Abstract
Background
Transarterial chemoembolization (TACE) has been used alone or in combination with three-dimensional conformal radiation therapy (3DCRT) for treating hepatocellular carcinoma (HCC). The overall survival rate of HCC patients undergoing both treatments, however, has not been systematically studied. The aim of this meta-analysis-based study was to evaluate the overall efficacy of the combined therapy or monotherapy, thereby providing information for clinical treatment.
Material/Methods
We searched Google Scholar, PubMed, and Chinese National Knowledge Infrastructure (CNKI) for eligible studies, and a total of 17 case-control studies (including HCC patients treated by TACE plus 3DCRT or TACE alone) were included to perform the meta-analysis. Based on the available data, we assessed the improvements of 1-year, 2-year, and 3-year survival rate for the combination therapy of TACE and 3DCRT or TACE alone. Furthermore, the analysis was also stratified by the tumor response: complete response (CR), partial response (PR), no response (NR) and progressive disease (PD). Statistical analysis was performed using STATA 12 (Stata Statistical Software: Release 12).
Results
The results show that HCC patients receiving combination therapy have significantly increased overall survival rate when compared to those receiving TACE alone (1-year survival rate: OR=1.95, 95% CI 1.54–2.47, p=7.3×10−8; 2-year survival rate: OR=1.87, 95% CI 1.49–2.34, p=1.6×10−7; 3-year survival rate: OR=2.00, 95% CI 1.52–2.64, p=1.8×10−6).
Conclusions
Assessment of tumor response demonstrates that the combination therapy can efficiently increase the tumor response rate (CR+PR: OR=2.29, 95% CI 1.70–3.08, p=1.1×10−7), with a lower rate of subsequent tumor development (PD: OR=0.25, 95% CI 0.15–0.40, p=5.5×10−8).
MeSH Keywords: Carcinoma, Hepatocellular; Chemoembolization, Therapeutic; Meta-Analysis
Background
Hepatocellular cancer (HCC), a primary malignancy of the liver, is one of the most common malignancies worldwide, especially in East Asian countries. For example, China, which has the highest overall mortality rate as well as a high incidence, has around 35 cases per 100 000 persons [1]. Numerous treatment strategies, including surgical resection, which is a widely accepted mainstay therapy, have been developed for HCC treatment [2]. Resection is able to remove the part of the liver that contains the hepatocellular carcinoma or malignant hepatoma surgically, and it largely depends on the size, number, and locations of the tumors [3]. For instance, hepatic resection is not suitable for patients with large tumors (>3 cm) or extensive disease progression [3]. Moreover, surgery is frequently accompanied by recurrence due to the intrahepatic dissemination of the multicentric carcinoma [3]. Among the various treatment strategies, TACE was developed for treating unresectable hepatocellular tumors or intermediate-stage HCC, thereby providing an additional option for patients [4,5]. TACE is a minimally invasive, real-time, image-guided technology that prevents tumor progression and improves survival through inducing tumor necrosis, which represses tumors locally. Compared with traditional surgery, TACE has lower risk of complications and takes less recovery time [4,5].
It has been documented that TACE frequently becomes ineffective due to tumor regression, even if TACE is currently considered as one of the primary approaches, especially for large tumors and metastatic liver cancer [6,7]. Therefore, it is necessary to develop other treatment options for favorable clinical outcomes. Previous work has demonstrated that three-dimensional conformal radiation therapy (3DCRT) is an effective adjunct for parallel treatment for HCC [8]. 3DCRT is a complex technology for creating a digitally reconstructed 3-D image of the tumor and normal adjacent tissue based on computers in conjunction with CT scans, initially developed to provide precise data for targeting the tumor and to reduce adverse events of other traditional treatments, such as radiotherapy [9]. To date, numerous clinical trials have been conducted to assess the efficacy of the combination of 3DCRT and TACE for HCC [10–26], and these studies have provided considerable evidence that the combination therapy is beneficial for HCC, whereas it remains unclear whether the combination therapy can improve overall patient survival. A previous meta-analysis [27] including several clinical trials has evaluated the efficacy of the combination of TACE and radiation therapy or TACE alone, but it did not specifically focus on TACE and 3DCRT. In the present study, we performed a meta-analysis to evaluate the efficacy of the combination of 3DCRT and TACE on the overall survival for HCC patients, thereby providing a reference for HCC therapy.
