Abstract
Variations in early life maternal care modulate hippocampal development to program distinct emotional–cognitive phenotypes that persist into adulthood. Adult rat offspring that received low compared with high levels of maternal licking and grooming (low LG offspring) in early postnatal life show reduced long term potentiation (LTP) and impaired hippocampal-dependent memory, suggesting a ‘detrimental' maternal effect on neural development. However, these studies focused uniquely on the dorsal hippocampus. Emerging evidence suggests a distinct role of the ventral hippocampus in mediating aggression, anxiety, and fear-memory formation, which are enhanced in low LG offspring. We report that variations in maternal care in the rat associate with opposing effects on hippocampal function in the dorsal and ventral hippocampus. Reduced pup licking associated with suppressed LTP formation in the dorsal hippocampus, but enhanced ventral hippocampal LTP. Ventral hippocampal neurons in low LG offspring fired action potentials at lower threshold voltages that were of larger amplitude and faster rise rate in comparison with those in high LG offspring. Furthermore, recordings of excitatory postsynaptic potential-to-spike coupling (E-S coupling) revealed an increase in excitability of ventral hippocampal CA1 neurons in low LG offspring. These effects do not associate with changes in miniature excitatory postsynaptic currents or paired-pulse facilitation, suggesting a specific effect of maternal care on intrinsic excitability. These findings suggest region-specific influences of maternal care in shaping neural development and synaptic plasticity.
Introduction
Abusive or neglectful parenting in humans stably influences cognitive–emotional development, which is then reflected in compromised academic achievement, increased stress reactivity, and increased risk of developing affective disorders (Binder et al, 2008; Young et al, 1997). Conversely, more nurturing parental care promotes resilience to abuse-related psychiatric disorders (Collishaw et al, 2007), highlighting the critical importance of early life parental interactions in shaping brain development and adult mental health. Similarly, the quality of mother–offspring interactions in rats directly affects neural development to stably influence cognitive–emotional function in adulthood. Variations in postnatal maternal care influence the capacity for hippocampal synaptic plasticity (Bagot et al, 2012a, 2012b; Champagne et al, 2008), a cellular correlate of learning and memory (Bliss and Collingridge, 1993). The adult offspring of mothers showing a decreased frequency of pup licking/grooming (low LG offspring) show reduced dendritic arborization (Bagot et al, 2009; Champagne et al, 2008) and long term potentiation (LTP) (Bagot et al, 2009; Champagne et al, 2008) in the dorsal hippocampus, and impaired performance in dorsal hippocampal-dependent tests of learning (Liu et al, 2000) in comparison with rats reared by high LG dams (high LG offspring). The effect is reversed with cross-fostering, revealing a direct influence of maternal care (Liu et al, 2000).
The hippocampus is heterogeneous along its longitudinal axis. Although the dorsal region is implicated in spatial learning (Moser et al, 1995), the ventral region is critical for fear-related signaling (Bannerman et al, 2002; Kjelstrup et al, 2002; Richmond et al, 1999), likely through circuit connections with the basolateral nucleus of the amygdala (Felix-Ortiz et al, 2013). Furthermore, the ventral hippocampus displays distinct properties of synaptic plasticity (Maggio and Segal, 2007) and intrinsic neuronal excitability (Dougherty et al, 2012), as compared with the dorsal hippocampus. Interestingly, low LG offspring are significantly more fearful than high LG offspring (Caldji et al, 1998; Weaver et al, 2006) and show enhanced fear-related learning and memory (Bagot et al, 2009; Champagne et al, 2008) and defensive behavior (Menard et al, 2004; Menard and Hakvoort, 2007). These findings suggest that rather than producing a global impairment in neural function, rearing by low LG mothers may specifically enhance neural processing for fearful stimuli through programming of ventral hippocampal hyperfunction as opposed to effects observed in the dorsal hippocampus.
We studied the relation between variations in maternal LG and dorsal and ventral hippocampal function in the CA1 region in adulthood. We found that variations in maternal LG in the rat have opposing effects on synaptic plasticity and intrinsic excitability in the dorsal and ventral hippocampus. Decreased pup licking in early postnatal life associated with enhanced LTP and intrinsic excitability in the ventral hippocampus of the offspring, while suppressing LTP in the dorsal hippocampus. Presynaptic function and basal synaptic input were unaffected. These findings suggest that variations in maternal care differentially program neural development and synaptic plasticity of the dorsal and ventral hippocampus.
Materials and Methods
Characterization of Maternal Behavior
Long–Evans rat dams (Charles River, St Constant, Quebec, Canada) were mated and singly housed with ad libitum food and water. Maternal behavior was scored during the first 6 postnatal days using behavioral observations (Champagne et al, 2003) to score the frequency of licking and grooming (LG). Dams for which the mean LG frequency lay 1 SD above or below the cohort mean were designated as high and low LG mothers, respectively. Weekly cage changing began only on postnatal day 7 and animals were weaned at postnatal day 21. Male offspring of high and low LG dams were pair-housed with littermates (ie of the same LG phenotype) after weaning and maintained with minimal handling until killed for the experiments in adulthood (approximately on postnatal day 120). Food and water were available ad libitum. All procedures conformed to the guidelines of the Canadian Council on Animal Care with protocols approved by the Facility Animal Care Committee at Douglas Mental Health University Institute.
Reagents
All reagents were obtained from Sigma-Aldrich (St Louis, Missouri, USA) unless otherwise specified.
Slice Preparation
Animals were anesthetized immediately following removal from the home cage by inhalation of isoflurane and rapidly decapitated. Brains were removed and cut into slices using a vibratome (Leica, Concord, Ontario, Canada) in slicing solution containing (in mM): 252 sucrose, 2.5 KCl, 4 MgCl2, 0.1 CaCl2, 1.25 KH2PO4, 26 NaHCO3, and 10 glucose (∼360 mOsmol/l). Slices of the dorsal hippocampus were prepared by making coronal blocking cuts at the posterior end of the forebrain and approximately one-third of the total length of the forebrain from the most anterior point. Brains were hemisected and mounted on the anterior surface created by this blocking cut for slicing. Slices of the ventral hippocampus were obtained by hemisecting the brain, laying it on its anterior surface, and making a blocking cut at ∼30° from the horizontal ventral surface. The surface created by this blocking cut was used to mount the brain for slicing (Dougherty et al, 2012). Hippocampal slices were cut at a maximum of 2 mm from either pole. Slices were incubated at 32 °C for 1 h in carbogenated (95% oxygen, 5% CO2) artificial cerebrospinal fluid containing (in mM): 125 NaCl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 25 glucose (∼310 mOsmol/l). Slices were allowed to recover at room temperature for 1 h before being transferred to a recording chamber for the start of recordings.
