Abstract
Background
This study, by regulating the expression level of microRNA-21 (miRNA-21) in antigen-1+ (Sca-1+) cardiac stem cells (CSCs), examined the role of miRNA-21 in migration, proliferation, and differentiation of Sca-1+ CSCs, and explored the use of miRNA-21 in treatment of heart-related diseases in mice.
Material/Methods
The CSCs of 20 healthy 2-month-old C57BL/6 mice were collected in our study. Immunomagnetic beads were used to separate and prepare pure Sca-1+ CSCs, which were further examined by flow cytometry. The samples were assigned to 4 groups: the blank group, the miRNA-21 mimic group, the miRNA-21 inhibitor group, and the negative control (NC) group. Quantitative real-time polymerase chain reaction (qRT-PCR), Transwell chamber assay, and the methyl thiazolylte-trazolium (MTT) assay were performed. Reverse transcriptase-polymerase chain reaction (RT-PCR) was used to measure the expression levels of GATA-4, MEF2c, TNI, and β-MHC differentiation-related genes.
Results
Immunomagnetic separation results indicated that Sca-1+ CSCs accounted for more than 87.4% of CSCs. RT-PCR results also showed that the expression level of miRNA-21 of the miRNA-21 mimic group was higher than those of the other groups (all P<0.05). Compared to the NC and the blank group, the migration of Sca-1+ CSCs was more active in the miRNA-21 mimic group and less active in the miRNA-21 inhibitor group (all P<0.05). Moreover, compared to the blank group, the proliferation of Sca-1+ CSCs was enhanced in the miRNA-21 mimic group and inhibited in the miRNA-21 inhibitor group (all P<0.05). The results of RT-PCR indicated that neither miRNA-21 mimics nor miR-21 inhibitors influenced the gene expression levels of GATA-4, MEF2c, TNI, or β-MHC.
Conclusions
Our study provides evidence that up-regulation of miRNA-21 can promote migration and proliferation of Sca-1+ CSCs to enhance the capacity of Sca-1+ CSCs to repair damaged myocardium, which may pave the way for therapeutic strategies directed toward restoring miRNA-21 function for heart-related diseases.
MeSH Keywords: Cell Differentiation, Cell Migration Assays, Cell Proliferation
Background
Cardiac stem cells (CSCs) are tissue-specific stem progenitor cells harbored within the adult mammalian heart, with the ability to differentiate into various cell types for myocardial regeneration, such as cardiomyocytes, vascular smooth muscle cells, and endothelial cells [1]. One category of CSCs, located in vascular niches distributed throughout the coronary circulation, mainly participates in the transformation of smooth muscle cells, vasculogenesis, and endothelial cells [2]. The other CSCs category, clustering in niches nested in the myocardial interstitium, regulates myocyte renewal and cardiomyogenesis [3]. Increasing evidence has suggested that the adult heart contains CSCs, which have been isolated in a number of species by detecting stem cell markers, including c-Kit, stem cell antigen-1 (Sca-1), Isl-1, and MDR1 [4]. Sca-1, as a member of the Ly-6 family, is a well-known surface marker of skeletal muscle cells, hematopoietic and CSCs [5]. Sca-1+ cells could be enriched to the functional collagen scaffold, both in vivo and in vitro, through the interaction between antigen and antibody [6]. In the last decade, microRNAs (miRNAs) have been shown to play crucial roles in the control of stem cell and tissue differentiation [7].
miRNAs, small non-coding RNAs about 19 to 25 nucleotides in length, can regulate the expression of their cognate target genes at the posttranscriptional level, and thus induce translational repression or transcript degradation [8]. miRNA-21 is one of the first miRNAs identified to be transcribed by RNA polymerase II, which was subsequently identified as a major driver of miRNA transcription [9]. miRNA-21 exhibits strong evolutionary conservation throughout a number of vertebrate species: mammalian, avian, and fish clades [10]. The small regulatory RNA, miRNA-21, participates in a number of biological functions, including growth, development, proliferation, and inflammation, as well cardiovascular diseases [11]. However, there were few studies that investigated the role of miRNA-21 in CSCs, which could restore cardiac function and reduce infarct size after cardiovascular diseases [12]. Furthermore, Sca-1+ CSCs accounts for 70% of the cells in the mouse heart after the depletion of cardiomyocytes [13]. Thereby, we hypothesized that up-regulation of miRNA-21 could promote migration and proliferation of Sca-1+ CSCs to enhance the capacity of Sca-1+ CSCs to repair damaged myocardium. We also investigated the exact role of miRNA-21 in migration, proliferation, and differentiation of Sca-1+ CSCs in mice by regulating the expression level of miRNA-21 in CSCs, which may pave the way for therapeutic strategies directed toward restoring miRNA-21 function for heart-related diseases.
