Abstract
Adherence with antipsychotic monitoring guidelines is notoriously low nationally. Without active monitoring and measures to improve metabolic abnormalities, more patients may develop related morbidity and mortality. An audit highlighted antipsychotic monitoring in this learning disability service in London did not match guideline recommendations. People with intellectual disability also experience health inequalities. Psychiatrists are well placed to provide advice and assistance that is suitable for those with complex communication, behaviour, and social needs.
The QI team tested ideas to increase rates of antipsychotic reviews. The focus was the follow up monitoring of all universal measures recommended by NICE 2014, collected at 2-weekly intervals. We trialled interventions in four broad categories; Intervention 1: to make monitoring more structured and planned; Intervention 2: to increase staff and patient awareness of healthy eating and exercise programs; Intervention 3: to increase the collection of diet and exercise histories from patients; Intervention 4: to improve the uptake of blood tests.
The interventions created an improvement in monitoring. There are lessons in the methodology for others carrying out similar projects.
Problem
The project was based in a Community Learning Disabilities Service in inner London. The team is integrated with social services and includes three psychiatrists and a range of other health professionals.
The quality improvement (QI) team informally identified barriers to antipsychotic monitoring. Certain equipment was not available and there was limited information about referral options for weight management. The psychiatrists usually rely on GPs to arrange blood tests, so it is unclear where barriers to this lie.
In June 2014 the team audited adherence with physical health monitoring recommendations in the Maudsley guidelines1 for 43 patients and found that adherence was poor. It could be expected that without active monitoring and measures to improve metabolic abnormalities, more patients would develop related morbidity and mortality.
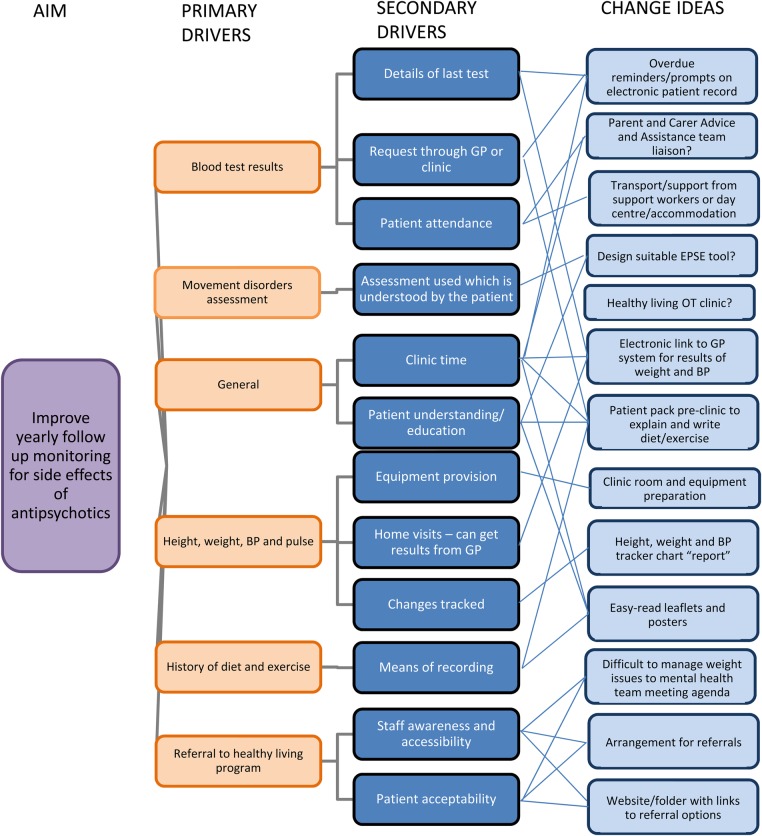
The QI team comprised psychiatrists, administrative staff, a physiotherapist, and occupational therapists. Using a driver diagram to generate ideas (see figure 2), the team tested changes to the service, which aimed to increase rates of antipsychotic reviews so that all patients taking antipsychotics would have a yearly review. The focus was the follow up monitoring of all universal measures recommended by NICE.2 The aim was to both discover, in liaison with primary care and patients, which improvement ideas increase rates of antipsychotic reviews, and to refine and use these methods to improve practice.
Figure 2.
Driver diagram of change ideas
Background
It is recognised that people with schizophrenia experience greater morbidity and live 10-25 years shorter than the general population.3 This is due to social consequences of the illness, lifestyle factors, and side effects of antipsychotics. Antipsychotics can indirectly cause weight gain, lipid abnormalities, and unregulated blood sugar, through a combination of reduced activity due to sedation and increased appetite, leading to higher rates of cardiovascular disease and diabetes.4 Further physical health side effects from antipsychotics include stigmatising and unpleasant extra-pyramidal side effects.