Electrophysiology
Field excitatory postsynaptic potentials (fEPSPs) and population spikes were evoked at 0.05 Hz using bipolar tungsten-stimulating electrodes placed in the stratum radiatum, and responses were detected by aCSF-filled glass recording electrodes. Recording electrodes were either placed in the stratum radiatum for LTP experiments or the stratum pyramidale for fEPSP-to-spike coupling (E-S coupling) experiments. Field recordings were performed in the presence of 5 μM bicuculline methobromide (Tocris, Bristol, UK) and 5 μM picrotoxin (Tocris) to block γ-Aminobutyric acid type A (GABAA) receptor-mediated inhibitory postsynaptic transmission unless otherwise specified.
To record action potentials, whole-cell patch clamp recording of CA1 pyramidal cells was performed in current clamp mode with pipettes containing (in mM): 120 K-Gluconate, 17.5 KCl, 2 MgCl2, 0.5 ethylene glycol tetraacetic acid (EGTA), 10 HEPES, 4 Na2-ATP, and with the pH adjusted to 7.2 with KOH (∼290 mOsm). Neurons recorded in current clamp mode were injected with 1000 ms long depolarizing current pulses in increasing steps of 10 pA to evoke action potentials. Action-potential properties were determined from the first spike elicited. The liquid junction potential was +13.3 mV.
To record α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated miniature excitatory postsynaptic currents (mEPSCs), whole-cell patch clamp recording of CA1 pyramidal neurons was performed in voltage clamp mode with pipettes containing (in mM): 110 Cs-gluconate, 17.5 CsCl, 10 HEPES, 2 MgCl2, 0.5 EGTA, 4 Na2-ATP, 5 QX-314, and 0.5% Biocytin (Tocris) with the pH adjusted to 7.2 with CsOH (∼290 mOsm). Neurons were held at −60 mV, and recordings of mEPSCs were performed in the presence of tetrodotoxin (0.5 μM; Alomone Labs, Jerusalem, Israel) to block action-potential formation, and 5 μM bicuculline (Tocris) to block GABAA receptor-mediated inhibitory currents.
No electronic compensation for series resistance was used. Access resistance was continuously monitored during recordings, and recordings were rejected if access resistance exceeded 25 MΩ. Recordings were amplified by Multiclamp 700B (Axon), low-pass filtered at 1 kHz, and sampled at 10 kHz. Recordings were digitized by Digidata 1400 (Axon), and stored in a PC for later offline analysis. All data were analyzed using Clampfit (Molecular Devices, Sunnyvale, CA, USA), except mEPSC recordings that were analyzed using Minianalysis (Synaptosoft, Decatur, GA, USA). Recordings were made at room temperature.
RNA Quantification
Brains were rapidly removed and hippocampi were dissected into one-thirds. The dorsal- and ventral-most one-third were snap frozen in isopentane and stored at −80 °C. RNA and DNA were separated simultaneously using an AllPrep DNA/RNA Mini kit (Qiagen, Venlo, The Netherlands). RNA was subsequently purified using on-column DNase digestion (Qiagen). RNA quality and yield were determined using a SmartSpec plus spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA). cDNA was then synthesized using reverse transcriptase avian myeloblastosis virus (Roche, Basel, Switzerland). Quantitative real-time PCR was performed using a LightCycler 480 (Roche) using SCN2A primers (forward primer 5′-ACCTGCACTGGAGACTGCTA-3′ and reverse primer 3′-CGTCCTTGCGTTCCTGTTTG-5′). β2 microglobulin (Qiagen) was amplified from the same samples and used as a reference gene for normalization.
Statistical Analysis
Statistical analyses were performed using SPSS 20.0. Graphs were plotted using Graphpad Prism 4. Data were analyzed using two-way ANOVA (with repeated measures when dependent variables were measured at multiple within-subject levels), and significant main effects and interactions interpreted using Bonferroni-corrected post hoc pairwise comparisons.
Results
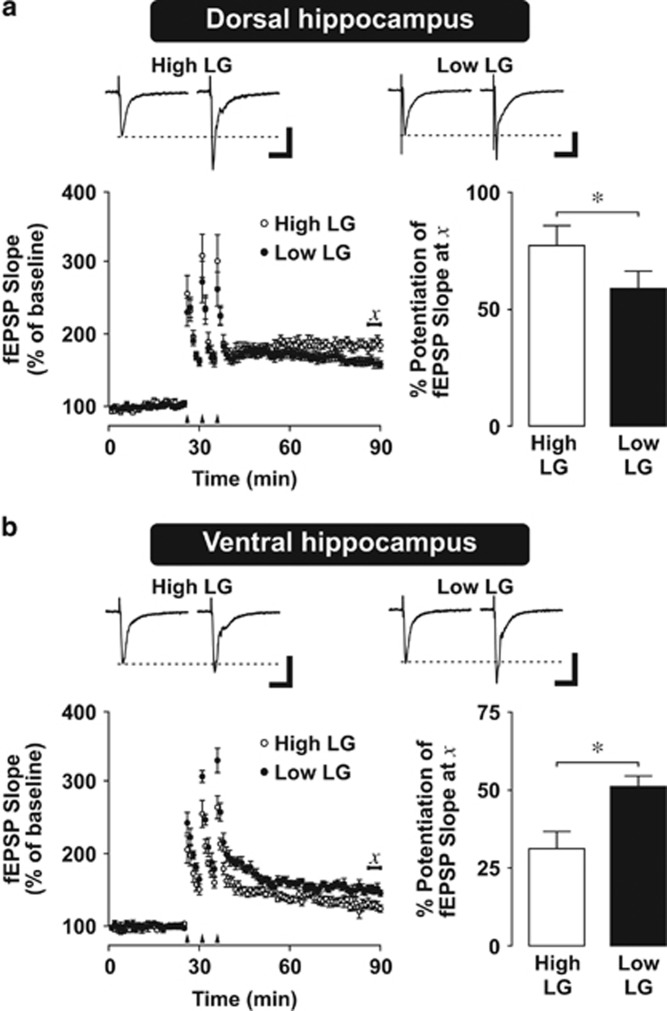
We first examined the effect of maternal care on the capacity for synaptic plasticity in the dorsal and ventral hippocampus of adult high and low LG offspring. We induced LTP of fEPSPs in area CA1 of the dorsal and ventral hippocampus by tetanic stimulation (3 trains of 100 pulses delivered at 100 Hz, separated by 5 min inter-train intervals). Analysis of fEPSP responses during the post-tetanic LTP period (t=55–60 min) relative to baseline (t=−5 to 0 min) revealed significantly reduced (P=0.001) LTP in slices of the dorsal hippocampus (Figure 1a) from low LG offspring compared with high LG offspring, as in previous studies (Bagot et al, 2009; Champagne et al, 2008). In contrast, ventral-LTP (Figure 1b) was significantly enhanced (P=0.03) in hippocampal slices from adult low LG offspring compared with high LG offspring, revealing a region-specific effect of maternal care (repeated measures ANOVA, (maternal care) x (hippocampal subregion) x (period) interaction, F(1,19)=13.751, P=0.001).