Material and Methods
Subjects
Experiments were performed on 20 healthy 2-month-old C57BL/6 mice weighing 20 to 25 g with half males and half females. The mice were provided by the Second Hospital of Shandong University Animal Center (certification No. SCXK 2009-0002). These mice were housed in cages with water freely available and given complete compound pellet feed in daylight condition with relative humidity of 55% and temperatures 24.3±2.6°C. The experiments in this study were approved by the Ethics Committee of the Second Hospital of Shandong University, which was conducted according to the Instructive Notions with Respect to Caring for Laboratory Animals issued by the Ministry of Science and Technology of the PRC [14]. All efforts were made to minimize the suffering of the mice.
Isolation of CSCs
The mice were sacrificed by dislocation of the cervical vertebra. After sterilization in ethyl alcohol (75%), their hearts were separated, and then placed in culture dishes containing phosphate-buffered saline (PBS), with the macrovessels removed. Subsequently, the hearts without macrovessels were washed twice, divided into tissue blocks (about 1 mm3), and placed into centrifuge tubes (50 ml). Type II collagenase (0.05%, 10 ml) (Sigma Company, St. Louis, MO, USA) was added into the tubes, and then the tissue blocks were digested at 37°C in a water bath for 5 min. After the digestive liquid was discarded, type II collagenase (0.05%, 10 ml) was added into the tubes again, and the tissue blocks were digested at 37°C for 8 min. The cell suspensions were then obtained and centrifuged at 1000 rpm for 5 min, with the supernatant discarded. Then samples were added into Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, and were pipetted into the cell suspensions. All the cell suspensions were filtered with a 200-mesh sieve to remove tissue blocks, collected, and finally incubated at 37°C in a humidified CO2 incubator (5% CO2) for subculturing.
Immunomagnetic separation of Sca-1+ CSCs
In the buffer solution used in immunomagnetic separation, 74.4 mg ethylenediamine tetraacetic acid (EDTA) diluted by phosphate buffer solution (PBS) was run on filter sterilization, and then 0.5 ml fetal bovine serum (FBS), and PBS was added to obtain a total volume of 100 ml. The buffer solution was finally obtained, containing 2 mmol/L EDTA and 0.5% FBS, and it was stored at 4°C for further use. After being resuspended in the buffer solution (1 mL), the cells were added into 20 μl fluorescein isothiocyanate (FITC)-labeled anti-mouse sca-1 and then incubated together at 4~8°C for 10 min in the dark. These cells washed with buffer solution (4 ml) were centrifuged twice at 1200 rpm at 4°C, each for 5 min. Subsequently, with the supernatant discarded, the cells were again suspended in the buffer solution (180 μl) together with 20 μl anti-FITC Multisort Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), and then this mixture was incubated at 4~8°C for 15 min in the dark. After being washed with buffer solution (4 ml) and centrifuged at 1200 rpm at 4°C for 5 min, the supernatants were discarded, and the cells were suspended in the buffer solution (500 μl) again. Before the immunomagnetic separation, the magnetic column (Miltenyi Biotec, Bergisch Gladbach, Germany) was washed with the buffer (500 μl). During the immunomagnetic separation, the magnetically labeled cells remained in the magnetic column, whereas the unlabeled cells passed directly through the magnetic column. Then the magnetic column was taken out and washed with the buffer (500 μl) for several times so that the retained Sca-1+ CSCs were washed down and collected into the centrifuge tubes for centrifugation (1200 rpm, 5 min).
Flow cytometry analysis
Sca-1+ CSCs separated by immunomagnetic beads were digested with 0.125% tryptase. After neutralization and centrifugation, these cells were resuspended with PBS (500 μl) in the 1.5 ml Eppendorf (EP) tubes, which were centrifuged (1000 rpm, 5 min) and washed with PBS. Subsequently, the mixtures continued to be suspended by PBS (500 μl) in the 1.5ml EP tubes, which were supplemented with labeled antibody (5 μl), mixed evenly, and incubated at room temperature for 20 min in the dark. The negative control group was set up. The incubated cells were resuspended with 2-ml buffer solution, followed by centrifugation at 1000 rpm for 5 min, and, after the removal of the supernatants, the remaining mixture was filtered (200 mesh) and resuspended with 0.5 ml PBS. After being fixed with the fixing liquid, the samples were subjected to flow cytometry analysis (FCM, BD FACSanto, USA).
