Abstract
Drug-associated cues elicit conditioned responses in human drug users, and are thought to facilitate a drug-seeking behavior. Yet, little is known about how these associations are acquired, or about the specificity of the conditioned response modalities. In this study, healthy, nondependent volunteers (N=90) completed a conditioning paradigm in which they received a moderate dose of methamphetamine paired with one stimulus and placebo with another stimulus, each on two separate occasions. Their responses to these cues were measured with a behavioral preference, self-reported ‘liking', emotional reactivity, and attentional bias measures, both before and after the conditioning. Following the conditioning procedure, subjects exhibited a behavioral preference, positive emotional reactivity, and attentional bias toward the methamphetamine-associated cue, compared with the placebo stimulus. In addition, subjects who reported greater positive subjective drug effects during the conditioning displayed a more robust conditioning. This work demonstrates that healthy nondependent volunteers readily acquire conditioned responses to neutral stimuli paired with a drug. The procedure has significant value to study individual variation in acquisition of conditioned responses as a possible risk factor for drug taking, and to study the neural basis of conditioned drug responses.
INTRODUCTION
Drug users appear to form strong associations between drugs and the environmental stimuli (cues) present during the drug-taking experience. These conditioned associations are central to many theories of addiction (Robinson and Berridge, 2008; Kalivas and Volkow, 2005; Koob and Le Moal, 2008) and appear to contribute to the acquisition, maintenance, and relapse to problematic drug use. Cues elicit craving in drug users, and facilitate drug seeking and consumption even after long periods of drug abstinence (Ehrman et al, 1992; O'Brien et al, 1992; Childress et al, 1999). Studies with laboratory animals have investigated both the acquisition and expression of drug conditioning, but in humans, most studies of drug cues investigate only the expression of drug-related responses, typically in currently or formerly dependent drug users. To date, only a few studies have examined the process of acquisition of conditioning with drugs in humans (eg, Winkler et al, 2011; Childs and de Wit, 2009; 2011). To address this gap, we have developed a novel human drug conditioning paradigm and demonstrated that healthy volunteers acquire a behavioral preference for a stimulus paired with a single dose of a known drug of abuse (ie, methamphetamine (MA); Mayo et al, 2013). In the prior study we examined only a single behavioral measure of cue preference, but in the present study we added measures of subjective, attentional and emotional components of the conditioned response to the MA-associated cue. Thus we sought to replicate our previous findings in a new sample of subjects, and extend the finding to investigate qualitatively different manifestations of the conditioned drug response.
Several outcome measures have been used to study responses to drug cues in established drug users. One indicator of conditioning in humans is attentional bias (for review, see Field and Cox 2008), which is based on the idea that drug cues acquire the ability to usurp attention, resulting in a change in verbal reaction time, behavioral reaction time, or direction of eye movement. In dependent users, high attentional bias is associated with propensity to relapse during abstinence (Waters et al, 2003), suggesting that it may to be an indicator of motivation to use drugs. A second measure of conditioned response to drug cues in humans is emotional reactivity, measured by detecting subtle facial movements with facial electromyography (EMG; Lang et al, 1993). Positive emotional stimuli activate the zygomatic muscle, the muscle activated in a smile, and negative emotional stimuli activate the corrugator muscle, which is activated in a frown. The corrugator muscle also relaxes in response to positive stimuli. In dependent drug users, drug cues increase reactivity of the zygomatic muscle reactivity and decrease corrugator muscle reactivity (Drobes and Tiffany, 1997; Geier et al, 2000), suggesting that the cues elicit positive emotional responses. Notably, presentation of drug-related cues also evokes self-reported ‘craving' in established drug users (O'Brien et al, 1992; Perkins et al, 1994). However, reports of craving appear to emerge only after prolonged use of a drug, and thus may not emerge during the early phases of conditioning. Moreover, the concept of craving is complex (eg, Tiffany and Carter, 1998; Rohsenow and Monti 1999; Franken, 2003); it is not easily assessed in animal models, and indeed may not be a simple consequence of Pavlovian conditioning (Skinner and Auben, 2010). Thus, in this investigation we focused on measures of preference for the stimuli, attention to the stimuli, and emotional responses to the stimuli.