People with intellectual disability (ID) also experience health inequalities and have more unmet health needs.5–7 Rates of schizophrenia are three times higher in people with ID and the illness often presents at a younger age.8 Psychiatrists might also prescribe antipsychotics for people with ID to help manage ‘severe challenging behaviour’ which 5-15% experience.9 The evidence for this use is limited, however.10,11 NICE reviewed the limited data and suggested people with ID suffer similar risks related to antipsychotics as those without ID.12
Barriers to health in those with schizophrenia include cognitive problems, social isolation,13 the physicians perceiving physical complaints as related to mental illness,14 and fragmented services.15 Barriers among people with ID include health literacy and a lack of accessible, affordable transport.16
Since the serious maltreatment of hospital patients with ID at Winterbourne View,17 there has been public health concern about inappropriate antipsychotic prescriptions in ID.18 19 In a primary care audit, 16% of adults with a diagnosis of ID were prescribed an antipsychotic without a recorded indication.20 Although new antipsychotic prescriptions are declining in this group, the rate of prescribing exceeds the numbers with severe mental illness, due to the high rates of prescribing for ‘challenging behaviour’.21 One would expect that improving side effect monitoring should also increase scrutiny over whether the medication is still required. Similar approaches to reducing unnecessary antipsychotic use through increasing monitoring are seen in people with behavioural and psychological symptoms of dementia.22
Psychiatrists are well placed to provide advice and assistance suitable for those with complex communication, behaviour, and social needs, and NICE recommends that psychiatrists carry out antipsychotic reviews for patients under their care.2 However, a national audit by the Royal College of Psychiatrists found that healthcare teams offered a comprehensive physical health check in 29% of people taking antipsychotics.23
A few studies have shown that structured, active monitoring effectively uncovers undetected illness, for example a service evaluation of a computerised GP cardiovascular risk template in schizophrenia detected significantly greater illness compared to a control.24 One fairly large study on psychiatric inpatients found it was cost-effective to screen lipids.25 One analysis found screening in severe mental illness detects diabetes five years earlier, and the avoided complications of diabetes made screening cost-effective.26
The principle of social inclusion advocates people with ID using public resources and mainstream community activities.27 Normalisation is related and aims to promote valued social roles and positive societal attitudes.28 The belief is that positive risk increases dignity for people with ID. Thus, on the one hand, people with ID have health inequalities, which could be targeted in a proactive way, and on the other hand, one must avoid creating division from mainstream society in addressing these differences.
Baseline measurement
This project used NICE guidelines for schizophrenia to set standards.2 These suggest all those on antipsychotics should have baseline and annual monitoring, which should include weight measurements, blood tests (for fasting blood glucose, glycosylated haemoglobin (HbA1c) and blood lipid profile), blood pressure (BP), pulse and assessment of movement disorders (extra-pyramidal side effects, EPSE), nutritional status, diet, and physical exercise. They also advise that mental health teams should offer referral to a healthy eating and exercise program.
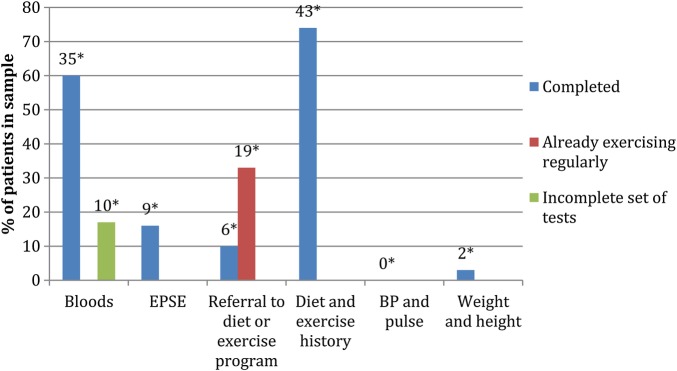
The baseline measurement was the proportion of 43 patients who had each measure of the antipsychotic monitoring over the previous 12 months, sampling those living in two of five possible postcodes (see figure 1). Of the 26 (60%) patients taking an antipsychotic, 19 (73%) had a recorded blood test, 12 (46%) had a recorded weight, and dietary and exercise advice was given in 12 (46%) and 8 (30%) respectively. The baseline results show that the most frequent intervention was diet and exercise history taking, followed by bloods. Clinicians rarely checked weight, height, BP, and pulse.
Figure 1.