Figure 1.
Synaptic plasticity in the dorsal and ventral hippocampus of high and low LG offspring. (a,b) Long term potentiation (LTP) in dorsal (a) and ventral (b) hippocampal slices. Top: representative traces of field excitatory postsynaptic potentials (fEPSPs) before and 1 h after tetanus. Dashed line indicates baseline fEPSP amplitude. Scale bars represent 0.2 mV (vertical) and 15 ms (horizontal). Bottom left: scatter plot of mean±SEM evoked fEPSP before and 1 hour after LTP induction. Arrows indicate tetanic stimulation (3 × 100 Hz, 100 pulses). Bottom right: mean±SEM normalized fEPSP slope 1 h after tetanus. Compared with high LG offspring, low LG offspring displayed an attenuated fEPSP slope in the dorsal hippocampus (a), but a larger fEPSP slope in the ventral hippocampus (b), 1 h after tetanic stimulation. Statistical analysis was performed on the average of values lying under χ reflecting LTP at 60 min following tetanic stimulation (n=5–6 slices from 4–6 rats per group). *P<0.05.
As LTP impairment in the dorsal hippocampus of low LG animals could be driven by increased expression and function of N-methyl-D-aspartate (NMDA) receptors in this region (Bagot et al, 2012a), we investigated the possibility that regulation of NMDA receptor expression might also associate with maternal regulation of LTP in the ventral hippocampus. Western blot analysis confirmed that expression of the GluN1 NMDA receptor subunit is increased in the dorsal hippocampus of low LG animals (P=0.03). In contrast, GluN1 expression in the ventral hippocampus was unaffected by maternal care (P=0.33), suggesting that alternative mechanisms drive differences in ventral hippocampal synaptic plasticity (Supplementary Figure S1, two-way ANOVA, (maternal care) x (hippocampal subregion) interaction, F(1,16)=5.691, P=0.03).
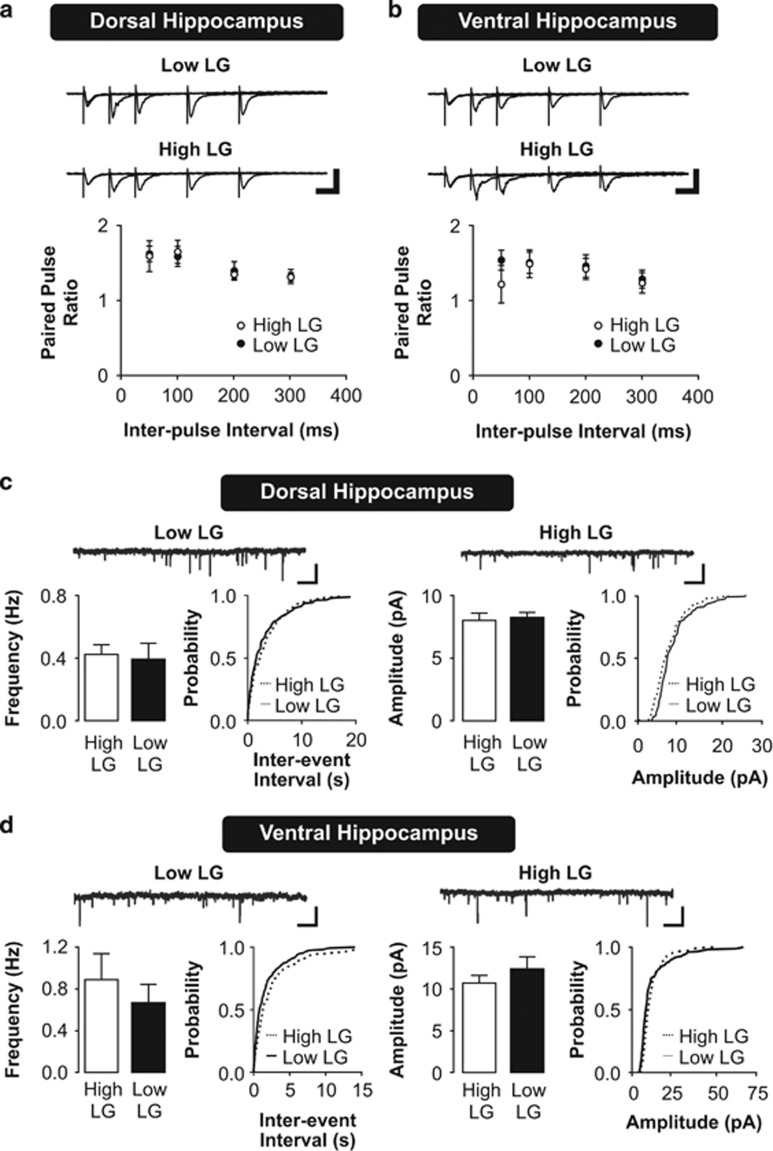
The observed effect of maternal care on synaptic plasticity in the dorsal and ventral hippocampus could be supported by changes in synaptic physiology. Increased presynaptic glutamate release could lead to greater activation of upstream regulators of synaptic plasticity, such as the NMDA-type and metabotropic postsynaptic glutamate receptors. To examine presynaptic glutamate release, we measured the facilitation of fEPSPs in response to paired stimulation pulses (paired-pulse facilitation (PPF)). We found no differences in PPF in slices from either the dorsal (Figure 2a; dorsal hippocampus PPF, repeated measures ANOVA, effect of maternal care, F(1,39)=0.002, P=0.968) or the ventral hippocampus from adult high and low LG offspring across a range of inter-stimulus intervals (Figure 2b; ventral hippocampus PPF, repeated measures ANOVA, effect of maternal care, F(1,36)=0.33, P=0.578). These findings suggest that variations in maternal care do not associate with changes in presynaptic glutamate release in CA1.