Most studies of cue responses in drug users rely on presentation of generic drug-related cues, consisting of images related to the users' drug of choice (ie, pictures of a beer bottle, cigarette, or drug paraphernalia) selected by the researcher. However, it is unclear if the responses evoked by these stimuli are truly the result of classical conditioning mechanisms, or whether they result from other learning or memory processes (eg, implicit or explicit memories or associations, or discriminative stimuli signaling drug availability (Schuster and Johanson, 1988)). To study the process of Pavlovian acquisition of these responses, we previously developed a novel drug conditioning paradigm (Mayo et al, 2013) to study the acquisition of de novo drug cues in healthy adults. The initial study showed that participants developed a behavioral preference for a cue paired with MA, compared with placebo (PBO) administration. In the present study, we sought first to replicate our original findings, and then to extend our measures of conditioning to include measures of attentional bias and emotional reactivity in a new group of healthy adults. This approach advances our understanding of the processes by which cues become associated with drug use and possibly lead to relapse. Using this approach, we can determine how contextual cues paired with drug administration become ‘conditioned' drug cues, how the conditioned responses are expressed (ie, self-reported, attentional, as well as emotional responses to the drug-paired stimuli) and set the stage to study ways to alter these conditioned responses to drug cues.
MATERIALS AND METHODS
Overall Design
This study was designed to investigate how a stimulus paired with a drug can elicit conditioned responses in healthy volunteers. Participants underwent a conditioning procedure in which one audiovisual stimulus (cue) was paired with the effects of a stimulant drug (oral 20 mg MA), and a different stimulus was paired with a PBO. We assessed the change in subjective, behavioral, and psychophysiological responses to the stimuli from before to after the conditioning (Figure 1a).
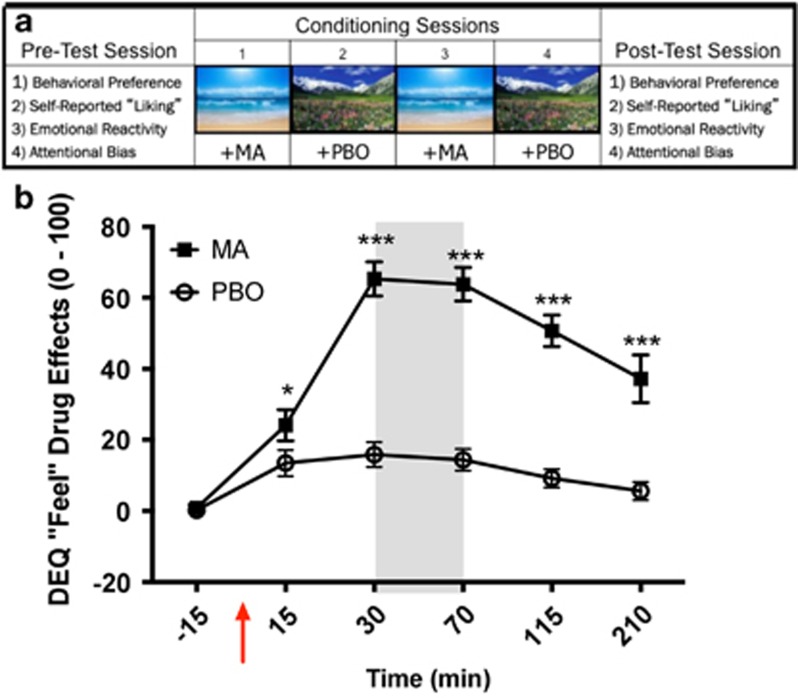
Figure 1.
Overall study design (1a; top) and illustration of methamphetamine (MA) and placebo (PBO) responses on a representative measure (1b: bottom). (a) Sequence of pre-test, four conditioning sessions and post conditioning test. Pictures show the two visual images that were paired with MA and PBO. (b) Time course of ratings of DEQ “feeling a drug effect” after MA and PBO administration during the conditioning sessions. Values are mean peak change scores from the baseline (±SEM) for the average of the two placebo sessions (circles) and two methamphetamine sessions (squares). The red arrow indicates administration of either placebo or methamphetamine. The gray shaded area indicates the 30-min task period, during which the cue is presented. *p<0.05, and ***p<0.001.
The study was also designed with a secondary goal to assess the potentially synergistic effect of extra monetary earnings during the conditioning sessions (none, low, high), but this manipulation was ineffective, so data from all subjects were combined and this distinction is not discussed further.
Participants
Healthy volunteers (N=90; Table 1) aged 21–35 were recruited from the community and screened with a psychiatric interview, electrocardiogram, and physical examination. Inclusion criteria were: BMI of 19–26 kg/m, high school education, fluency in English, resting blood pressure less than140/90 mm Hg, resting heart rate less than 80 bpm, and consumption of less than four standard alcohol or caffeinated drinks per day. Exclusion criteria were: current substance abuse or lifetime substance dependence, regular medication, history of cardiovascular illness, current major Axis I DSM-IV disorder (APA, 2004), mood disorder, or psychotic symptoms within the past year. Shift workers and pregnant or nursing mothers were also excluded. Women not on hormonal birth control were tested only during their follicular phase (White et al, 2002). The University of Chicago Biological Science Division Institutional Review Board approved the study.