The percentage of patients in the baseline audit that had each measure of the antipsychotic monitoring over the previous 12 months. For blood tests, the percentage of patients that had some tests, but not all those recommended by NICE, are shown. For referrals to diet and exercise programs, the percentage of patients who did not have a referral but reported exercising regularly are also shown. * The numbers of patients are above each bar
Following the baseline measurement, we then collected data on monitoring measures carried out during appointments in 2-week periods, as this was more practical. We identified the number of complete antipsychotic reviews including all monitoring measures recommended by NICE.2 For all patients receiving psychiatric input to have an antipsychotic review every 12 months, approximately five reviews should be conducted every two weeks, so this was the target. However, reporting the number of full reviews did not demonstrate smaller improvements so was not sensitive to change. Therefore we additionally started reporting on individual components of the monitoring.
Design
We used a literature review and suggestions from team discussions to create a driver diagram of change ideas (figure 2). As it was a small-scale project with a motivated team, we tested interventions if they seemed possible to achieve. We then adapted interventions, using rapid 2-weekly PDSA cycles.
Data were collected from clinic letters and GP summary records, cross-referenced with psychiatrists’ diaries, every two weeks.
Projects published by BMJ Quality Improvement Reports suggest benefits can result from improving equipment suitability and training for clinicians,29 introducing a patient-owned physical health booklet and a nurse-led antipsychotic monitoring clinic30 and forming new guidelines and a paper prompt for reviews at the time of admission to hospital.31
Other published studies indicate pharmacy checks on monitoring prior to dispensing the medication32 and combined projects between primary and secondary care33 can be effective.
Turner and Robinson summarised evidence of good practice in a review of annual health checks for people with ID.34 These include supporting people who are scared of needles; using ‘easy-read’ resources to make information more accessible; using electronic monitoring forms which are more compatible with GP record systems, and having the flexibility to carry out the checks at home if the nature of the disability makes it difficult to attend a clinic.
Although the results are not yet available, change ideas were described in the reports from the NHS Improving Quality and six nationwide ID teams’ pilot projects around antipsychotics. One Child and Adolescent Mental Health Team tried to improve monitoring through direct access to blood results and a monitoring booklet35 and one project in an ID inpatient unit developed an easy-read side effects screening tool.36
Strategy
Intervention 1
This intervention aimed to make the monitoring more structured and planned.
PDSA cycle 1
We revised the approach to monitoring, from ad-hoc to a planned, structured yearly antipsychotic review that aims to include all measures. This monitoring would be triggered when the blood tests are due. We implemented this following a team discussion. We anticipated this would improve both monitoring rates and ease of data collection.
PDSA cycle 2
We changed the format of the psychiatry follow-up templates on the electronic patient record to provide prompts and sections for each measure of the antipsychotic monitoring. The material is pre-populated into subsequent psychiatric records and a table of BPs and weights is created. We anticipated this would highlight when the reviews are due and avoid unnecessary duplication. An additional benefit is that the tables of BPs and weights would demonstrate changes over time so clinicians can act on these.
Intervention 2
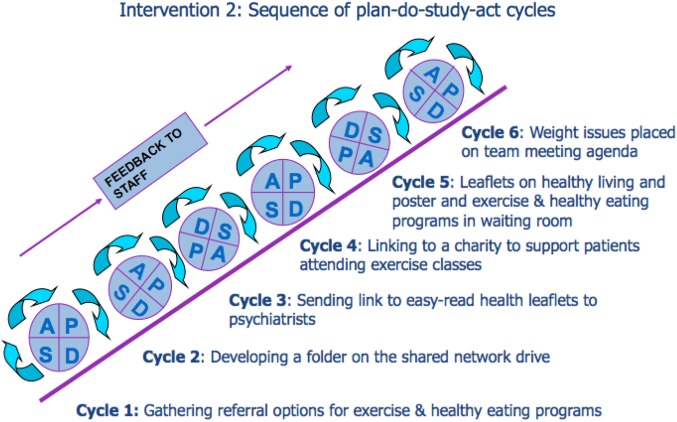
This intervention aimed to increase staff and patient knowledge around weight issues and increase awareness of referral options for healthy eating and exercise programs. We expected that increasing this knowledge and awareness would result in more staff and patients holding discussions around weight management. Figure 3 shows a diagram of this intervention.
Figure 3.
Sequence of plan-do-study-act cycles in intervention 2
PDSA cycle 1
Liaising with the wider team and external agencies, we gathered information about referral options for healthy eating and exercise programs suitable for people with ID. We aimed to gather feedback from all patients who attended these programs following referral to assess suitability. The referral options were initially shared between psychiatrists via email.
PDSA cycle 2
We felt there should be a more accessible place for information about healthy eating and exercise programs, which other staff could edit. A staff member took on the role of creating and updating a folder of this information on the shared network drive.