Figure 2.
Presynaptic function and synaptic input in dorsal and ventral hippocampal neurons of high and low LG offspring. (a,b) Paired-pulse facilitation (PPF) of excitatory synaptic responses in the dorsal (a) and ventral (b) hippocampus. Top: representative traces of facilitation of evoked fEPSPs in response to paired pulses delivered at a range of inter-stimulus intervals, average of five traces shown for each inter-stimulus interval. Scale bars represent 0.5 mV (vertical) and 40 ms (horizontal). Bottom: paired-pulse ratios are plotted as mean±SEM of fEPSP2/fEPSP1 (n=7–8 slices from 4 rats per group). (c, d) Miniature excitatory postsynaptic currents (mEPSCs) recorded from CA1 pyramidal neurons in the dorsal (c) or ventral (d) hippocampus. Top: representative traces of mEPSC recordings. Scale bars represent 15 pA (vertical) and 5 s (horizontal). Bottom: histograms depicting mean±SEM frequency and amplitude of mEPSCs (left) and their corresponding cumulative probability plots (right; n=6–8 slices from 3–5 rats per group).
Alternatively, increases in basal synaptic input may drive the observed differences in LTP. We used whole-cell patch clamp recordings of AMPA receptor-mediated mEPSCs to characterize the physiological properties of excitatory synaptic inputs on CA1 pyramidal neurons in the dorsal and ventral hippocampus. We compared the frequency and amplitude of mEPSCs recorded from dorsal (Figure 2c) and ventral (Figure 2d) hippocampal slices of high and low LG offspring. The frequency of mEPSCs is thought to reflect presynaptic vesicular release probability and the number of presynaptic active zones synapsing onto the recorded neuron, whereas mEPSC amplitudes are thought to depend on the synaptic AMPA receptor content and vesicle size (Tang et al, 1994). No significant effect of maternal care on mEPSC frequency (two-way ANOVA, F(1,20)=0.474, P=0.499) or amplitude (two-way ANOVA, F(1,20)=1.042, P=0.32) was detected. Thus, we conclude that the observed changes in synaptic plasticity resulting from early life maternal care are not related to changes in basal AMPA receptor-mediated excitatory synaptic transmission.
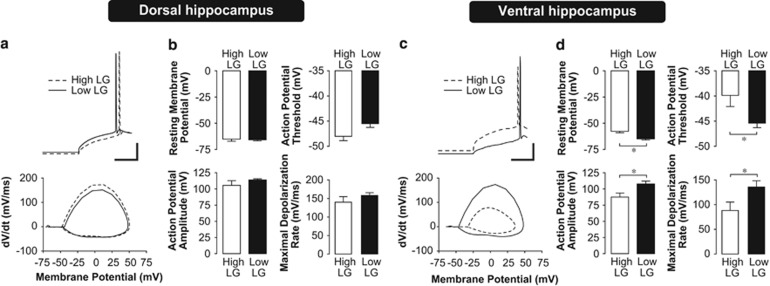
Changes in synaptic plasticity could also be shaped by intrinsic neuronal excitability. Thus, we characterized properties of action-potential firing in dorsal and ventral hippocampal CA1 pyramidal neurons using whole-cell patch clamp recording. We found a significantly lower action-potential threshold (P=0.003) in the ventral hippocampus of low LG offspring compared with high LG offspring (Figure 3c and d, Supplementary Table S1; two-way ANOVA, (maternal care) x (hippocampal subregion) interaction, F(1,30)=9.134, P=0.006). Furthermore, action potentials recorded in the ventral hippocampus of low LG offspring were larger in amplitude (P=0.009) and faster in maximal rise rate (P=0.02) compared with those recorded from high LG offspring (two-way ANOVA, main effect of maternal care, AP amplitude, F(1,30)=8.218, P=0.008; maximal rise rate, F(1,30)=7.433, P=0.012). There was a significant hyperpolarization of the resting membrane potential in the ventral hippocampus of low LG offspring (P=0.001), suggesting that the enhancement of excitability is specific to action-potential properties, and does not associate with a more depolarized basal resting membrane potential ((maternal care) x (hippocampal subregion) interaction, F(1,30)=4.479, P=0.045). In contrast, we found that maternal care had no effect on these measures in the dorsal hippocampus, suggesting that these effects are specific to the ventral hippocampus.
Figure 3.
Action-potential properties in dorsal and ventral hippocampal neurons of high and low LG offspring. (a,c) Representative traces and corresponding phase plane plots (Vm vs dV/dt) of action potentials evoked by current step injection in a CA1 pyramidal neuron in the dorsal (a) or ventral (c) hippocampus. Phase plane plots illustrate the lower action-potential threshold, increased maximal depolarization rate, and larger action-potential amplitude in the ventral hippocampus of low LG offspring, compared with high LG offspring (c). Conversely, note that action-potential threshold is not significantly different in dorsal hippocampus of high and low LG offspring (a). Scale bars represent 20 mV (vertical) and 80 ms (horizontal). (b,d) Mean±SEM resting membrane potential, action-potential threshold, amplitude, and rise rate for pyramidal cells from the dorsal (b) or ventral (d) hippocampal slices (n=7–10 cells from 4–5 rats per group). *P<0.05.