Material and Methods
Data collection
PubMed, Google Scholar, and Chinese National Knowledge Infrastructure (CNKI) databases were searched with the following terms: “hepatocellular cancer”, “transarterial chemoembolization”, and “Three-dimensional conformal radiation therapy”. The search included studies published before September 2014. A total of 81 results (8 from PubMed, 58 from Google Scholar, and 15 from CNKI) were retrieved. Only studies meeting the following inclusion criteria were pooled for our meta-analysis: (i) case-control studies that focused on hepatocellular cancer and TACE/3dCRT therapy; (ii) source information available for both case and control groups; (iii) the diagnosis of hepatocellular cancer confirmed by clinical examinations; (v) studies published in English. The exclusion criteria were: (i) irrelevant and duplicated articles; (II) study without case-control information; (iii) survival rate and tumor response information was unavailable; (iv) article type such as meta-analysis, letter, or editorial. A total of 17 studies were analyzed in our meta-analysis. From eligible articles, the following data were collected: the first author’s last name, year of publication, number of patients, overall survival rate (1-, 2-, and 3-year survival rates), and tumor response information. The procedure for study collection is shown in Figure 1.
Figure 1.
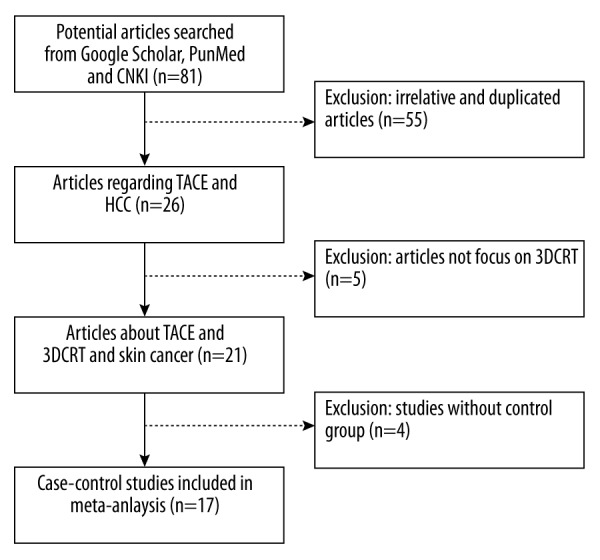
Flow diagram of eligible studies included in the meta-analysis.
Statistical methods
In this study, STATA software (Release 12 College Station, TX: StataCorp LP) was used for data analyses. The improvement between case group (the combination therapy of 3DCRT and TACE) and control group (TACE alone) was assessed by pooled odds ratios (ORs) and 95% confidence intervals (CIs). Three overall survival rates (1-, 2-, and 3-year survival rates) were used to evaluate the efficacy. Subgroup analysis was conducted with respect to tumor response (complete response, partial response, no response, and progressive disease). The heterogeneity was assessed by I2 index; a higher I2 indicates more significant heterogeneity. When I2≤25%, we assumed that there was no significant heterogeneity between pooled data. I2>75% was regarded as significant heterogeneity. We used a Mantel-Haenszel (M-H) fixed-effects model for calculation unless there was a significant heterogeneity, in which case a DerSimonian and Laird (D-L) random-effects model was used. In our meta-analysis, we used an M-H fixed-effects model to test the heterogeneity first, and then we chose a different model based on the test results. Forest plots were generated to summarize the results. Begg’s funnel plots and Egger’s regression asymmetry test were used to evaluate potential publication bias. All reported P values were two-sided, and P<0.05 was considered to be statistically significant.