PDSA cycle 3
We distributed a link to a website containing easy-read leaflets about weight and health issues between the psychiatrists. We anticipated that staff might more readily discuss weight management if they have easy-read information as prompts.
PDSA cycle 4
We made links with a charity project (Motivate East) and organised a meeting between them, a lead physiotherapist, lead OT, and psychiatrist to decide on a strategy for disseminating information about accessible physical activity opportunities for people with ID as well as any opportunities for our team to assist the charity’s program development team in providing resources for people with ID attending such activity groups. Changes planned by the charity include a new exercise group, equipment such as a throw down activity surface, events and promotional campaigns, as well as transport to such activities. The team will be liaising about these opportunities so that our patients can participate. Representatives from several community activity programs were also invited but due to a major strike affecting transport, all except one were unable to attend. The plan is to continue such meetings.
PDSA cycle 5
From those few who provided feedback, one patient spoke positively about the dietician service he had been referred to and said he had lost weight. We incorporated this service and some exercise program options into an easy-read poster placed in the waiting room. We also printed some easy-read leaflets with information about healthy weight, nutrition, and weight management. We then added a less complex leaflet as the first type contained a lot of information, which might not be suitable for patients with lower reading ability. We made them available in the waiting room and office for staff to use as prompts for communication with patients. We anticipated that patients might become more aware of these health issues and ask about referrals to the services listed.
Intervention 3
This intervention aimed to increase the collection of diet and exercise histories from patients.
PDSA cycle 1
We designed and piloted a healthy living questionnaire to send to all patients with their appointment invites to explain about antipsychotic monitoring, provide information about cardiovascular side effects, and gather a diet and exercise history. We liaised with speech and language therapy to develop a suitable easy-read questionnaire. We anticipated it would allow quicker and more detailed collection of health information. Another, not formally tested benefit of this intervention might be improved patient awareness of the side effects of their medications. Patients who are engaged in their healthcare are more likely to have better outcomes.37
We tested the questionnaire on 10 patients and collected feedback about its suitability. According to the feedback, patients found the questionnaire helpful and acceptable. Some minor alterations were suggested; one patient wanted the psychiatrists’ name or photo and the time of the appointment on the questionnaire to indicate which appointment it was for. Some wrote helpful information on the questionnaires and one checked his BP and weight through his GP.
PDSA cycle 2
We revised the questionnaires, incorporating the feedback and sent them to all patients with up-coming appointments.
This system will be revised further, as patients will otherwise be sent questionnaires for several different appointments. Additionally, many patients do not take antipsychotics. Streamlining this will ensure that only patients due reviews are sent questionnaires.
Intervention 4
We aimed to improve the uptake of blood tests. We carried out process mapping, speaking to GP surgeries, patients, and their families or support workers to identify the sequence of events when blood tests were missed. It became apparent that two ‘missing’ blood results had in fact, been carried out but were on a different pathology system. This left six patients who hadn’t had a blood test. Two of these had requests from the psychiatrist to “continue yearly blood tests” but no such arrangement was made, for unclear reasons. A third request was more specific but it was unclear why the test was not done. Three others were frightened of needles. In these patients, plans were put in place by psychology and nursing around desensitisation and explanation of the test process to lessen patients’ fear.
We didn’t discover any patients who were invited for a test but couldn’t attend, for reasons other than being frightened. GPs advised that engaging patients’ support workers seems to improve attendance at blood tests.