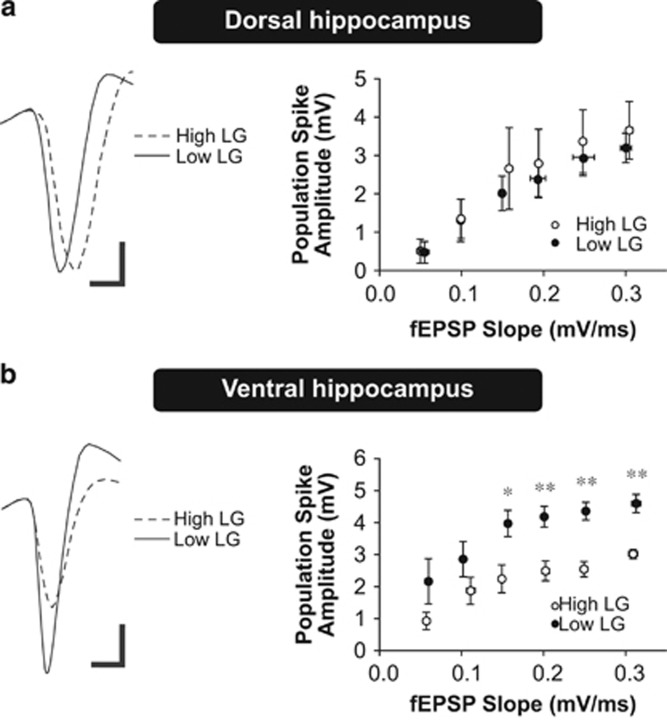
Intrinsic excitability (Figure 3) is significantly enhanced at the single-cell level in adult low LG offspring in the absence of changes in synaptic input (Figure 2), which could support changes in synaptic plasticity by enhancing the integrative function of neurons to transform synaptic input into spike output. Thus, we predicted that synaptic input of equivalent magnitude would elicit larger action-potential outputs in the ventral CA1 of low LG offspring. We assessed the input–output function by measuring the coupling relationship between evoked fEPSPs and population spike amplitudes (E-S coupling) in the CA1 region of the dorsal and ventral hippocampus in high and low LG offspring. The fEPSP reflects the depolarizing synaptic input, whereas the population spike represents the action-potential output triggered by the fEPSP. We found no significant difference in E-S coupling in the dorsal hippocampus (Figure 4a; repeated measures ANOVA, effect of maternal care, F(1,35)=0.16, P=0.70) between high and low LG offspring. In contrast and consistent with the changes observed in intrinsic excitability, we found a significant enhancement in E-S coupling in the ventral hippocampus in low compared with high LG offspring (Figure 4b; effect of maternal care, F(1,40)=9.738, P=0.014).
Figure 4.
fEPSP-to-spike (E-S) coupling in the dorsal and ventral hippocampus of high and low LG offspring. (a,b) E-S coupling in dorsal (a) or ventral (b) hippocampal slices. Left: representative traces of positive-going evoked fEPSPs and corresponding negative-going population spikes recorded from slices of high and low LG offspring. Compared with high LG offspring, larger population spikes were elicited by equivalently-sized evoked fEPSPs in the ventral hippocampus of low LG offspring (b). Scale bars represent 1 mV (vertical) and 2 ms (horizontal). Right: scatter plots depict the coupling relationship between input (fEPSP slope) and output (population spike amplitude; n=4–5 slices from 3 rats per group). *P<0.05, **P<0.01.
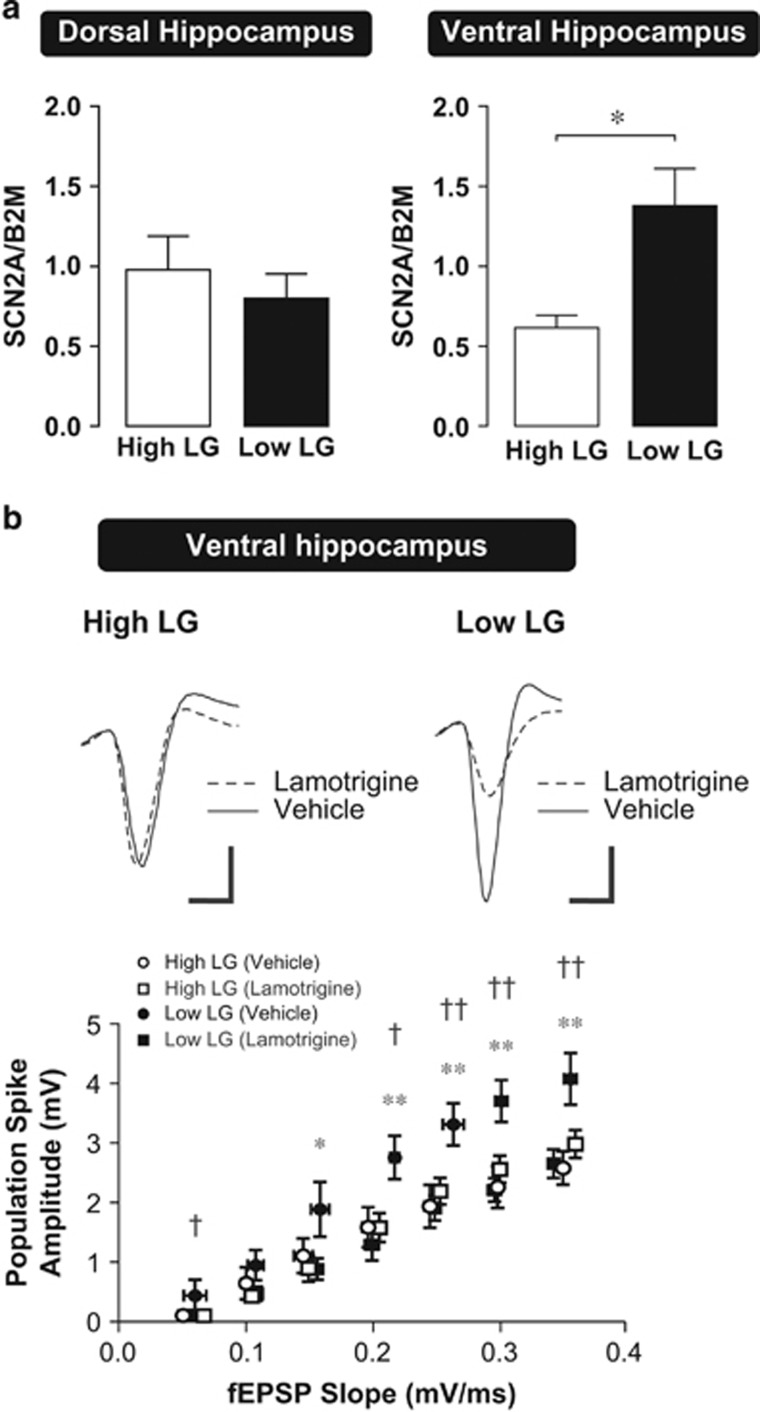
Increased excitability of action-potential firing in the ventral hippocampus of low LG compared with high LG offspring suggests the involvement of the voltage-gated sodium current, which drives the action-potential rising phase. Changes in sodium current conductance may result from changes in the level of expression of voltage-gated sodium channels. Using quantitative real-time polymerase chain reaction (qRT-PCR), we found that expression of the SCN2A mRNA transcript, which codes for the NaV1.2 voltage-gated sodium-channel subunit, was significantly (P=0.006) upregulated in the ventral hippocampus of low LG compared with high LG offspring. In contrast, there was no significant difference in SCN2A expression in the dorsal hippocampus, consistent with a ventral hippocampus-specific effect of maternal care on intrinsic excitability (Figure 5a; two-way ANOVA, (maternal care) x (hippocampal subregion) interaction, F(1,29)=6.457, P=0.017).