Table 1. Participant Demographics and Current and Lifetime Drug Use (N=90).
| Percent (N) or mean±SEM | |
|---|---|
| Gender | |
| Female/Male | 50/40 |
| Race | |
| Caucasian | 59% (53) |
| African-American | 18% (16) |
| Asian | 13% (12) |
| Other | 10% (9) |
| Age (years) | 24.6 (3.1) |
| Education (years) | 15.0 (1.8) |
| BMI | 22.6 (1.2) |
| Current drug use | |
| Caffeinated beverages per day | 1.9 (0.4) |
| Cigarettes per week | 0.3 (1.9) |
| Alcohol beverages per week | 6.8 (0.6) |
| Lifetime drug use (ever used; nonmedical use only) | |
| Marijuana | 81% (73) |
| Opiates | 51% (46) |
| Stimulants | 32% (29) |
| Hallucinogens | 27% (24) |
| MDMA | 21% (19) |
| Sedatives | 10% (9) |
Drug
Methamphetamine (MA; 20 mg oral; Desoxyn, Lundbeck) was selected because of its relatively fast onset and reliable subjective effects (Martin et al, 1971; Cook et al, 1992). To speed the absorption, tablets were crushed and administered in 10-ml sugar-free syrup (Ora-Sweet and Ora-Plus, Paddock Laboratories, Minneapolis, MN). PBO consisted of a 10-ml sugar-free syrup alone.
Session Procedures
Orientation session
During an orientation session, qualifying participants were informed of the study procedures and provided informed consent. They then completed practice versions of the study tasks to be completed at subsequent sessions. Participants were told they could be given a placebo, stimulant, or sedative drug to minimize expectancy effects during potential drug administration sessions. They were instructed to abstain from drugs for 48 h (marijuana for 7 days), but to consume their normal amount of caffeine or nicotine before subsequent sessions. Compliance was assessed with breath-alcohol levels (Alco-SensorIII, Intoximeters, St Louis, MO), urine drug test (ToxCup, Branan Medical Corporation, Irvine, CA) and, for women, pregnancy test (AimStickPBD, hCG professional, Craig Medical Distribution, Vista, CA). Sessions took place in comfortably furnished rooms with a computer, television, and video player. When not completing study procedures, participants were allowed to relax, watch selected movies, or read. Participants were compensated for their participation.
Pretest session (Session 1)
At this 2 h session, participants completed tasks to assess baseline responses toward the to-be-conditioned cues, including behavioral preference, subjective ‘liking', emotional reactivity (acquired with facial EMG) and attentional bias (assessed via monitoring eye gaze by using electrooculography; EOG). The cues consisted of an ocean or mountain background image on a computer screen, accompanied by appropriate sounds (waves crashing or birds chirping). Cues were presented with E-Prime 2.0 (PST, Pittsburg, PA).
Conditioning Sessions (Sessions 2–5)
Four 4-h conditioning sessions were conducted from 0900–1300 h at least 48 h apart. The order of the conditioning measures was randomized across subjects, but consistent within the subjects. At each session, participants first completed compliance tests, pre-drug mood ratings, and physiological measures, and then ingested the drug (20 mg MA) or PBO in syrup (see below) in a mixed order under double blind conditions. Thirty minutes later, participants performed three simple computer tasks for 30 min (see Mayo et al, 2013), while the cues (ocean or mountain) were presented as background screens behind a smaller central panel presenting the tasks. One background screen (mountain or ocean) was always present during MA sessions, while the other (ocean or mountain; whichever was not paired with MA) was present during PBO sessions. Tasks were displayed by using Presentation software (Neurobehavioral Systems, Berkeley, CA). Sessions always alternated between MA and PBO. The order of session (MA or PBO first) and cues (MA-paired: ocean or mountain) were counterbalanced across subjects. The tasks were included to ensure that subjects attended to the cue on the screen and ensure uniform exposure to the cues. Participants also completed standardized mood and drug effect questionnaires and cardiovascular measures, 15 before and 15, 30, 70, 115, and 210 min after drug administration. They left at 1 pm if they were no longer affected by the drug.
Posttest session (Session 6)
A posttest session was conducted at least 48 h after the last conditioning session, at the same time of day as the pretest. The procedure was identical to the pretest, and provided the primary measures of change in responses to the cues from before to after conditioning.
Conditioning Measures
All conditioning measures were completed at the pretest (Session 1) and posttest (Session 6).