miRNA-21 transfection and grouping
Sca-1+ CSCs were cultured for 3 days and then plated at a density of 5×104/well in 24-well plates. Within 24 h of cell fusion (60~70%), transfection was conducted according to the operating manual of HiPerFect transfection reagents. Four groups were set up: (1) the blank group (control group, no transfection); (2) the miRNA-21 mimic group (experiment group, the cells transfected with miRNA-21 mimics); (3) the miRNA-21 inhibitor group (experiment group, the cells transfected with miRNA-21 inhibitors); and (4) the negative control group (NC group, experiment group, the cells were transfected with microRNA negative control). miRNA mimics were used to mimic miRNAs in vivo, and can enhance endogenous miRNA function. miRNA inhibitors were chemically modified to inhibit specific targeted miRNAs in cells. Sca-1+ CSCs were cultured for 48 h after transfection and prepared for the following experiments.
Quantitative real time-polymerase chain reaction (qRT-PCR)
The isolation of total RNA from the cells obtained from the previous step was conducted with the single-step Trizol method (Molecular Research Center, Cincinnati, Inc., USA). RNA quality was assessed by an ultraviolet (UV) spectrophotometer, and its integrity was examined with agarose gel electrophoresis. The reverse transcription was conducted by use of the TaqMan® microRNA reverse transcription kit (Applied Biosystems, Foster City, USA). PCR amplification was performed with the TaqMan® microRNA assays kit (Applied Biosystems, Foster City, USA), with the primers synthesized by Shanghai Sangon Bio-engineering Co., Ltd and U6 snRNA as an internal reference (Table 1). The amplification protocol consisted of 1 cycle of 95°C for 10 min followed by 50 cycles of 95°C for 15 s and 60°C for 1 min. The qRT-PCR results were calculated using 2−ΔΔCT method.
Table 1.
The primer sequences of GATA-4, MEF2c, TNI, β-MHC, β-actin, miR-21 and U6 snRNA.
| Forward | Reverse | Accession number | |
|---|---|---|---|
| GATA-4 | 5′-CGGAAGCCCAAGAACCTGAATA-3′ | 5′-TTGCTGGAGTTACCTACTGAA-3′ | NM_001310610 |
| MEF2c | 5′-GTACAACGAGCCGCACGAGA-3′ | 5′-AGACTCAGGGCTGACCTACTGAA-3′ | NM_001170537 |
| TNI | 5′-AGGGCCCACCTCAAGCA-3′ | 5′-GGCCTTCCATGCCACTCA-3′ | NM_009406 |
| β-MHC | 5′-GCTACGCTTCCTGGATGATCT-3′ | 5′-CCTCTTAGTGTTGACAGTCTTCC-3′ | NM_080728 |
| β-actin | 5′-ACCCACACTGTGCCCATCTA-3′ | 5′-CACGCTCGGTCAGGATCTTC-3′ | NM_007393 |
| miR-21 | 5′-TCCGAAGTTGTAGTCAGACT-3′ | 5′-GTGCAGGGTCCGAGGT-3′ | NR_029738 |
| U6 | 5′-CTCGCTTCGGCAGCACA-3′ | 5′-AACGCTTCACGAATTTGCGT-3′ | NM_001110101 |
Transwell chamber assay
After 48 h of transfection in the groups, the cells were subjected to 12 h of starvation by DMEM without serum. After the digestion with pancreatin and the neutralization with 10% fetal calf serum (FCS)-containing DMEM, the cells were centrifuged at 1000 r/pm for 5 min with the supernatants removed. The cell concentration was adjusted to 1×105 cells/ml. Each Transwell chamber (8 μl) (Corning Costar Company, Corning, NY, USA) was added with 1% DMEM (100 μl)-contained cell suspensions in the upper layer and with 10% FCS-contained DMEM (600 μl) in lower layer in the condition with 5% CO2 at 37°C for 12 h. After staining with diamidino-phenyl-indole (DAPI), the number of migrated cells was counted.