Figure 5.
Voltage-gated sodium-channel expression and pharmacological inhibition in high and low LG offspring. (a) SCN2A mRNA expression determined using qRT-PCR and normalized to β2 microglobulin (B2M) from dorsal and ventral hippocampus of high (n=8 rats) and low (n=8–9 rats) LG offspring. Expression levels of SCN2A were significantly increased in the ventral hippocampus of low LG offspring (right), but was not significantly different in the dorsal hippocampus (left). Bars represent mean+SEM. *P<0.05. (b) fEPSP-to-spike (E-S) coupling in the ventral hippocampus of high and low LG offspring in the presence of lamotrigine or aCSF vehicle. Recordings were performed in the absence of GABAA receptor antagonists. Top: representative traces of positive-going evoked fEPSPs and corresponding negative-going population spikes recorded from slices of high and low LG offspring. Increased E-S coupling in slices from low LG compared with high LG offspring is maintained in the absence of GABAA receptor antagonists. Bath application of lamotrigine reduced the population spike amplitude elicited by equivalently-sized evoked fEPSPs in the ventral hippocampus of low LG offspring, but not in high LG offspring. Scale bars represent 1 mV (vertical) and 2 ms (horizontal). Bottom: scatter plots depict the coupling relationship between input (fEPSP slope) and output (population spike amplitude; n=5–10 slices from 4–6 rats per group). *denotes differences between vehicle and lamotrigine-treated slices from low LG offspring. †denotes differences between vehicle-treated slices from high and low LG offspring. *P<0.05, **P<0.01, †P<0.05, ††P<0.01.
GABAergic modulation can affect the E-S coupling relationship (Chavez-Noriega et al, 1989). To rule out the possibility that ventral hippocampal E-S coupling differences (Figure 4) result from differential sensitivity to GABAergic blockade, we measured the ventral hippocampal E-S coupling relationship in high and low LG offspring in the absence of GABAA receptor antagonists. Under these conditions, ventral hippocampal E-S coupling in low LG offspring remains significantly higher compared with high LG offspring, further supporting the role of intrinsic excitability, rather than inhibition, in driving differences in coupling. We then adopted a pharmacological approach to investigate whether differences in ventral hippocampal E-S coupling could be eliminated by inhibiting voltage-gated sodium channel function. Bath application of the sodium-channel inhibitor lamotrigine (50 μM) (Tocris) significantly reduced E-S coupling in ventral hippocampal slices from low LG offspring, but did not have a significant effect in ventral hippocampal slices from high LG offspring (Figure 5b; repeated-measures ANOVA, (maternal care) x (drug) interaction, F(1,26)=6.664, P=0.016).
Discussion
We present evidence for a differential association between early life maternal care and the capacity for synaptic plasticity, as reflected in the expression of LTP, in the dorsal and ventral hippocampus in adulthood. We previously demonstrated that dorsal hippocampal LTP is reduced in adult low LG offspring (Bagot et al, 2009, 2012a; Champagne et al, 2008) and associates with inferior performance on tests of dorsal hippocampal-related spatial- and object-recognition learning (Bredy et al, 2003; Liu et al, 2000) when compared with high LG offspring. Our present findings replicate the previously reported reduction in dorsal hippocampal LTP and present striking evidence that ventral hippocampal LTP is simultaneously increased in adult low LG offspring. Such activity-dependent LTP of synaptic responses is a candidate cellular mechanism of learning (Bliss and Collingridge, 1993; Squire and Alvarez, 1995). In the dorsal hippocampus, LTP formation supports the consolidation of memory traces into downstream cortical projection sites linked to locomotor control and spatial cognition (Swanson and Cowan, 1977; Whitlock et al, 2006). Conversely, the ventral hippocampus forms reciprocal connections with amygdalar nuclei and downstream projections to hypothalamic nuclei, forming neural networks that process fear and threat-response signals (Cenquizca and Swanson, 2007; Fanselow and Dong, 2010). Therefore, LTP formation in the ventral hippocampus likely supports experience-dependent encoding and consolidation of fearful and emotional memories in these stress-related networks (Maggio and Segal, 2012). Indeed, synaptic plasticity in the ventral hippocampus is increased by glucocorticoids and stress (Maggio and Segal, 2007, 2011), in contrast to the dorsal hippocampus. Thus, the observed increase in ventral hippocampal LTP suggests that hippocampal information encoding in low LG offspring is biased towards ventral hippocampal networks.