Conditioning Task 1: behavioral preference measure
This measure assessed subjects' preferences for the two study cues (Mayo et al, 2013). In this task, each cue (ocean or mountain) was presented in combination with images from each of the three computer tasks (from the conditioning sessions). In each trial, participants first viewed two separate combinations of background image and computer task image (ie, ocean+task 1; mountain+task 1) presented on the screen individually. The pairs of images were then placed side-by-side, and subjects had to quickly indicate their preferred combination by pressing the corresponding mouse button. They viewed a total of 15 pairs of cue and task images, in a full-factorial design. We assessed preference for task images with the same cue to rule out biases for the tasks at baseline or after conditioning. No task bias was observed, so we collapsed the tasks for analysis. Trials in which the cue (ocean or mountain) was the same gave us no information regarding cue preference (ie, ocean+task 1; ocean+task 2), so these were removed from the analysis, leaving nine trials providing information about preference for the cues (ie ocean+task 2; mountain+task 2). The primary outcome measure was the change in number of MA-paired cue selections (0–9) from before to after the conditioning.
Conditioning Task 2: subjective rating measure
This measure assessed subjects' ‘liking' of study cues before and after conditioning. In this task, the two cues (cue image+sound) were presented individually and participants rated how much they liked each on a scale of 0 (do not like at all) to 9 (like very much) by pressing the appropriate numeric key.
Conditioning Task 3: emotional reactivity measure
Emotional reactivity was assessed by measuring corrugator and zygomatic reactivity in response to each cue (Lang et al, 1993; Drobes and Tiffany, 1997; Geier et al, 2000). For this task, each cue (with accompanying sound) was presented for 6 s, ten times, in a randomized, counterbalanced order. Presentations were separated by a variable intertrial interval (4.5–5 s) during which a fixation cross was presented on the screen. Responses were quantified as mean EMG activation in the corrugator and zygomatic muscles during the 6 s presentation minus the mean EMG activation during the 1 s before the cue was presented. EMG was measured over left brow and cheek with 4 mm Ag/AgCl electrodes and an 8-mm gel-filled Ag/AgCl ground sensor on the forehead. EMG signals were amplified, 10–500 Hz band pass filtered, digitized at 1000 Hz, 60 Hz band stop filtered, rectified, and integrated over 20 ms by using EMG100C amplifiers, and MP150 Data Acquisition System and Acqknowledge software from Biopac Systems (Goleta, CA, USA).
Conditioning Task 4: attention measure
The attention bias measure consisted of a modified visual probe task using the two visual cues (ie, Wardle et al, 2012). During this task, each trial began with a 1 s fixation cross, followed by presentation of the two study cues on the right and left of the screen, for 2 s. Both cues then disappeared and were replaced by gray rectangles of the same size, one of which contained a white circle or square visual probe. Subjects were instructed to classify the probe as a circle or square as quickly as possible by pressing a key. After a response, or 10 s with no response, an intertrial interval (750–1250 ms) began. Probe shape, location, and cue location were counterbalanced across trials. The 40 trials were presented in random, counterbalanced order.
The primary outcome was attentional bias toward the MA-paired cue, quantified in two ways: (1) Initial attention, or the direction of the first gaze when the cues appeared and (2) sustained attention, calculated as the average amount of sustained/dwell time directed toward each cue. Gaze was quantified by using electrooculography with attached 1.5 cm from the outer canthus of each eye, and data were treated similar to EMG data. Trained raters discarded trials in which: (1) gaze was not centrally fixated prior to the trial, (2) initial fixation was <100 ms after picture onset (reflecting anticipatory eye movements), (3) noise obscured eye movements.
Subjective Drug Effect Measures
Subjective effects of the drug were assessed during conditioning sessions (Sessions 2–5) by using the Drug Effects Questionnaire (DEQ; Johanson and Uhlenhuth, 1980) and Profile of Mood States (POMS; McNair et al, 1971).
Cardiovascular Drug Effect Measures
HR and BP were monitored during the conditioning sessions (Sessions 2–5) at regular intervals.
Statistical Analysis
Direct effects of drug
The subjective and physiological effects of the drug during the conditioning sessions were assessed using a repeated-measures ANOVA (RM-ANOVA), with time (baseline, five time points after drug administration) and treatment (MA, PBO) as within-subjects factors. Differences at individual time points were evaluated using Bonferroni's post hoc testing. Similar RM-ANOVA testing with time and session (first vs second session) was used to test for differences between the two MA sessions, as well as the two PBO sessions.
To explore correlations among subjective drug effects and conditioning measures, peak change scores from baseline for MA and PBO sessions (average of two PBO sessions, two MA sessions) were also calculated (Table 2).