The methyl-tetrazolium (MTT) assay
Sca-1+ CSCs in the groups were collected and then plated in 96-well plates at a density of 5×103/well with 100 μL cell suspension in each well and 6 wells for each group. With 20 μL MTT solution (5mg/ml) (Sigma Company, USA) added to each well, the cells continued to be cultured in 5% CO2-contained incubator at 37°C for 4 h, and then were taken out and observed under an inverted microscope. After the medium was completely removed, dimethyl sulfoxide (DMSO, 200 μl) was added to each well, followed by incubation at room temperature for 3 h in the dark. Subsequently, the optical density (OD) value at 450 nm was measured in each well using an enzyme-labeling measuring instrument at 24 h, 48 h, and 72 h, and the OD rates were used to represent the speed of cell proliferation. The average values of 3 wells in each group were obtained, and the curve of cell proliferation was plotted for each group. The experiment was repeated 3 times. Cell proliferation rate=(ODexperiment group/ODcontrol group – 1) ×100%.
Relevant gene expression by reverse transcriptase-polymerase chain reaction (RT-PCR)
After Sca-1+ CSCs were transfected for 2 weeks, the total RNAs were extracted, and the cDNAs were synthesized through reverse transcription, with β-actin as an internal reference. By the use of the same RT-PCR reagent and method as described above, the expression levels of the genes, GATA-4, MEF2c, TNI, and β-MHC, all of which are important cardiac transcription factors, were measured to assess the effects of miRNA-21 on the differentiation of Sca-1+ CSCs in vitro [15,16]. The primer sequences of GATA-4, MEF2c, TNI, and β-MHC are displayed in Table 1.
Statistical analysis
All statistical tests were performed with SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Continuous data are presented as mean ± standard deviation, and 1-way analysis of variance (ANOVA) was used for comparisons. Categorical data are presented as percentage or rate, with comparisons conducted by chi square test. All the tests were 2-sided, with P<0.05 considered statistically significant.
Results
Isolation, culture, and identification of Sca-1+ CSCs
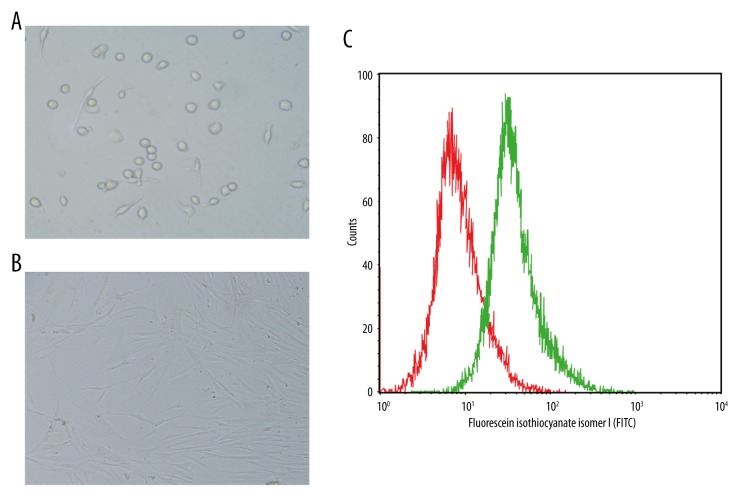
Sca-1+ CSCs obtained from the immunomagnetic separation were transferred into 6-well plates and were cultured for 1 day at 37°C in a 5% CO2 environment. Most of the cells adhered to the wall, exhibiting a round shape. After being cultured for 3 days, most cells changed from round to spindle-shaped and grew larger (Figure 1A, 1B). Flow cytometry found that Sca-1+ CSCs accounted for more than 87.4% of CSCs obtained from the immunomagnetic separation (Figure 1C).
Figure 1.
Isolation and culture of Sca-1+ CSCs and identification of Sca-1+ CSCs by flow cytometry (A – After 1 day of culture, the majority of cells were found to adhere to the wall, with round shapes; B – After 3 days of culture, the majority of cells were spindle-shaped and grew larger; C – identification of Sca-1+ CSCs by flow cytometry).
Expression levels of miRNA-21 in Sca-1+ CSCs after transfection
The expression levels of miRNA-21 in Sca-1+ CSCs of the groups after transfection are presented in Figure 2. The data indicated that the miRNA-21 mimic group had higher expression level of miRNA-21 than the other groups (all P<0.05). The miRNA-21 inhibitor group had lower expression level of miRNA-21 compared to the NC and the blank groups (both P>0.05), while there was no significant difference observed in the expression level of miRNA-21 between the NC group and the blank group (P>0.05).
Figure 2.
