Abstract
BACKGROUND:
Recurrent miscarriage (RM) is the most common pregnancy loss in the first trimester affecting approximately 0.5–2% of women. It is a heterogeneous condition and remains an enigma as the underlying cause is still difficult to track down.
AIM:
This study was aimed to investigate the distribution of tumor necrosis factor-alpha (TNF-α) 308G/A polymorphism and its association with RM in females. The comparative picture was also demonstrated by comparing genotyping results with healthy control women having no history of miscarriage.
METHODS:
This clinical study was conducted among 115 women aged 21–44 years with history of recurrence of miscarriage. The samples were collected from women attending the outpatient departments of various hospitals, nursing homes, and infertility clinics of this region. In the present study, 111 fertile healthy women aged 24–46 years with at least one live birth and no history of miscarriage were also included.
RESULTS:
Mean age of women with RM was found to be 28 ± 5.6 years by recall method, whereas it was found to be 30 ± 7.4 in context to healthy women with no history of pregnancy loss. In the present study, 66% of women with RM had homozygous wild type genotype (GG) while 30% and 4% of women had heterozygous (GA) and homozygous mutant genotype (AA), respectively. Among control group, 79%, 16%, and 5% of women showed GG, GA, and AA genotype, respectively.
CONCLUSION:
The current study supports the concept of TNF-α 308G/A variant in particular with reproductive failure, GG and GA alleles showing 1-fold risk association with RM (odds ratio: 1.86 and 1.43, respectively).
KEY WORDS: Polymorphism, recurrent miscarriage, reproductive failure, tumor necrosis factor
INTRODUCTION
Recurrent miscarriage (RM) is defined as two or more (first trimester) pregnancy losses before 20 weeks of gestation.[1] It is a common complication of pregnancy affecting 0.5–2% of women of reproductive age and is an important health issue of women.[2,3] In most of the cases, the cause of RM often remains elusive. In lieu, the idiopathic behavior of RM dysregulated immunity has been proposed as a potential underlying mechanism, highlighted by altered cytokines production and increased frequency of antiphospholipid antibody positivity.[4,5,6] Success of pregnancy is dependent on a fine balance between feto-maternal interactions and embryonic implantation, which is controlled by maternally and fetally derived cytokines and growth factors. It has been proposed that proinflammatory cytokine such as tumor necrosis factor-alpha (TNF-α) may lead to inflammation-induced miscarriage.[7] Many studies have reported for association of TNF-α polymorphism and level of cytokine production for premature birth, but their role for miscarriages/reproductive failures is still debatable.[8,9,10,11,12] The present study is aimed to investigate the distribution of TNF-α 308G/A (rs1800629) polymorphism and association with RM in a group of 115 well-defined RM women having at least two consecutive spontaneous first trimester miscarriages of unknown reason and compared genotyping results with a group of 111 matched healthy control women having no history of miscarriage.
METHODS
The present study recruited a total of 226 women, in which 115 women (mean age 28.6, ranging from 21 to 44 years) with a clinical history of recurrence of miscarriage. All the samples were collected from outpatient department of different hospitals. The patients having any positivity for anatomical anomalies as revealed by ultrasonography, endocrinal disorders, hepatitis B, torch infections, and autoimmune diseases were excluded from the study. A total of 111 unrelated fertile healthy women (mean age 30.4 years, ranging from 24 to 46) with at least one live birth and no history of miscarriage have been recruited. The study was approved by the Institutional Ethical Committee, with the principles embodied in the Declaration of Helsinki. After taking written informed consent from each participant, blood sample was obtained from each woman and was stored at − 20°C for further analysis.
Genomic DNA was extracted using phenol-chloroform method with slight modifications[13] and quantified using NanoDrop™ 2000/200c spectrophotometer (Thermo Scientific™, Pittsburgh, USA). The TNF-α 308G/A (rs1800629) polymorphism was done with polymerase chain reaction (PCR)-restriction fragment length polymorphism method. The purified DNA was amplified using primers for TNF-α G/A forward 5’ AGGCAATAGGTTTTGAGGGCCAT’3; reverse 5’ TCCTCCCTGCTCCGATTCCG’3.[14] The PCR mixture contained 20 ng of genomic DNA, 0.2 μM of each primer, 200 μM of each deoxynucleotide, 1.5 mM of tris-HCl buffer (pH 9.0), 1.5 mM of MgCl2, and 0.024 unit of Taq DNA polymerase in a final reaction volume of 20 μl. The amplification cycle was performed on Mastercycler (ABI 2720, Applied Biosystems, USA). After initial denaturation at 95°C for 5 min, the DNA was amplified by 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for s, extension at 72°C for 30 s, and final extension at 72° C for 10 min. The PCR products were visualized under ultraviolet transilluminator after staining with ethidium bromide. The amplified PCR products were digested with restriction enzyme NcoI (New England Biolabs, USA) at 37°C for 16 h giving restriction products of 87 and 20 bp which were separated by electrophoresis on 3.5% agarose gel.