Table 2. Mean (SEM) Scores for Subjective Ratings and Cardiovascular Measures Averaged Across the Conditioning Sessions with Placebo or Methamphetamine.
|
Placebo |
MA |
||||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | T-value | |
| Subjective effects | |||||
| DEQ | |||||
| Feel | 18.21 | 1.91 | 46.85 | 2.71 | 12.55*** |
| Like | 20.89 | 2.52 | 62.51 | 3.35 | 12.68*** |
| Dislike | 23.81 | 2.67 | 25.49 | 2.43 | 0.533 |
| High | 11.08 | 1.7 | 35.57 | 3.01 | 9.223*** |
| More | 17.861 | 2.65 | 59.37 | 3.59 | 12.23*** |
| POMS | |||||
| Friendliness | −1.9 | 0.373 | 1.56 | 0.499 | 6.08*** |
| Anxiety | −0.17 | 0.177 | 0.462 | 0.301 | 1.77 |
| Elation | −1.25 | 0.281 | 2.676 | 0.366 | 8.87*** |
| Anger | −0.242 | 0.186 | −0.33 | 0.143 | −0.384 |
| Fatigue | 1.18 | 0.303 | −0.984 | 0.296 | 5.58*** |
| Depression | −0.28 | 0.19 | −0.566 | 0.22 | −1.15 |
| Confusion | 0.571 | 0.221 | −0.225 | 0.192 | −2.87** |
| Vigor | −2.29 | 0.359 | 2.7 | 0.567 | 7.85*** |
| Cardiovascular effects | |||||
| Blood pressure | 2.41 | 3.77 | 10.5 | 3.26 | 1.56 |
| Heart rate | −7.25 | 1.01 | 4.84 | 0.27 | 8.74*** |
Abbreviations: DEQ, Drug Effects Questionnaire, POMS, Profile of Mood States. Blood pressure is represented as Mean Arterial Pressure.
Note: **p<0.01, and ***p<0.0001.
Effects of conditioning
The primary outcome measures were change in response to the study cues from before to after conditioning on the measures of behavioral preference, subjective liking, emotional reactivity, and attentional bias. Behavioral preference was analyzed as the change in the number of MA-paired cue selections from before to after conditioning, by using a paired t-test. Subjective liking, emotional reactivity (corrugator, zygomatic reactivity), and attention (initial, sustained) were analyzed by using RM-ANOVA with phase (pre- and postconditioning) and cue (MA-, PBO-paired) as within-subjects factors.
RESULTS
Direct effects of MA During Conditioning Sessions
Initially, we compared subjects' responses to MA on the first and second pair of conditioning sessions to assess changes in responses to the drug across the two administrations. Subjective and cardiovascular responses to MA did not differ between the pairs, so the two MA sessions and the two PBO sessions were averaged. MA produced its expected subjective effects on both the DEQ and POMS (Table 2). Specifically, MA increased ratings of ‘feel' drug effects, ‘like' drug, ‘high', and ‘want more' on the DEQ. Post hoc test show that the drug effects were present within 15 min of drug administration and peaked during the time of cue presentation, 30–70 min post drug administration (ie, Figure 1b). MA also increased ratings of Friendly, Anxious, Elation, and Vigor compared with PBO sessions, while decreasing ratings of Confusion and Fatigue (Table 2). These effects peaked between 30 and 70 min post drug administration, and were present during the time of cue presentation. The drug did not affect ratings of anger or depression.
Conditioning Measures
Behavioral preference for the MA-associated cue increased from before conditioning (mean preference 4.01±0.22 SEM) to after conditioning (mean preference 4.81±0.24 SEM; t(89)=3.75, p<0.0001, Cohen's d=0.38). However, self-reported ‘liking' of the MA and PBO images did not change. Although subjects' ratings of liking of the two cues decreased from before to after conditioning (main effect of phase F(1,89)=7.253, p=0.008), the decrease was similar for the MA-paired and PBO-paired cues (cue*phase interaction F(1,89)=0.65, p=0.42, ηp2=0.001).
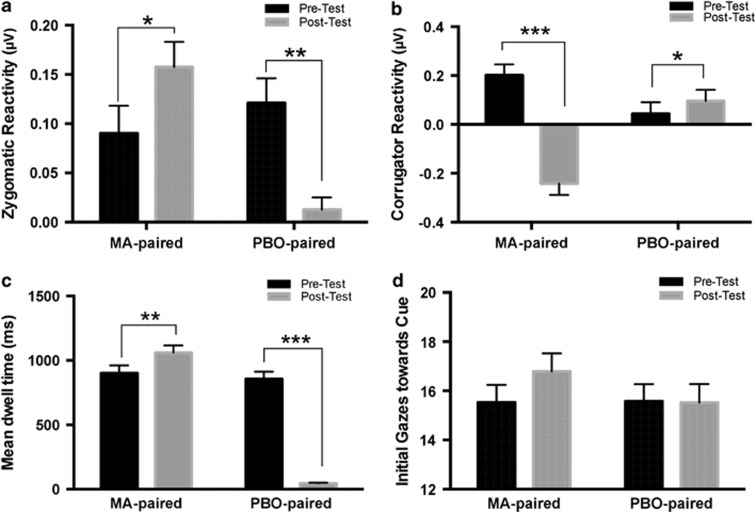
For the emotional reactivity analysis, one participant was eliminated due to equipment malfunction, resulting in n=89. Positive emotional reactivity to the MA-paired cue increased after conditioning, whereas positive emotional reactivity to the PBO-paired cue decreased (see Figure 2a). That is, zygomatic reactivity in response to the MA-paired cue increased after conditioning, whereas zygomatic reactivity with the PBO-paired cue decreased (cue*phase interaction F(1,88)=23.277, p<0.0001, ηp2=0.23). The conditioning procedure also had a significant effect on corrugator reactivity (Figure 2b). Corrugator reactivity to the MA-paired cue decreased following conditioning, indicating a decrease in negative emotional reactivity. Corrugator reactivity increased in response to the PBO-paired cue, suggesting an increase in negative emotional reactivity (cue*phase interaction F(1,88)=31.836, p<0.0001, ηp2=0.31).