Results
Characteristics of studies
A total of 17 eligible case-control studies [10–26] were included for our meta-analysis, and the characteristics of these studies are shown in Table 1. A total of 1652 patients that had been randomized into combined or monotherapy groups were enrolled in this analysis, and among them, 654 cases received the combination therapy of TACE and 3DCRT (case group) and 998 received TACE alone (control group). The overall survival rates are presented in Table 1 with period length from 1 year to 3 years. Irrespective of the None-Applicable cases (N/A cases), in case groups, the 1-year survival rate ranged from 32.5% to 86.7%, 2-year survival rate ranged from 20.8% to 60%, and 3-year survival rate ranged from 25% to 34%. In control groups, the 1-year survival rate ranged from 25% to 78%, 2-year survival rate ranged from 12.5% to 44%, and 3-year survival rate ranged from 6.3% to 35%.
Table 1.
Characteristics of studies included in the meta-analysis.
| Author | Year | Survival rate (%) in (TACE+3dCRT)/TACE | No. of patients | Tumor response in TACE | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1-year | 2-year | 3-year | CR | PR | NR | PD | |||
| Lu | 2014 | 62.4/56.5 | 20.8/18.8 | N/A | 30/33 | 5/5 | 16/10 | 6/11 | 3/7 |
| Gong | 2011 | 79.2/58.3 | 54.2/33.3 | 25/16.7 | 24/24 | 1/0 | 16/13 | 5/8 | 2/3 |
| Xiao | 2011 | 45/25 | 27.5/12.5 | N/A | 40/40 | 6/4 | 24/14 | 8/14 | 2/8 |
| Ning | 2009 | 61.8/46.9 | 41.2/18.8 | 29.4/6.3 | 34/32 | 0/0 | 26/15 | 5/11 | 3/6 |
| Zhao | 2009 | 95/78 | N/A | N/A | 40/50 | 7/4 | 28/30 | 4/12 | 1/4 |
| Zhang | 2009 | 32.5/6.9 | N/A | N/A | 16/29 | N/A | N/A | N/A | N/A |
| Xiao | 2008 | 86.7/53.3 | 53.3/36.7 | 33.3/16.7 | 30/30 | 5/1 | 22/18 | 2/7 | 1/4 |
| Shang | 2007 | 78/50 | 60/32 | 34/18 | 40/36 | 14/1 | 16/12 | 8/7 | 2/9 |
| Chung | 2006 | 65/58 | 35/44 | 26/35 | 64/276 | N/A | N/A | N/A | N/A |
| Zhao | 2006 | 82/55 | 63/28 | 43/15 | 49/47 | 17/8 | 18/11 | 9/17 | 5/11 |
| Liu | 2005 | 66.5/53.9 | 48.4/37.2 | 37.4/17.8 | 54/60 | N/A | N/A | N/A | N/A |
| Lan | 2005 | 57.1/61.7 | 40.5/30 | 26.2/16.7 | 42/60 | 3/0 | 20/22 | 19/38 | 0/0 |
| Shim | 2005 | N/A | 36.8/14.3 | N/A | 38/35 | 0/0 | N/A | N/A | 1/8 |
| Zeng | 2004 | 71.5/59.6 | 42.3/26.5 | 24/11.1 | 54/149 | 3/1 | 38/45 | 13/68 | 0/35 |
| Wu | 2004 | 90.2/89.7 | 75.6/58.7 | 44.6/24 | 41/40 | 7/4 | 28/22 | 4/6 | 2/8 |
| Li | 2003 | 73.2/54.8 | 58.7/27.3 | 41.9/12.8 | 41/41 | 4/1 | 32/23 | 3/14 | 2/3 |
| Cheng J | 2001 | N/A | 58/56 | N/A | 17/16 | N/A | N/A | N/A | N/A |
The results for tumor response are shown in Table 1, which contain 4 types of responses: complete response (CR, complete clearance of the lesion), partial response (PR, lesion decreased more than 50%), no response (NR, lesion decreased less than 50% or increased less than 25%), and progressive disease (PD, lesion increased more than 25%). Specifically, despite the N/A cases, 72, 309, 98, and 24 of 654 patents had CR, PR, NR, and PD, respectively, in case groups and the percentage in each arm was 11.01%, 47.25%, 14.98%, and 3.67%, respectively. However, 29, 235, 213, and 106 of 998 patients had CR, PR, NR, and PD, respectively, in control groups, and the percentage in each arm was 2.91%, 23.55%, 21.34%, and 10.62%, respectively. Based on the data, the patients in case groups had higher percentages in CR and PR categories, but lower percentages in NR and PD compared with control groups.