Results
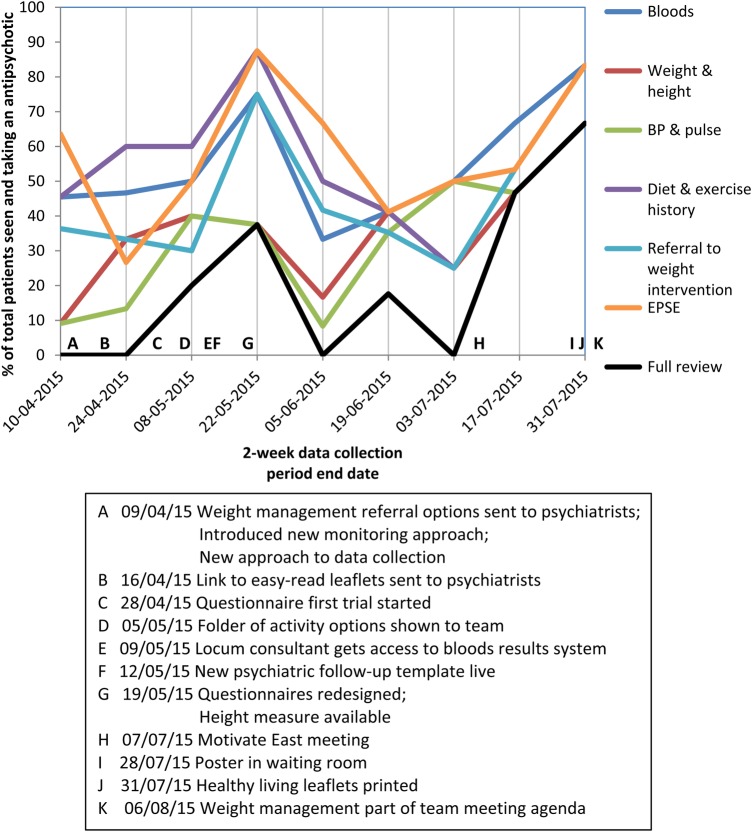
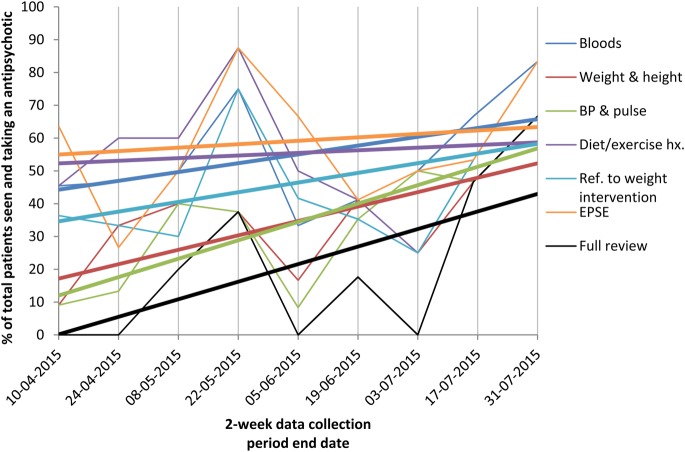
The run chart is available in figures 4 and 5. The overall improvement in monitoring is difficult to assess in this chart, since the data do not reflect yearly antipsychotic reviews, but those seen within a 2-week period that had checks done in that time. Additionally, many patients seen later in the QI project who did not have monitoring measures carried out may have already been seen and had these earlier in the project period. Repeating the initial data – the number of patients with monitoring measures over a 12-month period – would demonstrate the improvement in yearly monitoring better but collecting this was very time consuming and therefore not currently possible to repeat.
Figure 4.
The proportion of patients that had each measure of the antipsychotic monitoring completed, out of those taking antipsychotics with appointments in each 2-week data collection period. The letters demonstrate the approximate timing of each intervention to improve monitoring, shown in the box.
Figure 5.
The proportion of patients that had each measure of the antipsychotic monitoring completed, out of those taking antipsychotics with appointments in each 2-week data collection period, with trend lines added for each measure.
Monitoring rates (figure 4 and table 1) initially increase and then decrease in June. In July monitoring rates then once again increase, perhaps due to renewed interventions at this time. The decrease in June may be partly due to the lack of new interventions, but perhaps also improved monitoring earlier in the study. We were not expecting very high proportions to have reviews, as they are often not clinically indicated; hence the target of five every two weeks. Thus the decrease isn’t necessarily problematic. Improved monitoring in April among patients given 2- or 3-month follow-up appointments would affect results in June and July.
Table 1.
The number of times each measure was carried out in each 2-week data collection period. Also shown are the cumulative figures for measures carried out, the total number of appointments in which a patient taking an antipsychotic was seen in each period, and the numbers of these that were seen in clinic and the community.
| 2-week data collection period end date | 10/04/2015 | 24/04/2015 | 08/05/2015 | 22/05/2015 | 05/06/2015 | 19/06/2015 | 03/07/2015 | 16/07/2015 | 31/07/2015 |
|---|---|---|---|---|---|---|---|---|---|
| Seen and taking antipsychotic, no. (cumulative) | 11 (11) | 15 (26) | 10 (36) | 8 (44) | 12 (56) | 17 (73) | 4 (77) | 15 (92) | 6 (98) |
| Clinic | 3 | 6 | 7 | 3 | 3 | 6 | 1 | 10 | 3 |
| Community | 8 | 9 | 3 | 5 | 9 | 11 | 3 | 5 | 3 |
| Bloods, no. (cumulative) | 5 (5) | 7 (12) | 5 (17) | 6 (23) | 4 (27) | 7 (34) | 2 (36) | 10 (46) | 5 (51) |
| Weight & height, no. (cumulative) | 1 (1) | 5 (6) | 4 (10) | 3 (13) | 2 (15) | 7 (22) | 1 (23) | 7 (30) | 4 (34) |
| BP & pulse, no. (cumulative) | 1 (1) | 2 (3) | 4 (7) | 3 (10) | 1 (11) | 6 (17) | 2 (19) | 7 (26) | 4 (30) |
| Hx. diet/ exercise, no. (cumulative) | 5 (5) | 9 (14) | 6 (20) | 7 (27) | 6 (33) | 7 (40) | 1 (41) | 8 (49) | 5 (54) |
| Ref. health intervention, no. (cumulative) | 4 (4) | 5 (9) | 3 (12) | 6 (18) | 5 (23) | 6 (29) | 1 (30) | 8 (38) | 5 (43) |
| EPSE, no. (cumulative) | 7 (7) | 4 (11) | 5 (16) | 7 (23) | 8 (31) | 7 (38) | 2 (40) | 8 (48) | 5 (53) |
| Full, no. (cumulative) | 0 (0) | 0 (0) | 2 (2) | 3 (5) | 0 (5) | 3 (8) | 0 (8) | 7 (15) | 4 (19) |
The number of patients seen in clinic rather than the community affected the data (see figure 6). This may be due to the resources available in clinic, such as written patient information and equipment.