Figure 2.
Responses to methamphetamine-paired and placebo-paired stimuli before (pre) and after (post) conditioning. *p<0.05, **p<0.01, and ***p<0.001. (a) Zygomatic reactivity (mean activation ±SEM) during the pre- and post-tests, for stimuli paired with MA or PBO. Zygomatic reactivity to the stimulus paired with MA increased from pre to post, whereas reactivity to the PBO stimulus decreased over time. (b) Corrugator reactivity (mean activation±SEM) during the pre- and post test, for stimuli paired with MA or PBO. Corrugator reactivity in response to the MA-paired cue is decreased following conditioning, while corrugator reactivity is enhanced in response to the PBO-paired cue. (c) Sustained attention (mean dwell time±SEM ) to the stimulus paired with MA and PBO pre- and post-test. Sustained attention is increased toward the MA-paired cue, and decreased toward the PBO cue. (d) Initial gazes (mean number of gazes±SEM) to the stimulus paired with MA and PBO, pre- and post-test. Initial gazes toward the methamphetamine-paired cue were marginally increased after conditioning, but this increase did not reach significance.
On the attention task, only data with valid gazes in at least 50% of trials were included, which excluded 15 participants, while equipment malfunction excluded three more. Thus, 72 participants were included in analysis. Sustained attention toward the MA-paired cue increased from before to after conditioning, while attention toward the PBO cue decreased (cue*phase interaction F(1,71=250.9, p<0.0001, ηp2=0.80; Figure 2c). Although there was a trend toward increased initial gazes at the MA-paired cue, this did not reach significance (cue*phase interaction F(1,72)=2.19, p=0.12, ηp2=0.03).
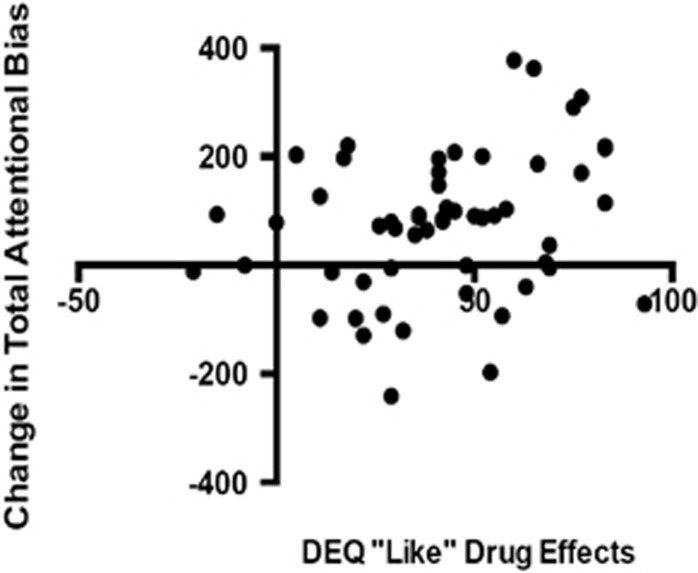
Interestingly, change in sustained attention bias (change in sustained attention toward MA cue minus change in sustained attention toward PBO cue) was positively correlated with subjective drug effects (ie DEQ ‘feel' effects: Pearson's r=0.27, p=0.020; DEQ ‘like' drug effects: Pearson's r=0.28, p=0.019), such that those reporting greater subjective drug effects showed the greatest increase in total attentional bias (Figure 3). There was no effect of sex, age, level of education, or previous drug use (including stimulant use) on any of the conditioning measures, and a majority of subjects (N=84; 93%) were unaware of the specific drug-cue pairings.
Figure 3.