To further explore potential neurophysiological mechanisms supporting differences in LTP formation, we studied synaptic function at the level of presynaptic glutamate release, synaptic input, and intrinsic excitability of postsynaptic neurons. Strikingly, we found a significant enhancement of intrinsic neuronal excitability in a direction that is consistent with differences in LTP formation in the ventral hippocampus. Action potentials fired from ventral hippocampal CA1 neurons were significantly reduced in firing threshold, and increased in amplitude and maximal depolarization rate in the low LG offspring. The observed hyperpolarization of the resting membrane potential in ventral hippocampal CA1 neurons of the low LG offspring suggests that increased excitability is specific to action-potential properties rather than subthreshold depolarization. Action-potential characteristics are largely shaped by ion channels such as voltage-gated sodium and calcium channels. In particular, the observed increase in peak depolarization rate (Figure 3c and d) suggests a contribution of voltage-gated sodium-channel function. As the major voltage-gated sodium-channel subtype expressed in the hippocampus is NaV1.2 (Westenbroek et al, 1989), we measured the expression of the NaV1.2-coding mRNA transcript, SCN2A, and found its expression to be significantly upregulated in the ventral hippocampus of low LG offspring. Furthermore, bath application of lamotrigine at a concentration effective on voltage-gated sodium channels (Xie et al, 1995) attenuated E-S coupling in ventral hippocampal slices from low LG offspring to levels similar to that found in high LG offspring. Lamotrigine has been shown to reduce intrinsic excitability in hippocampal CA1 pyramidal neurons (Englund et al, 2011). Taken together, these data suggest that enhancement of intrinsic excitability in the ventral hippocampus of low LG offspring is mediated at least in part through changes in sodium current. Increased sodium-channel function could increase depolarization in postsynaptic membranes, driving calcium conductance through NMDA receptors (Collingridge et al, 1983; Yu and Salter, 1998) or voltage-gated calcium channels (Miyakawa et al, 1992). This calcium influx into postsynaptic sites is critical for the initiation of signaling cascades that lead to the formation of synaptic plasticity (Lynch et al, 1983). Indeed, voltage-gated calcium channels have been implicated as a regulatory target in modulation of LTP in the ventral hippocampus (Maggio and Segal, 2007). Alternatively, more efficacious spike generation could modulate synaptic calcium influx and synaptic plasticity formation through the facilitation of backpropagating action potentials (Magee and Johnston, 1997). However, the stringent temporal constraints of such spike timing-dependent plasticity make it unlikely to fully account for differences in the tetanically-induced LTP examined in the present studies.
A reduction in action-potential firing threshold predicts an increased likelihood of action-potential firing in response to synaptic depolarization, which is confirmed by the enhancement of E-S coupling observed in this region. Although GABAergic signaling can modulate E-S coupling (Chavez-Noriega et al, 1989), differences in E-S coupling were maintained when inhibitory influences were pharmacologically excluded. Thus, this difference in E-S coupling is likely shaped by increased intrinsic excitability resulting in increased sensitivity to synaptic input. This increase in neuronal excitability and input–output efficacy occurs in the absence of changes in basal excitatory synaptic input, as indicated by mEPSC recordings. This is consistent with previous studies reporting no change in field recordings of the synaptic AMPA receptor-mediated input–output function in the dorsal hippocampus (Bagot et al, 2012a). Our results extend these findings by showing no changes in spontaneous excitatory synaptic activities. We also observed no difference in PPF, a form of short-term plasticity that reflects changes in presynaptic glutamate release. Taken together, our findings suggest that enhancement of ventral hippocampal LTP by low LG rearing is related to an increase in the intrinsic excitability and E-S coupling properties of postsynaptic CA1 neurons, rather than changes in presynaptic glutamate release or synaptic input.
The excitability of ventral hippocampal neurons has been linked to anxious behaviors (Kheirbek et al, 2013). An increase in excitability, input–output transformation and LTP in the ventral hippocampus observed in adult low LG offspring could lead to an increase in sensitivity to incoming afferent signals originating from fear-signaling regions. Notably, activation of afferent glutamatergic synapses from the basolateral nucleus of the amygdala onto ventral CA1 neurons enhances anxious behavior (Felix-Ortiz et al, 2013). Increased activity in these circuits is consistent with previous findings wherein low LG offspring show enhanced fearful behavior (Bredy et al, 2004; Caldji et al, 1998), increased fear-related learning (Champagne et al, 2008), and aggression (Menard et al, 2004; Menard and Hakvoort, 2007). Thus, differential regulation of dorsal and ventral hippocampal function by maternal care might underlie the distinct cognitive–emotional phenotypes observed in the adult offspring.
Increased activity in the ventral hippocampus associates with anxiety-like behaviors (Felix-Ortiz et al, 2013; Kheirbek et al, 2013) and an increased capacity for synaptic plasticity in ventral hippocampus could predispose individuals towards anxious and depressive vulnerability through aberrant over-representation of fear signals in memory traces. Such effects might involve connections of the ventral hippocampus to the amygdala or to the prefrontal cortex (Adhikari et al, 2010). Indeed, selective lesions of the ventral hippocampus reveal that the ventral, but not the dorsal hippocampus is implicated in the expression of anxiety-related behavior (Bannerman et al, 2002; Kjelstrup et al, 2002). As the low LG maternal phenotype is associated with stressful environmental conditions (ie, chronic stress decreases the frequency of pup LG (Champagne and Meaney, 2006)), our findings suggest that this form of maternal care derive from conditions of adversity and subsequently contribute to increased fearfulness and enhanced fear-related learning and memory in the offspring. It is important to note that increased fearfulness, which typifies the male and female offspring of low LG mothers, may be adaptive under conditions of adversity. Increased pituitary-adrenal stress reactivity in rats associates with a greater capacity for counter-regulation of pro-inflammatory responses to bacterial infection and may be protective against sepsis (Shanks et al, 2000). Increased fearfulness is also protective against predation (Dingemanse et al, 2004; O'Steen et al, 2002). Indeed, there is evidence for context-specific adaptive value of increased fearfulness in humans: behavioral inhibition predicts successful outcomes amongst young males living in impoverished, high-crime environments (Farrington et al, 1988; Haapasalo and Tremblay, 1994). We suggest that a decreased level of maternal licking/grooming may serve to alter neural development in a manner that is subsequently adaptive for animals contending with an increased level of environmental adversity. This idea is consistent with previous studies in both rodents and humans suggesting accelerated development of amygdala function and connectivity in response to severe early life adversity (Gee et al, 2013; Landers and Sullivan, 2012). Early maturation of amygdala-dependent fear systems might similarly be adaptive when development occurs under conditions of persistent adversity.