Figure 6.
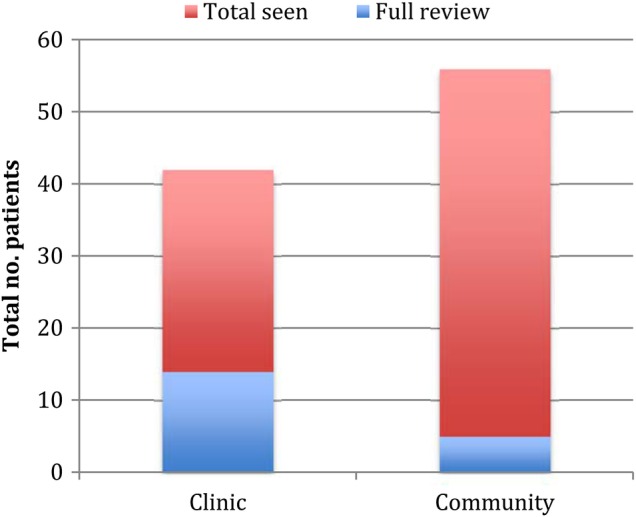
The number of patients seen and taking an antipsychotic and the number that had a full antipsychotic review across the whole project period, amoung those seen in the community and in clinic.
Lessons and limitations
The fact that the baseline data were not comparable to subsequent data means it is not easy to tell if the changes resulted in an improvement. This resulted from adapting and improving the data collection method as the project progressed. The rates at which individual measures were carried out, however, did improve (figure 5) and overall numbers of interventions were higher during the 18-week data collection period than in the 12-month baseline data (figure 1 and table 1). Unless interventions were repeated in patients, this would indicate an improvement.
Additionally certain interventions were introduced in the same data collection period, making it difficult to assess the relative effect of each. Staggering interventions might have made the results easier to interpret, but wasn’t always practical.
It became apparent during the project that it is important to address reasons for health inequality, such as by increasing support for patients. However, these issues are complex and for this project, we tried to focus on aims that would be achievable over the relatively short timeframe. We were able to influence how a patient could use their existing social support to get an intervention (e.g. have a blood test or attend an exercise activity). Through discussion at team meetings we were able to raise awareness of the issues with the team social workers.
We tried to engage a GP surgery in this QI project, initially through an intervention on blood tests. Several problems arose when trying to run a PDSA cycle in this area. After limited success getting information from GPs and practice managers during process mapping, we relied on information from GP practice secretaries. From this we could only establish certain information such as blood test invites. One reason why it may have been difficult to engage GPs was that each practice has low numbers of patients with ID who are also taking an antipsychotic, which would not have made interventions in this area worthwhile for them. An additional issue was there were too few patients who experienced problems having blood tests to interpret meaningfully. However this also demonstrates missed blood tests aren’t a large issue for our patients.
Conclusion
The combination of interventions has created an improvement in monitoring and changes appear to be sustained throughout the project. It is difficult to draw conclusions about individual interventions from these data, since the timing of some interventions meant that more than one were introduced between data collection periods and external factors such as repeat appointments affected the data. It may take over a year to test the success of certain changes such as changes to the structuring of the antipsychotic reviews and psychiatric records, since the reviews are carried out yearly.
This project is on-going and is sustainable due to the rapid method of data collection. Next steps involve refining the use of the health information questionnaires and streamlining the way GP information is accessed before appointments.
Although these results are specific to this team, there are lessons for others carrying out QI projects, such as suggestions for interventions and how to plan data collection to be practical and meaningful.