Individual subjects' ratings of MA liking in relation to their change in attention toward the MA cue. Values shown indicate the change in sustained attention ([sustained attention towards MA cue] minus [sustained attention towards PBO cue]) and subjective “liking” of MA effects (DEQ “Like Drug Effects” mean peak change score at MA session—DEQ “Like Drug Effects” peak change score at PBO session scores) had a positive significant relationship. Subjective ratings of “liking” MA predicted increased attentional bias. Pearson's r=0.28, p=0.019.
DISCUSSION
Here, we report that two pairings between an environmental stimulus (cue) and administration of MA resulted in conditioned responses to the drug-associated cue. This replicated our previous findings demonstrating an increase in behavioral preference for a MA-paired cue (Mayo et al, 2013), and extended them to two additional measures of conditioning: emotional reactivity and attentional bias.
An important novel finding from this study was that emotional reactivity to the drug-associated stimulus increased after conditioning. This is consistent with the hypothesis that drug-paired conditioned stimuli produce positive affect in healthy adults, just as they do in experienced drug users. The MA-paired stimulus increased zygomatic activity and decreased corrugator reactivity, both indicators of positive affective response. Studies with dependent populations also report positive emotional reactivity in response to drug cues (Drobes and Tiffany, 1997; Geier et al, 2000). Our findings indicate that the pattern of affective response to drug-paired stimuli in healthy adults, after just two pairings with the drug, is similar to the responses to cues seen in dependent users after extensive use.
Interestingly, the PBO-paired stimulus produced the opposite responses, such that subjects exhibited a decrease in positive affect in response to the PBO stimulus. This could be a result of habituation; emotional reactivity to a stimulus decreases when the stimulus is presented repeatedly (Bradley et al, 1993). At the pretest session, our stimuli were novel, and novel stimuli have the potential to elicit psychophysiological responses. Then, with repeated presentations, the stimuli may have become less salient, resulting in a decrease in positive emotional reactivity. In the case of the MA-paired stimulus, the acquired appetitive conditioned response may override the possible habituation due to repeated exposure. Another possible explanation could be a ‘contrast' effect to the MA conditioning trials. For example, subjects may have felt less alert and less positive during the PBO sessions, relative to the MA sessions. This relatively negative state may have conferred some conditioned negative affect to the PBO-paired stimuli.
A second significant finding was the increase in sustained attention toward the drug-paired cue after conditioning. It has been well established that dependent drug users demonstrate a bias in attention toward drug cues. Here, we demonstrate healthy adults also acquire this attentional bias after just two pairings with the drug. Although sustained attention toward the PBO cue significantly decreases after conditioning, likely because of the decrease in novelty of the cue, attention towards the MA cue increases after conditioning. We also see that there is no difference in initial gazes toward the PBO-paired cue after conditioning, even though sustained attention toward that cue is decreased. Therefore, participants look toward the cue the same amount (at least, with their initial gaze), but spend less time overall looking at this cue. Meanwhile, sustained attention toward the MA cue is significantly increased, suggestion a bias in attention toward this cue following conditioning.
Interestingly, we also found that positive subjective responses to MA during the conditioning sessions predicted the change in attentional bias, such that those participants reporting the greatest subjective effects in response to MA also demonstrated the greatest increase in attentional bias toward the MA cue. It has been suggested that attentional bias toward drug-associated cues can serve as a proxy of motivation to use drugs (Field and Cox, 2008). Among currently- or formerly dependent drug users, attentional bias to drug cues is related to both craving and relapse to drug use (Franken, 2003; Waters et al, 2003), although the attentional bias may be either a consequence of extended drug use or a determinant. Our finding that attentional bias was correlated with positive subjective response to the drug in healthy nonusers supports the idea that attentional bias may be associated with motivation to use drugs, as positive subjective drug responses are associated with likelihood to repeat the drug use. In addition, these findings suggest that individual variation in attentional responses to drug cues may exist even before dependence develops, such that certain individuals may be more susceptible to the influence of drug cues on attention.
We found that subjects did not report ‘liking' the MA-paired cue more after conditioning, even though we found changes in behavioral preference, emotional reactivity, and attention. The lack of increased self-reported liking of the stimuli fits with similar work with dependent populations. For example, Winkler et al, 2011 found that, although smoking-related cues elicited enhanced positive emotional reactivity using the facial EMG, subjects did not report liking the cues. It is possible that emotional reactions that are too subtle to reach subjective experience still may influence behavior (Childress et al, 2008; Winkielman et al, 2005). It is also possible that the subjective ratings of the drug-related stimuli would increase if there were more conditioning sessions, or higher doses of the drug.
The study also had limitations. First, the participants were light recreational drug users, most of whom had no previous experience with methamphetamine. As a result, is it unclear whether these findings on the acquisition of conditioned responses would also apply to more experienced drug users. However, comparisons of acquisition among healthy volunteers and more experienced users may highlight important differences between drug users and nonusers. As only a small proportion of those who use drugs progress to dependence, our findings would still apply to a large percentage of the drug using population, which could provide insight into mechanisms associated with recreational drug use. A second limitation relates to the dose and the number of conditioning trials. More robust conditioning may occur with higher doses, other routes of administration, or more conditioning trials. These parametric manipulations in future studies will improve the sensitivity and validity of the conditioning procedure.