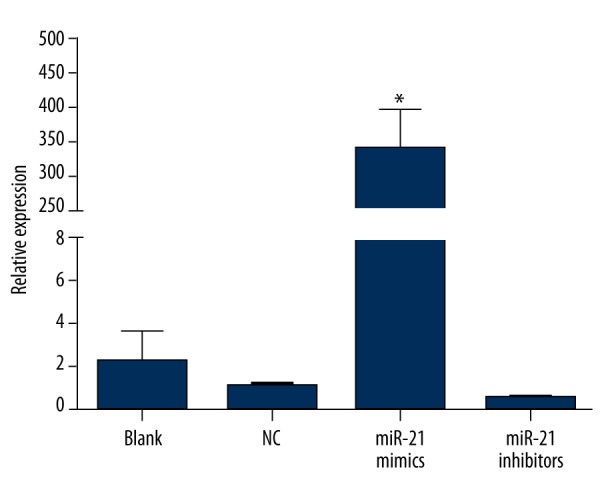
The expression levels of miRNA-21 in Sca-1+ CSCs after transfection in blank, negative control, miRNA-21 mimic, and miRNA-21 inhibitor groups (* – the miRNA-21 mimic group was compared with the other 3 groups, P<0.05). The miRNA-21 mimic group had a higher expression level of miRNA-21 than other groups.
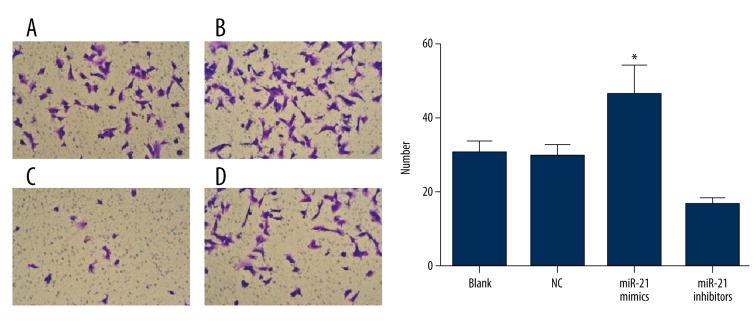
The effects of miRNA-21 on migration of Sca-1+ CSCs in vitro
After 48 h of culture, no remarkable difference was found in the effects of miRNA-21 on the migration of Sca-1+ CSCs between the NC group and the blank group (P>0.05). However, compared to the NC and the blank groups, the migration of Sca-1+ CSCs was more active in the miRNA-21 mimic group but less active in the miRNA-21 inhibitor group (all P<0.05) (Figure 3).
Figure 3.
The migration of Sca-1+ CSCs in vitro after transfection by the Transwell chamber assay in blank, negative control, miRNA-21 mimic, and miRNA-21 inhibitor groups (× 200, DAPI staining; * – the miRNA-21 mimic group was compared with the other 3 groups, P<0.05) (A – blank group; B – miRNA-21 mimic group; C – miRNA-21 inhibitor group; D – negative control group).
The effects of miRNA-21 on proliferation of Sca-1+ CSCs in vitro
Compared to the blank group, the proliferation of Sca-1+ CSCs was obviously enhanced in the miRNA-21 mimic group but inhibited in the miRNA-21 inhibitor group (all P<0.05). No significant difference was detected at any time in the proliferation of Sca-1+ CSCs between the NC group and the blank group (all P>0.05) (Table 2, Figure 4).
Table 2.
The reproduction rate of Sca-1+ CSCs at 24 h, 48 h and 27 h after transfection by the MTT assay in blank, NC, miRNA-21 mimic, and miRNA-21 inhibitor groups.
| Group | Reproduction rate (%) | ||
|---|---|---|---|
| 24 h | 48 h | 72h | |
| Blank | 100 | 100 | 100 |
| NC | −6.79* | −7.99* | −8.75* |
| miR-21 mimic | 17.67* | 19.6* | 35.10* |
| miR-21 inhibitor | −20.25* | −27.48* | −30.63* |
MTT – methyl thiazolylte-trazolium; NC – negative control; CSCs – cardiac stem cells
Compared to blank group, P<0.05.
Figure 4.
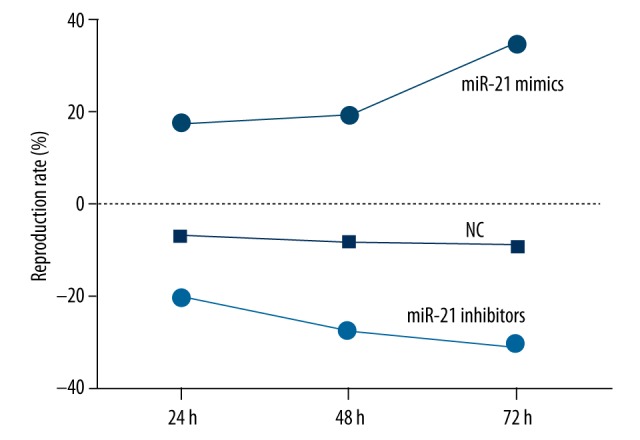
The reproduction rate of Sca-1+ CSCs in vitro after transfection by the methyl thiazolylte-trazolium (MTT) assay in blank, negative control, miRNA-21 mimic, and miRNA-21 inhibitor groups at different time points. The proliferation of Sca-1+ CSCs was significantly enhanced in the miRNA-21 mimic group but was inhibited in the miRNA-21 inhibitor group compared to the blank group.