Acknowledgments
Dr Genevieve Holt, Dr Flora Greig, Dr Aruna Sahni, Dr Kate Highton, Silvia Barellini, Elaine Galton, Julie Askew, Melanie Smith-Lyons, Dr Anton Canagasabey, Laura Copsey, Sarah Bellringer.
Footnotes
Declaration of interests: Nothing to declare.
Ethical approval: This was a quality improvement study and did not deviate from accepted, local clinical practice. Local policy meant that ethical approval was not required.
References
- 1.Taylor D, Paton C, Kapur S. The South London and Maudsley NHS Foundation Trust Oxleas NHS Foundation Trust: Prescribing Guidelines [Internet]; 2010. [cited 2015 Dec 16]. Available from: http://www.scribd.com/doc/51180647/Maudsley-Prescribing-Guidelines- 10th-edition. [Google Scholar]
- 2.National Institute for Health and Clinical Excellence. Psychosis and schizophrenia in adults: treatment and management NICE clinical guideline 178 [Internet]; 2014. [cited 2015 Dec 16]. Available from: guidance.nice.org.uk/cg178. [Google Scholar]
- 3.Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25:83–8. [DOI] [PubMed] [Google Scholar]
- 4.Hassiotis A, Barron DA, Hall I. Intellectual Disability Psychiatry: a practical handbook, Ch 10. Chichester: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 5.Heslop P, Blair P, Fleming P, Hoghton M, Marriott A, Russ L. Confidential Inquiry into premature deaths of people with learning disabilities (CIPOLD) [Internet]; 2013. [cited 2015 Dec 16]. Available from: http://www.bristol.ac.uk/media-library/sites/cipold/migrated/documents/fullfinalreport.pdf. [DOI] [PubMed] [Google Scholar]
- 6.Mencap. Death by indifference: Following up the Treat me right! Report [Internet]; 2007. [cited 2015 Dec 16]. Available from: https://www.mencap.org.uk/sites/default/files/documents/2008-03/DBIreport.pdf. [Google Scholar]
- 7.Michael J. Healthcare For All: Report of the independent inquiry into access to healthcare for people with learning disabilities [Internet]; 2008. [cited 2015 Dec 16]. Available from: http://webarchive.nationalarchives.gov.uk/20130107105354/http:/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_106126.pdf. [Google Scholar]
- 8.Deb S, Thomas M, Bright C. Mental disorder in adults with intellectual disability. 1: Prevalence of functional psychiatric illness among a community-based population aged between 16 and 64 years. J Intellect Disabil Res. 2008;45:495–505. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clinical Excellence. Challenging behaviour and learning disabilities: final scope [Internet]; 2013. [cited 2015 Dec 16]. Available from: http://www.nice.org.uk/guidance/gid-cgwave0654/documents/challenging-behaviour-and-learning-disability-final-scope3. [Google Scholar]
- 10.Tsiouris JA. Pharmacotherapy for aggressive behaviours in persons with intellectual disabilities: treatment or mistreatment? J Intell Disabil Res. 2010;54:1–16. [DOI] [PubMed] [Google Scholar]
- 11.Matson JL, Bamburg J, Mayville EA et al. . Psychopharmacology and mental retardation: a ten-year review (1990-1999). Res Dev Disabil. 2000;21:263–96. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Clinical Excellence. Challenging behaviour and learning disabilities: prevention and interventions for people with learning disabilities whose behaviour challenges (NG11) [Internet]; 2015. [cited 2015 Dec 16]. Available from: http://www.nice.org.uk/guidance/NG11. [PubMed] [Google Scholar]
- 13.Phelan M, Stradins L, Morrison S. Physical health of people with severe mental illness. BMJ. 2001;322:443–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence D, Stephen K. Inequalities in health care provision for people with severe mental illness. J Psychopharmacol. 2010;24:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischhacker WW, Cetkovich-Bakmas M, De Hert M et al. . Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69:514–9. [DOI] [PubMed] [Google Scholar]
- 16.Abbott S, Mcconkey R. The barriers to social inclusion as perceived by people with intellectual disabilities. J Intellectual Disabil Res. 2006;10:275. [DOI] [PubMed] [Google Scholar]
- 17.Flynn M. Winterbourne View Hospital: A serious case review. South Gloucestershire Adult Safeguarding Board [Internet]; 2012. [cited 2015 Dec 16]. Available from: Available at: http://hosted.southglos.gov.uk/wv/summary.pdf. [Google Scholar]
- 18.NHS England. Winterbourne View - time for change [Internet]; 2014. [cited 2015 Dec 16]. Available from: http://www.england.nhs.uk/wp-content/uploads/2014/11/transforming-commissioning-services.pdf. [Google Scholar]