Taken together, the findings discussed here provide a critical ‘proof of principle' demonstration of the acquisition of conditioned associations with a mood-altering drug in healthy adults. We have shown that not only do humans, like laboratory animals, form associations between cues and psychoactive drugs in a manner consistent with classical conditioning. Notably, these associations were formed prior to any drug dependence (ie only after two drug-cue pairings). This study makes the crucial link between classical conditioning studies in animals, many of which form the basis of our theories of addiction, and indirect evidence of conditioned cue responses in dependent populations. This procedure will be useful to study individual differences in conditioned responding to drug cues, and to investigate the neural processes implicated during the conditioned responses. The procedure may also be useful to study conditioning that occurs with non-drug-induced positive mood states.
FUNDING AND DISCLOSURE
This reserach was supported by DA02812. In addition, LMM is supported by fellowships from the Chicago Biomedical Consortium, the Society for Neuroscience Scholars Program, and DA32015 (Diversity Supplement). The authors declare no conflict of interest.
Acknowledgments
We gratefully acknowledge Markus Heilig for his valuable intellectual contributions and technical support of this research.
References
- Bradley MM, Lang PJ, Cuthbert BN (1993). Emotion, novelty, and the start reflex: habituation in humans. Behav Neurosci 107: 970–980. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Erhman RN, Wang Z, Li Y, Sciortino N, Hakun J et al (2008). Prelude to passion: limbic activation by ‘unseen' drug and sexual cues. PLoS One 3: e1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, de Wit H (2009). Amphetamine-induced place preference in humans. Biol Psychiatry 65: 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, de Wit H (2011). Contextual conditioning enhances the psychostimulant and incentive properties of d-amphetamine in humans. Addict Biol 18: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Sadler BM, Hill JM, Voyksner RD, Pugh DE et al (1992). Pharmacokinetics of oral methamphetamine and effects of repeated daily dosing in humans. Drug Metab Dispos 20: 856–862. [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST (1997). Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol 106: 15–25. [DOI] [PubMed] [Google Scholar]
- Ehrman R, Robbins SJ, Childress AR, O'Brien CP (1992). Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 107: 523–529. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM (2008). Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend 97: 1–20. [DOI] [PubMed] [Google Scholar]
- Franken IH (2003). Drug craving and addiction: integrating psychophysiological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry 27: 563–579. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P (2000). Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology 150: 283–291. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH (1980). Drug preference and mood in humans: d-amphetamine. Psychopharmacology (Berl) 71 p 275–279. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403–1413. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2008). Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci 363: 3113–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Greenwalk MK, Bradley MM, Hamm AO (1993). Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 30: 261–273. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR (1971). Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharm Ther 12: 245–258. [DOI] [PubMed] [Google Scholar]
- Mayo LM, Fraser D, Childs E, Momenan R, Hommer DW, Heilig M (2013). Conditioned preference to a methamphetamine-associated contextual cue in humans. Neuropsychopharmacology 38: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. (1971) Profile of Mood States. Educational and Industrial Testing Service: San Diego, CA. [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Herman R (1992). Classical conditioning in drug-dependent humans. Ann NY Acad Sci 654: 400–415. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe J, Fonte C (1994). Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol Biochem Behav 47: 107–112. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2008). Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363: 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM (1999). Does urge to drink predict relapse after treatment? Alcohol Res Health 23: 225–232. [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE (1988). Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser 4: 161–175. [DOI] [PubMed] [Google Scholar]
- Skinner MD, Auben HJ (2010). Craving's place in addiction theory: contributions of the major models. Neurosci Biobehav Rev 34: 606–623. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL (1998). Is craving the source of compulsive drug use? J Psychopharmacol 12: 23–30. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Garner MJ, Munafo ME, de Wit H (2012). Amphetamine as a social drug: effects of d-amphetamine on social processing and behavior. Psychopharmacology (Berl) 223: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH (2003). Attentional bias predicts outcome in smoking cessation. Health Psychol 22: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H (2002). Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 73: 729–741. [DOI] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL (2005). Unconscious affective reactions to makes happy versus angry faces influence consumption behavior and judgments of value. Pers Soc Psychol Bull 31: 121–135. [DOI] [PubMed] [Google Scholar]
- Winkler MH, Weyers P, Mucha RF, Stippekohl B, Stark R, Pauli P (2011). Conditioned cues for smoking elicit preparatory responses in healthy smokers. Psychopharmacology 231: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]