Statistical analysis was performed using SPSS (version 17.0; SPSS Inc., Chicago) and online Web-Assotest program. Genotype and allele frequencies of TNF-α gene in case and control groups were compared and tested using Pearson's Chi-square. Deviations from Hardy–Weinberg equilibrium (HWE) were calculated using a goodness of fit. Analyses have also been done for dominant, co-dominant (additive), and recessive genetic models, and their odds ratio (OR) with 95% confidence interval (CI) ranges and corresponding P values using the Web-Assotest program.
RESULTS
A total of 226 samples (115 cases and 111 controls) were genotyped. The mean age of women included in the study was 28 ± 5.6 years and that of control females was 30 ± 7.4 years. Table 1 represents TNF-α G/A allele and genotype frequency distribution of case and control subjects. In the present study, 66% of women with RMs had homozygous wild type genotype (GG) while 30% and 4% of women had heterozygous (GA) and homozygous mutant genotype (AA), respectively. Among control group, 79%, 16%, and 5% of women showed GG, GA, and AA genotypes, respectively.
Table 1.
Distribution of tumor necrosis factor-alpha polymorphism among recurrent miscarriages women
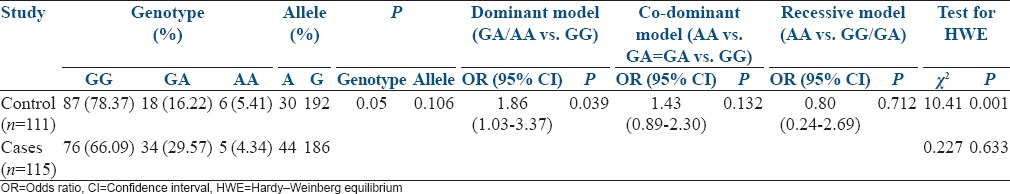
The genotype distribution of TNF-α G/A polymorphism has shown significant difference between case and control groups (P = 0.05), whereas allele distribution did not differ significantly between case and control groups (P = 0.106). However, we observed a significant deviation from HWE with control group (P = 0.001) while no deviation was observed in case group (P = 0.633). There was a suggestive evidence of an association in dominant model (GA/AA vs. GG; OR: 1.86, 95% CI: 1.03–3.37, P = 0.039) of TNF-G/A polymorphism with the occurrence of RMs among women. However, no significant association has been observed in co-dominant model as well as recessive model.
DISCUSSION
Spontaneous abortion is the most common form of pregnancy failure, with 20% of pregnancies culminating in detectable spontaneous abortions. RM is a heterogeneous disorder in which almost 40% of women do not have a known underlying reason for the pregnancy failure.[15] A fine balance between Th1 and Th2 type of cytokine is necessary for a successful pregnancy.[1,16,17,18] TNF-α is encoded by TNF-α gene, and many functional polymorphisms in proximal and distal regions of gene have been found.[14] A fine balance between Th1 and Th2 type of cytokine is necessary for a successful pregnancy.[19]
The present study describes the significant association between the TNF-α 308G/A polymorphism and recurrence of miscarriages in females. In a previous study by Kaur and Kaur,[20] no association was found between the 308G/A polymorphism and RM probably due to smaller sample size. The findings of the present study were in consistency with the report by Kamali-Sarvestani et al.[21] Their results indicated a significant association between the 308 G/A polymorphism and RM in Iranian women. However, TNF-α gene variants have been involved in the pathogenesis of reproductive failure although many studies have observed a nonsignificant association between TNF-α polymorphism and pregnancy complications.[8,11,12,22,23] A meta-analysis by Bombell and McGuire[24] also observed nonsignificant associations with TNF-α variants. Gupta et al.[25] applied dominant and recessive models of inheritance, showing no association among the TNF-α 308G/A polymorphism and RM in North Indian women.