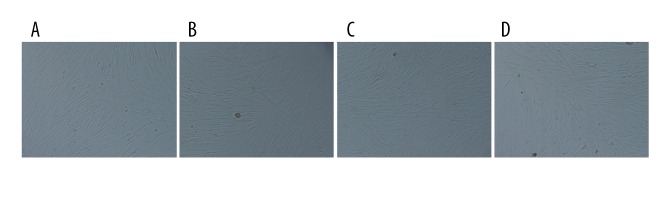
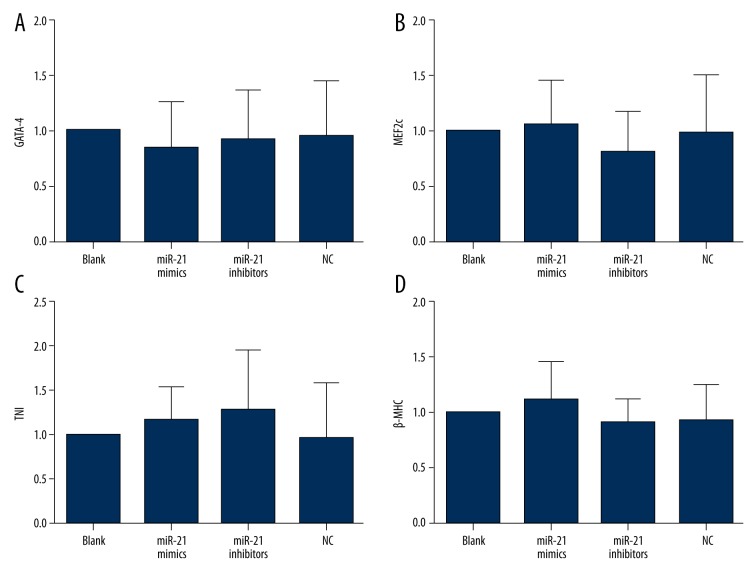
The effects of miRNA-21 on differentiation of Sca-1+ CSCs in vitro
Under the microscope, Sca-1+ CSCs in the blank, the miRNA-21 mimic, the miRNA-21 inhibitor, and the NC groups did not differentiate into functional cardiomyocytes (Figure 5). RT-PCR results indicated that neither miRNA-21 mimics nor miR-21 inhibitors influenced the expression levels of the genes, GATA-4, MEF2c, TNI and β-MHC, suggesting that CSCs did not differentiate into cardiomyocytes (Figure 6).
Figure 5.
The differentiation of Sca-1+ CSCs under the microscope (A – blank group; B – miRNA-21 mimic group; C – miRNA-21 inhibitor group; D – negative control group). Under the microscope, the Sca-1+ CSCs in blank, miRNA-21 mimic, miRNA-21 inhibitor, and NC groups did not differentiate into functional cardiomyocytes.
Figure 6.
The relative gene expression levels of GATA-4, MEF2c, TNI, and β-MHC in blank, negative control, miRNA-21 mimic, and miRNA-21 inhibitor groups (A – GATA-4; B – MEF2c; C – TNI; D – β-MHC). Neither miRNA-21 mimics nor miR-21 inhibitors influenced the expression of GATA-4, MEF2c, TNI, or β-MHC.
Discussion
For the first time, we investigated the role of miRNA-21 in migration, proliferation, and differentiation of Sca-1+ CSCs by regulating the expression level of miRNA-21. Our overall results mainly demonstrated that by up-regulating the expression level of miRNA-21, the migration and proliferation of Sca-1+ CSCs were enhanced, which thereby could promote the capacity of Sca-1+ CSCs to repair damaged myocardium.
In the past the heart was considered as an organ incapable of regenerating, with only terminally differentiated cardiomyocytes [17]. However, recent evidence has suggested that the heart still has a limited capability of regenerating by activating and recruiting a stem/progenitor cell population that is resident in human and murine hearts [18]. After isolation, culture, and identification of Sca-1+ CSCs, we found that Sca-1+ CSCs accounted for more than 87.4% of CSCs. Liu et al. reported that an estimated proportion of Sca-1+ CSCs was 83.5±1.8% [19].