- 19.NHS England. The use of medicines in people with learning disabilities. Publications Gateway Reference 03689 [Internet]; 2015. [cited 2015 Dec 16]. Available from: http://www.england.nhs.uk/wp-content/uploads/2015/07/med-advice-ld-letter.pdf. [Google Scholar]
- 20.Public Health England. Prescribing of psychotropic drugs to people with learning disabilities and/or autism by general practitioners in England [Internet]; 2015. [cited 2015 Dec 16]. Available from: http://www.improvinghealthandlives.org.uk/securefiles/150730_0854//Psychotropic%20medication%20and%20people%20with%20learning%20disabilities%20or%20autism.pdf. [Google Scholar]
- 21.Sheehan R, Hassiotis A, Walters K, Osborn D, Strydom A, Horsfall L. Mental illness, challenging behaviour, and psychotropic drug prescribing in people with intellectual disability: UK population based cohort study. BMJ. 2015;351:4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee S. The use of antipsychotic medication for people with dementia: time for action. London: Department of Health; 2009. [Google Scholar]
- 23.Royal College of Psychiatrists. Report of the National Audit of Schizophrenia (NAS) 2012. London: Healthcare Quality Improvement Partnership; 2012. [Google Scholar]
- 24.Yeomans D, Dale K, Beedle K. Systematic computerised cardiovascular health screening for people with severe mental illness. Psychiatr Bull. 2014;38:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arce-Cordon R, Perez-Rodriguez MM, Baca-Baldomero E, Oquendo MA, Baca-Garcia E. Routine laboratory screening among newly admitted psychiatric patients: is it worthwhile? Psychiatr Serv. 2007;58:1602–1605. [DOI] [PubMed] [Google Scholar]
- 26.Bruggeman R, Schorr S, Van der Elst K, Postma M, Taxis K. Cost- effectiveness of screening for diabetes in a cohort of patients with schizophrenia. Schizophr Res. 2008;102:161–2. [Google Scholar]
- 27.Burchardt T, Le Grand J, Piachaud D. Degrees of Exclusion: Developing a Dynamic Multidimensional Measure in Hills J, Le Grand J, Piachaud D (eds) Understanding Social Exclusion Oxford: Oxford University Press; 2002. [Google Scholar]
- 28.Wolfensberger W. The Principle of Normalization in Human Services. Toronto, Canada: National Institute on Mental Retardation; 1972. [Google Scholar]
- 29.Jeffrey J. Quality improvement in resident education: a pilot project to mitigate metabolic side effects from atypical antipsychotic medications in youth. BMJ Qual Improv Report. 2015;4: doi:10.1136/bmjquality.u208804.w3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowlands C. Improving physical monitoring for patients' taking antipsychotic medication [Internet]; 2013. [cited 2015 Dec 16]. Available from: http://www.rcpsych.ac.uk/pdf/QIF% 20poster.pdf. [Google Scholar]
- 31.Pasha N, Saeed S, Drewek K. Monitoring of physical health parameters for inpatients on a child and adolescent mental health unit receiving regular antipsychotic therapy. BMJ Qual Improv Report. 2015;4: doi:10.1136/bmjquality.u202645.w3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peh ALH. Safety monitoring of patients on atypical antipsychotics. Qual Saf Health Care. 2008;17:469–472. [DOI] [PubMed] [Google Scholar]
- 33.Modi RN, Ledingham D. Cardiovascular health monitoring in patients with psychotic illnesses: A project to investigate and improve performance in primary and secondary care. BMJ Qual Improv Report. 2013;2: doi:10.1136/bmjquality.u632.w640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner S, Robinson C. Health checks for people with learning disabilities: Implications and actions for commissioners. Evidence into practice report No. 2. Improving Health and Lives Learning Disabilities Observatory: Stockton on Tees; 2010. [Google Scholar]
- 35.Simmons H. Improving medicines monitoring in children and adolescents with a learning disability [Internet]; 2015. [cited 2015 Dec 16]. Available from: http://www.nhsiq.nhs.uk/media/2641823/wandsworth_camhs_final_report_2015.pdf. [Google Scholar]
- 36.Sussex Partnership NHS Foundation Trust. Spotlight Project - Medicines Management at the Selden Centre [Internet]; 2015. [cited 2015 Dec 16]. Available from: http://www.nhsiq.nhs.uk/media/2641818/sussex_partnership_nhs_ft_final_report_2015.pdf. [Google Scholar]
- 37.Hibbard JH, Greene J. What The Evidence Shows About Patient Activation: Better Health Outcomes And Care Experiences; Fewer Data On Costs. Health Aff. 2013;32:207–214. [DOI] [PubMed] [Google Scholar]