CONCLUSION
The current study supports the concept of significant association of TNF-α 308G/A variants, in particular with reproductive failure. The association observed in the present study between TNF-α variant and reproductive failure might suggest a positive role of the cytokine in coagulation activation which has been reported in a study by Guadagni et al.[26] The functional aspect is that the trophoblast antigens initiate the lymphocytes of RM susceptible women to synthesize TNF-α which is embryotoxic cytokine.[25,27] It is also well known that TNF-α activity produces a series of complications such as necrosis of the implanted embryo, reduced blood supply, and fetal expulsion due to uterine contractions. All these factors ultimately cause pregnancy loss. However, very scanty literature is available to support the associations between TNF-α 308G/A polymorphism and pregnancy loss, except a few such as in Tunisian and Chinese populations.[9,28] In the present study, GG and GA genotypes showed more than 1-fold risk association with RM (OR: 1.86 and 1.43, respectively). In the present study results, there is an apparent increase in the frequency of GG genotype among control group (as compare to case group 78.37% vs. 66.09%, respectively); however, there is no apparent increase in the occurrence of AA genotype in RM cases which calls for further study with bigger sample size in the same population.
There is a need for further studies to confirm the association between TNF-α 308G/A variant with reproductive failure. It is necessary to evaluate worldwide frequency of the variant allele that may cause the TNF-α dysfunction and to establish a data bank of information for further study of RM.
Financial support and sponsorship
We are thankful to the Department of Science and Technology for providing financial assistance and fellowship to Ms. Neha Sudhir, through DST-PURSE (9535/Estt./A2) program.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge Dr. Tajinder Kaur, Dr. S.S. Chawla and Dr. Neerat Aggrawal for providing samples with RM and all the controls.
REFERENCES
- 1.Stephenson M, Kuteh W. Evaluation and management of recurrent early pregnancy loss. Clin Obstet Gynecol. 2007;50:132–45. doi: 10.1097/GRF.0b013e31802f1c28. [DOI] [PubMed] [Google Scholar]
- 2.Choudhury SR, Knapp LA. Human reproductive failure I: Immunological factors. Hum Reprod Update. 2001;7:113–4. doi: 10.1093/humupd/7.2.113. [DOI] [PubMed] [Google Scholar]
- 3.Sierra S, Stephenson M. Genetics of recurrent pregnancy loss. Semin Reprod Med. 2006;24:17–4. doi: 10.1055/s-2006-931797. [DOI] [PubMed] [Google Scholar]
- 4.Raghupathy R. Th-1 type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–2. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 5.Hill JA, Polgar K, Anderson DJ. T-helper1-type immunity to trophoblast in women with recurrent spontaneous abortion. J Am Med Assoc. 1995;273:1933–6. [PubMed] [Google Scholar]
- 6.Sater MS, Finan RR, Mustafa FE, Al-Khateeb GM, Almawi WY. Anti-annexin V IgM and IgG autoantibodies and the risk of idiopathic recurrent spontaneous miscarriage. J Reprod Immunol. 2011;89:78–3. doi: 10.1016/j.jri.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+) CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmirotta R, Farina FL, Ferroni P, Ludovici G, Nigro C, Savonarola A, et al. TNFA gene promoter polymorphisms and susceptibility to recurrent pregnancy loss in Italian women. Reprod Sci. 2010;17:659–66. doi: 10.1177/1933719110366603. [DOI] [PubMed] [Google Scholar]
- 9.Zammiti W, Mtiraoui N, Khairi H, Gris JC, Almawi WY, Mahjoub T. Associations between tumor necrosis factor-alpha and lymphotoxin-alpha polymorphisms and idiopathic recurrent miscarriage. Reproduction. 2008;135:397–403. doi: 10.1530/REP-07-0322. [DOI] [PubMed] [Google Scholar]