Our results also showed that, after transfection, the miRNA-21 mimic group had the highest expression level of miRNA-21, while the miRNA-21 inhibitor group had the lowest expression level of miRNA-21. miRNA mimics (miRNA precursor molecules) are small, chemically modified double-stranded RNA molecules that are adapted to mimic endogenous mature miRNAs, which allows miRNA functional experiments through an increase in miRNA activity. miRNA inhibitors are chemically modified, single-stranded nucleic acids that are specifically adapted to bind to and inhibit endogenous miRNA molecules, which allows loss-of-function analysis by the down-regulation of miRNA activity [20].
Interestingly, the most important result in our study was that miRNA-21 can promote the migration and the proliferation of Sca-1+ CSCs in vitro, while no significant role of miRNA-21 was identified in the differentiation of Sca-1+ CSCs in vitro. miRNA-21 is a candidate to prevent heart damage by controlling the proteins programming cell death and some recognized cardioprotective mediators such as endothelial nitric-oxide synthase, HSP-70, activator protein-1, and heat shock transcription factor-1 [21,22]. Up-regulation of miRNA-21 also promotes cell survival by inhibiting phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which is essential for the activation of the prosurvival Akt kinase pathway, and inhibiting miRNA-21 elevates PTEN expression in cardiac fibroblasts, while miRNA-21 oligonucleotide suppresses it [23]. miRNA-21 can regulate the expression level of matrix metalloproteinase-2 through PTEN, indicating the potential role of miRNA-21 in the heart [24]. Richart et al. previously reported the therapeutic potential of miRNA-21 in ischemic diseases and found that miR-21-deficient mice presented a number of impaired infiltrated monocytes, with a defective angiogenic phenotype [25]. Collectively, these findings support that up-regulation of miRNA-21 can enhance capacity of Sca-1+ CSCs to repair damaged myocardium by inducing migration and proliferation of Sca-1+ CSCs in vitro, which may pave the way for therapeutic strategies directed toward restoring miRNA-21 function for heart-related diseases. Purvis et al. published a review article in which they stated that the use of miRNAs to CSCs to treat myocardial infarction was a newly emerging treatment strategy to regulate the proliferation and differentiation of CSCs [13]. Menasche et al. reported that if the benefit of stem cell transplantation could be recapitulated through the broad administration of their secretome, these stem cells could be exclusively utilized in vitro for producing the factors that would be the only therapeutics given to patients with heart failure [26]. As for the effects of miRNA-21 on the differentiation of Sca-1+ CSCs in vitro, our study found that under the microscope, Sca-1+ CSCs in the blank, the miRNA-21 mimic, the miRNA-21 inhibitor, and the NC groups did not differentiate into functional cardiomyocytes. Differentiation of stem cells into cardiomyocytes is one of the major challenges of cardiovascular regenerative medicine [27]. Nuclear transport of transcription factors is a critical step in stem cell commitment to a tissue-specific lineage [28]. Dai et al. and Arminan et al. found that the overexpression of GATA4 MEF2c, TNI, and β-MHC, all of which are important cardiac transcription factors, could physically and synergistically activate cardiac gene expression [15,16]. These factors drive the cardiac genetic program in embryonic stem cells, as well as adult stem cells recapitulating the molecular mechanism that functions during embryonic heart development [28,29]. In our study, neither miRNA-21 mimics nor miR-21 inhibitors influenced the expression levels of GATA-4, MEF2c, TNI, or β-MHC.
Conclusions
Our study provides evidence that the up-regulation of miRNA-21 has great potential to promote migration and proliferation of Sca-1+ CSCs to enhance capacity of Sca-1+ CSCs to repair damaged myocardium, which may pave the way for therapeutic strategies directed toward restoring miRNA-21 function for heart-related diseases. Nevertheless, we suggest that more prospective studies with larger sample sizes be conducted to provide more precise estimates to confirm the effects of miRNA-21 on the differentiation of Sca-1+ CSCs in vitro, to get closer to the goal of developing new genetic therapeutic strategies for the treatment of heart diseases.
Acknowledgements
We would like to acknowledge the helpful comments from our reviewers on this paper.
Footnotes
Source of support: Departmental sources
Competing interests
We declare that we have no conflicts of interest.
