables using fMRI and carotid stimulation. Computing in Cardiology 37: 529–532. [Google Scholar]
- Cannon WB (1927). The James-Lange theory of emotions. Am J Psychol 39: 115–1124. [PubMed] [Google Scholar]
- Cooper VL, Hainsworth R (2009). Carotid baroreflex testing using the neck collar device. Clin Auton Res 19: 102–112. [DOI] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley HD (2005. a). Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493: 154–166. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2002). Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33: 653–663. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ (2005. b). Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage 24: 751–762. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshstein P, Öhman A, Dolan RJ (2004). Neural systems supporting awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1994) Descartes' Error: Emotion, Reason and the Human Brain. Grosset/Putnam: New York. [Google Scholar]
- Dolan RJ (2002). Emotion, cognition, and behavior. Science 298: 1191–1194. [DOI] [PubMed] [Google Scholar]
- Edwards L, Inui K, Ring C, Wang X, Kakigi R (2008). Pain-related evoked potentials are modulated across the cardiac cycle. Pain 137: 488–494. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV (1974). Detecting deception from body or face. J Pers Soc Psychol 29: 288–298. [Google Scholar]
- Ekman P, Levenson RW, Friesen WV (1983). Autonomic nervous system activity distinguishes among emotions. Science 221: 1208–1210. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM, Raven PB (2003). Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol 88: 671–680. [DOI] [PubMed] [Google Scholar]
- Friedman BH (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol 74: 185–199. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J (2002). Classical and Bayesian inference in neuroimaging: applications. Neuroimage 16: 484–512. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Barrett AB, Minati L, Dolan RJ, Seth AK, Critchley HD (2013). What the heart forgets: Cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiology 50: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Minati L, Gray MA, Seth AK, Dolan RJ, Critchley HD (2014). Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. J Neurosci 34: 6573–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD (2012). Brain systems for baroreflex suppression during stress in humans. Hum Brain Mapp 33: 1700–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Beacher FD, Minati L, Nagai Y, Kemp AH, Harrison NA et al (2012). Emotional appraisal is influenced by cardiac afferent information. Emotion 12: 180–191. [DOI] [PubMed] [Google Scholar]
- Hamann S, Mao H (2002). Positive and negative emotional verbal stimuli elicit activity in the left amygdala. NeuroReport 13: 15–19. [DOI] [PubMed] [Google Scholar]
- Harrison N, Singer T, Rotshtein P, Dolan RJ, Critchley HD (2006). Pupil size modulates the empathic experience of sadness. Soc Cogn Affect Neurosci 1: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD (2009). Inflammation causes mood change through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 66: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use (1996). Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 17: 354–381. [PubMed] [Google Scholar]
- Howorka K, Pumprla J, Jirkovska A, Lacigova S, Nolan J (2010). Modified orthostatic load for spectral analysis of short-term heart rate variability improves the sensitivity of autonomic dysfunction assessment. J Diabetes Complications 24: 8–54. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Abercrombie ED, Fornal CA, Levine ES, Morilak DA, Stafford IL (1991). Single-unit and physiological analyses of brain norepinephrine function in behaving animals. Prog Brain Res 88: 159–165. [DOI] [PubMed] [Google Scholar]
- James W (1894). Physical basis of emotion. Psychol Rev 1: 516–529 (reprinted in 1994. Psychological Rev 101: 205–210). [DOI] [PubMed] [Google Scholar]
- Kimmerly Ds,, O'Leary DD, Menon RS, Gati JS, Shoemaker JK (2005). Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol 569: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriath JJ, Lindholm E, Landers DM (1987). Cardiac-related cortical activity during variations in mean heart rate. Int J Psychophysiol 5: 289–299. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. (2012). Neuroimaging of the periaqueductal gray: state of the field. Neuroimage 60: 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Camm AJ (1995). Heart Rate Variability. Futura Armonk, NY. pp 393–406.
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray MA et al (2009). From threat to fear: The neural organization of defensive fear systems in humans. J Neurosci 29: 12236–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Fornal C, Jacobs BL (1986). Single unit activity of noradrenergic neurons in locus coeruleus and serotonergic neurons in the nucleus raphe dorsalis of freely moving cats in relation to the cardiac cycle. Brain Res 399: 262–270. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I (2004). Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage 21: 768–780. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48: 175–187. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ et al (1997). A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Herbert BM, Matthias E, Schandry R (2007). Heart rate response after emotional picture presentation is modulated by interoceptive awareness. Int J Psychophysiol 63: 117–124. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, Outhred T, Hickie IB, Kemp AH (2012). Heart rate variability is associated with emotion recognition: direct evidence for a relationship between the autonomic nervous system and social cognition. Int J Psychophysiol 86: 168–172. [DOI] [PubMed] [Google Scholar]
- Sagawa K (1983). Baroreflex control of systemic arterial pressure and vascular bed. In Shepard JT, Abboud FM, Geiger SR (eds). Handbook of Physiology, The Cardiovascular System: Peripheral Circulation and Organ Blood Flow. American Physiological Society: Bethesda, MD. pp 453–496. [Google Scholar]
- Schachter S, Singer JE (1962). Cognitive, social and physiological determinants of emotional state. Psychol Rev 69: 379–399. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA. [Google Scholar]
- Srinivasan K, Ashok MV, Vaz M, Yeragani VK (2002). Decreased chaos of heart rate time series in children of patients with panic disorder. Depress Anxiety 15: 159–167. [DOI] [PubMed] [Google Scholar]
- Thayer J, Brosschot J (2005). Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology 30: 1050–1058. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH (2009). Heart rate variability, prefrontal neural function and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med 37: 141–153. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 61: 201–216. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Wilkinson M, McIntyre D, Edwards L (2013). Electrocutaneous pain thresholds are higher during systole than diastole. Biol Psychol 94: 71–73. [DOI] [PubMed] [Google Scholar]
- Zald DH (2003). The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev 41: 88–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.