- 10.Yu XW, Li X, Ren YH, Li XC. Tumour necrosis factor-alpha receptor 1 polymorphisms and serum soluble TNFR1 in early spontaneous miscarriage. Cell Biol Int. 2007;31:1396–9. doi: 10.1016/j.cellbi.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Daher S, Shulzhenko N, Morgun A, Mattar R, Rampim GF, Camano L, et al. Associations between cytokine gene polymorphisms and recurrent pregnancy loss. J Reprod Immunol. 2003;58:69–77. doi: 10.1016/s0165-0378(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 12.Reid JG, Simpson NA, Walker RG, Economidou O, Shillito J, Gooi HC, et al. The carriage of pro-inflammatory cytokine gene polymorphisms in recurrent pregnancy loss. Am J Reprod Immunol. 2001;45:35–40. doi: 10.1111/j.8755-8920.2001.450106.x. [DOI] [PubMed] [Google Scholar]
- 13.Adeli K, Ogbonna G. Rapid purification of human DNA from whole blood for potential application in clinical chemistry laboratories. Clin Chem. 1990;36:261–4. [PubMed] [Google Scholar]
- 14.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor a promoter on transcriptional activation. Proc Natl Acad Sci U S A. 1997;94:3195–9. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephenson MD. Frequency of factors associated with habitual abortions in 197 couples. Fertil Steril. 1996;66:24–9. [PubMed] [Google Scholar]
- 16.Makhseed M, Raghupathy R, Azizieh F, Omu A, Al-Shamali E, Ashkanani L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum Reprod. 2001;16:2219–26. doi: 10.1093/humrep/16.10.2219. [DOI] [PubMed] [Google Scholar]
- 17.Quenby S, Vince G, Farquharson R, Aplin J. Recurrent miscarriage: A defect in nature's quality control? Hum Reprod. 2002;17:1959–63. doi: 10.1093/humrep/17.8.1959. [DOI] [PubMed] [Google Scholar]
- 18.Zhu XY, Zhou YH, Wang MY, Jin LP, Yuan MM, Li DJ. Blockade of CD86 signaling facilitates a Th2 bias at the maternal-fetal interface and expands peripheral CD4CCD25C regulatory T cells to rescue abortion-prone fetuses. Biol Reprod. 2005;72:338–45. doi: 10.1095/biolreprod.104.034108. [DOI] [PubMed] [Google Scholar]
- 19.El-Shazly S, Makhseed M, Azizieh F, Raghupathy R. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am J Reprod Immunol. 2004;52:45–52. doi: 10.1111/j.1600-0897.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaur A, Kaur A. Recurrent pregnancy loss: TNF-α and IL-10 polymorphisms. J Hum Reprod Sci. 2011;4:91–4. doi: 10.4103/0974-1208.86090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamali-Sarvestani E, Zolghadri J, Gharesi-Fard B, Sarvari J. Cytokine gene polymorphisms and susceptibility to recurrent pregnancy loss in Iranian women. J Reprod Immunol. 2005;65:171–8. doi: 10.1016/j.jri.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Baxter N, Sumiya M, Cheng S, Erlich H, Regan L, Simons A, et al. Recurrent miscarriage and variant alleles of mannose binding lectin, tumour necrosis factor and lymphotoxin alpha genes. Clin Exp Immunol. 2001;126:529–34. doi: 10.1046/j.1365-2249.2001.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babbage SJ, Arkwright PD, Vince GS, Perrey C, Pravica V, Quenby S, et al. Cytokine promoter gene polymorphisms and idiopathic recurrent pregnancy loss. J Reprod Immunol. 2001;51:21–7. doi: 10.1016/s0165-0378(01)00069-9. [DOI] [PubMed] [Google Scholar]
- 24.Bombell S, McGuire W. Cytokine polymorphisms in women with recurrent pregnancy loss: Meta-analysis. Aust N Z J Obstet Gynaecol. 2008;48:147–54. doi: 10.1111/j.1479-828X.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 25.Gupta R, Prakash S, Parveen F, Agrawal S. Association of CTLA-4 and TNF-α polymorphism with recurrent miscarriage among North Indian women. Cytokine. 2012;60:456–62. doi: 10.1016/j.cyto.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Guadagni F, Ferroni P, Basili S, Facciolo F, Carlini S, Crecco M, et al. Correlation between tumor necrosis factor-alpha and D-dimer levels in non-small cell lung cancer patients. Lung Cancer. 2004;44:303–10. doi: 10.1016/j.lungcan.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Yamada H, Polgar K, Hill JA. Cell mediated immunity to trophoblast antigens in women with recurrent spontaneous abortions. Am J Obstet Gynecol. 1994;170:1339–44. doi: 10.1016/s0002-9378(94)70153-9. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Wang J, Zhou S, Wang B, Ma X. Association between-238 but not-308 polymorphism of tumor necrosis factor alpha (TNF-alpha) and unexplained recurrent spontaneous abortion (URSA) in Chinese population. Reprod Biol Endocrinol. 2010;28:114–8. doi: 10.1186/1477-7827-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]