References
- 1.Hosseinkhani H, Hosseinkhani M, Hattori S, et al. Micro and nano-scale in vitro 3D culture system for cardiac stem cells. J Biomed Mater Res A. 2010;94:1–8. doi: 10.1002/jbm.a.32676. [DOI] [PubMed] [Google Scholar]
- 2.Bearzi C, Leri A, Lo Monaco F, et al. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci USA. 2009;106:15885–90. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.D’Amario D, Fiorini C, Campbell PM, et al. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108:857–61. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Gu H, Yu Q, Manukyan MC, et al. Sca-1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF-1 following myocardial ischemia/reperfusion. PLoS One. 2011;6:e29246. doi: 10.1371/journal.pone.0029246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai T, Matsuura K, Komuro I. Cardiac side population cells and Sca-1-positive cells. Methods Mol Biol. 2013;1036:63–74. doi: 10.1007/978-1-62703-511-8_5. [DOI] [PubMed] [Google Scholar]
- 6.Shi C, Li Q, Zhao Y, et al. Stem-cell-capturing collagen scaffold promotes cardiac tissue regeneration. Biomaterials. 2011;32:2508–15. doi: 10.1016/j.biomaterials.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Bras-Rosario L, Matsuda A, Pinheiro AI, et al. Expression profile of microRNAs regulating proliferation and differentiation in mouse adult cardiac stem cells. PLoS One. 2013;8:e63041. doi: 10.1371/journal.pone.0063041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baglio SR, Devescovi V, Granchi D, Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527:321–31. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8:706–13. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37:918–25. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 11.Jazbutyte V, Thum T. MicroRNA-21: from cancer to cardiovascular disease. Curr Drug Targets. 2010;11:926–35. doi: 10.2174/138945010791591403. [DOI] [PubMed] [Google Scholar]
- 12.Williams AR, Hatzistergos KE, Addicott B, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–23. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purvis N, Bahn A, Katare R. The Role of MicroRNAs in Cardiac Stem Cells. Stem Cells Int. 2015;2015:194894. doi: 10.1155/2015/194894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi XP, Zong AN. Study of instructive notions with respect to caring for laboratory animals. Journal of China Medical University. 2007;36:493–93. [Google Scholar]
- 15.Arminan A, Gandia C, Garcia-Verdugo JM, et al. Cardiac transcription factors driven lineage-specification of adult stem cells. J Cardiovasc Transl Res. 2010;3:61–65. doi: 10.1007/s12265-009-9144-3. [DOI] [PubMed] [Google Scholar]
- 16.Dai YS, Cserjesi P, Markham BE, Molkentin JD. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J Biol Chem. 2002;277:24390–98. doi: 10.1074/jbc.M202490200. [DOI] [PubMed] [Google Scholar]
- 17.Anversa P, Leri A. Innate regeneration in the aging heart: healing from within. Mayo Clin Proc. 2013;88:871–83. doi: 10.1016/j.mayocp.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chimenti I, Gaetani R, Barile L, et al. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol Biol. 2012;879:327–38. doi: 10.1007/978-1-61779-815-3_19. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Wang Y, Du W, Yu B. Sca-1-positive cardiac stem cell migration in a cardiac infarction model. Inflammation. 2013;36:738–49. doi: 10.1007/s10753-013-9600-8. [DOI] [PubMed] [Google Scholar]
- 20.Tong BD, Xiao MY, Zeng JX, Xiong W. MiRNA-21 promotes fibrosis in orbital fibroblasts from thyroid-associated ophthalmopathy. Mol Vis. 2015;21:324–34. [PMC free article] [PubMed] [Google Scholar]
- 21.Dong S, Cheng Y, Yang J, et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–25. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. 2009;104:572–75. doi: 10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy S, Khanna S, Hussain SR, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol. 2011;80:558–64. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richart A, Loyer X, Neri T, et al. MicroRNA-21 coordinates human multipotent cardiovascular progenitors therapeutic potential. Stem Cells. 2014;32:2908–22. doi: 10.1002/stem.1789. [DOI] [PubMed] [Google Scholar]
- 26.Menasche P. Stem cells for the treatment of heart failure. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0373. pii: 20140373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Laake LW, Hassink R, Doevendans PA, Mummery C. Heart repair and stem cells. J Physiol. 2006;577:467–78. doi: 10.1113/jphysiol.2006.115816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Terzic C, Faustino RS, Boorsma BJ, et al. Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S68–76. doi: 10.1038/ncpcardio0763. [DOI] [PubMed] [Google Scholar]
- 29.Arminan A, Gandia C, Bartual M, et al. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009;18:907–18. doi: 10.1089/scd.2008.0292. [DOI] [PubMed] [Google Scholar]