In summary, we show that maternal care differentially programs the capacity for synaptic plasticity and intrinsic neuronal excitability in the dorsal and ventral hippocampus in the rat. The present data indicate that, rather than globally impairing neural function (Bagot et al, 2012a; Champagne et al, 2008; Liu et al, 2000), rearing by low LG mothers exerts a region-specific effect to enhance ventral hippocampal function while reducing dorsal function. These studies support the role of low LG rearing in programming hippocampal function in a region-specific manner to promote aversive and stress-related signaling. Although poor quality parent–offspring interactions predict vulnerability for anxiety and depression in humans and are commonly thought to compromise neural development and impair learning through a reduced capacity for neural plasticity, our findings suggest instead that reduced maternal care shapes neural development with a region-specific increase in neural plasticity in the ventral hippocampus, which may underlie increased fearfulness. Such developmental outcomes may be potentially adaptive in a context-specific manner, but would nevertheless associate with the potential costs of an increased risk for later adverse mental health outcomes.
FUNDING AND DISCLOSURE
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (38868-2013), the Canadian Institutes for Health Research (8632) and the Hope for Depression Research Foundation (48465) to MJM. MJM is a Fellow of the Bank of Montréal Research of the Canadian Institute for Advanced Research. RCB was supported by a graduate fellowship from the Gouvernement du Québec (116071). The authors declare no conflict of interest.
Acknowledgments
We thank Alice S. Wong for assistance with immunoblotting and Dr. Yiu Chung Tse for assistance with electrophysiology.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adhikari A, Topiwala MA, Gordon JA (2010). Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65: 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Tse YC, Nguyen HB, Wong AS, Meaney MJ, Wong TP (2012. a). Maternal care influences hippocampal N-methyl-D-aspartate receptor function and dynamic regulation by corticosterone in adulthood. Biol Psychiatry 75: 491–498. [DOI] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M (2009). Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem 92: 292–300. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Zhang TY, Wen X, Nguyen TT, Nguyen HB, Diorio J et al (2012. b). Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proc Natl Acad Sci USA 109(Suppl 2): 17200–17207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN (2002). Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci 116: 884–901. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB et al (2008). Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299: 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ (2003). Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience 118: 571–576. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Zhang TY, Grant RJ, Diorio J, Meaney MJ (2004). Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. Eur J Neurosci 20: 1355–1362. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95: 5335–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW (2007). Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev 56: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER et al (2008). Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci 28: 6037–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ (2003). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 79: 359–371. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ (2006). Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry 59: 1227–1235. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Bliss TV, Halliwell JV (1989). The EPSP-spike (E-S) component of long-term potentiation in the rat hippocampal slice is modulated by GABAergic but not cholinergic mechanisms. Neurosci Lett 104: 58–64. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H (1983). Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol 334: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B (2007). Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abuse Negl 31: 211–229. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Both C, Drent PJ, Tinbergen JM (2004). Fitness consequences of avian personalities in a fluctuating environment. Proc Biol Sci 271: 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KA, Islam T, Johnston D (2012). Intrinsic excitability of CA1 pyramidal neurones from the rat dorsal and ventral hippocampus. J Physiol 590(Pt 22): 5707–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund M, Hyllienmark L, Brismar T (2011). Effect of valproate, lamotrigine and levetiracetam on excitability and firing properties of CA1 neurons in rat brain slices. Cell Mol Neurobiol 31: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington DP, Gallagher B, Morley L St, Ledger RJ, West DJ (1988). Are there any successful men from criminogenic backgrounds? Psychiatry 51: 116–130. [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79: 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH et al (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 110: 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo J, Tremblay RE (1994). Physically aggressive boys from ages 6 to 12: family background, parenting behavior, and prediction of delinquency. J Consult Clin Psychol 62: 1044–1052. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE et al (2013). Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77: 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB (2002). Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA 99: 10825–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM (2012). The development and neurobiology of infant attachment and fear. Dev Neurosci 34: 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ (2000). Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci 3: 799–806. [DOI] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F (1983). Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305: 719–721. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D (1997). A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275: 209–213. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M (2007). Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus. J Neurosci 27: 5757–5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio N, Segal M (2011). Persistent changes in ability to express long-term potentiation/depression in the rat hippocampus after juvenile/adult stress. Biol Psychiatry 69: 748–753. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M (2012). Steroid modulation of hippocampal plasticity: switching between cognitive and emotional memories. Front Cell Neurosci 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard JL, Champagne DL, Meaney MJ (2004). Variations of maternal care differentially influence 'fear' reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience 129: 297–308. [DOI] [PubMed] [Google Scholar]
- Menard JL, Hakvoort RM (2007). Variations of maternal care alter offspring levels of behavioural defensiveness in adulthood: evidence for a threshold model. Behav Brain Res 176: 302–313. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Ross WN, Jaffe D, Callaway JC, Lasser-Ross N, Lisman JE et al (1992). Synaptically activated increases in Ca2+ concentration in hippocampal CA1 pyramidal cells are primarily due to voltage-gated Ca2+ channels. Neuron 9: 1163–1173. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG (1995). Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA 92: 9697–9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Steen S, Cullum AJ, Bennett AF (2002). Rapid evolution of escape ability in Trinidadian guppies (Poecilia reticulata). Evolution 56: 776–784. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J et al (1999). Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci 113: 1189–1203. [DOI] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD et al (2000). Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci USA 97: 5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Alvarez P (1995). Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol 5: 169–177. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM (1977). An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172: 49–84. [DOI] [PubMed] [Google Scholar]
- Tang CM, Margulis M, Shi QY, Fielding A (1994). Saturation of postsynaptic glutamate receptors after quantal release of transmitter. Neuron 13: 1385–1393. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M (2006). Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA 103: 3480–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Merrick DK, Catterall WA (1989). Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron 3: 695–704. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF (2006). Learning induces long-term potentiation in the hippocampus. Science 313: 1093–1097. [DOI] [PubMed] [Google Scholar]
- Xie X, Lancaster B, Peakman T, Garthwaite J (1995). Interaction of the antiepileptic drug lamotrigine with recombinant rat brain type IIA Na+ channels and with native Na+ channels in rat hippocampal neurones. Pflugers Arch 430: 437–446. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Curtis GC, Nesse RM (1997). Childhood adversity and vulnerability to mood and anxiety disorders. Depress Anxiety 5: 66–72. [PubMed] [Google Scholar]
- Yu XM, Salter MW (1998). Gain control of NMDA-receptor currents by intracellular sodium. Nature 396: 469–474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.