Survival rates
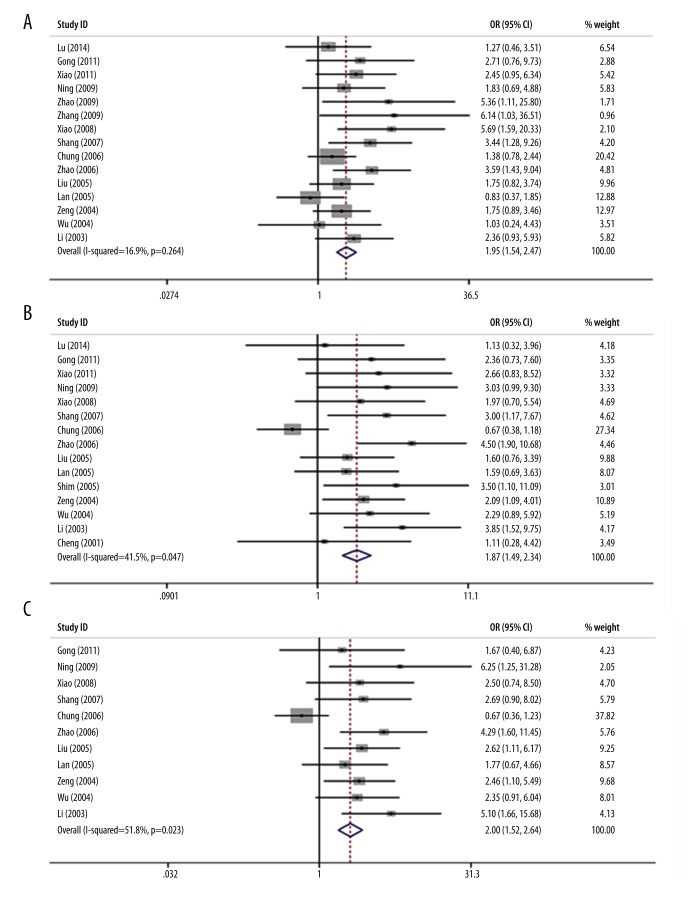
We further compared the survival rates between case and control groups and the results are shown in Table 2. We used random-effects models, and the 1-, 2-, and 3-year survival rates were analyzed. Overall, we found significant survival rate improvement for the combination of TACE and 3DCRT over TACE alone. Specifically, the combination of TACE plus 3DCRT was associated with higher 1-year (OR=1.95, 95% CI 1.54–2.47, p=7.3×10−8), 2-year (OR=1.87, 95% CI 1.49–2.34, p=1.6×10−7), and 3-year survival rates (OR=2.00, 95% CI 1.52–2.64, p=1.8×10−6) compared with TACE treatment. These results indicate that the HCC patients with the additive treatment of 3DCRT generally had nearly 2-fold better survival rate than those who received TACE treatment alone. Also, we noted that the largest overall OR occurred in 3-year survival rate, implying that this advantage becomes more apparent with longer observation. Forest plots for survival rates are shown in Figure 2.
Table 2.
Meta-analysis of entire database with respect to different survival rates.
| Survival rate | No. of studies | Analysis method | Heterogeneity | OR | Publication bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | p-value# | Overall | Lower | Upper | p-value* | Begg | Egger | |||
| 1-year | 15 | Fixed | 16.9 | 0.264 | 1.95 | 1.54 | 2.47 | 7.3×10−8 | 0.075 | 0.028 |
| 2-year | 15 | Fixed | 41.5 | 0.047 | 1.87 | 1.49 | 2.34 | 1.6×10−7 | 0.921 | 0.075 |
| 3-year | 11 | Fixed | 51.8 | 0.023 | 2.00 | 1.52 | 2.64 | 1.8×10−6 | 0.436 | 0.013 |
P-value from heterogeneity test;
P-value from OR test.
Figure 2.
Forest plot of meta-analysis for the comparison of the combined therapy with TACE alone in terms of overall survival rates. (A) Meta-analysis of 1-year survival. (B) Meta-analysis of 2-year survival. (C) Meta-analysis of 3-year survival.
Tumor response
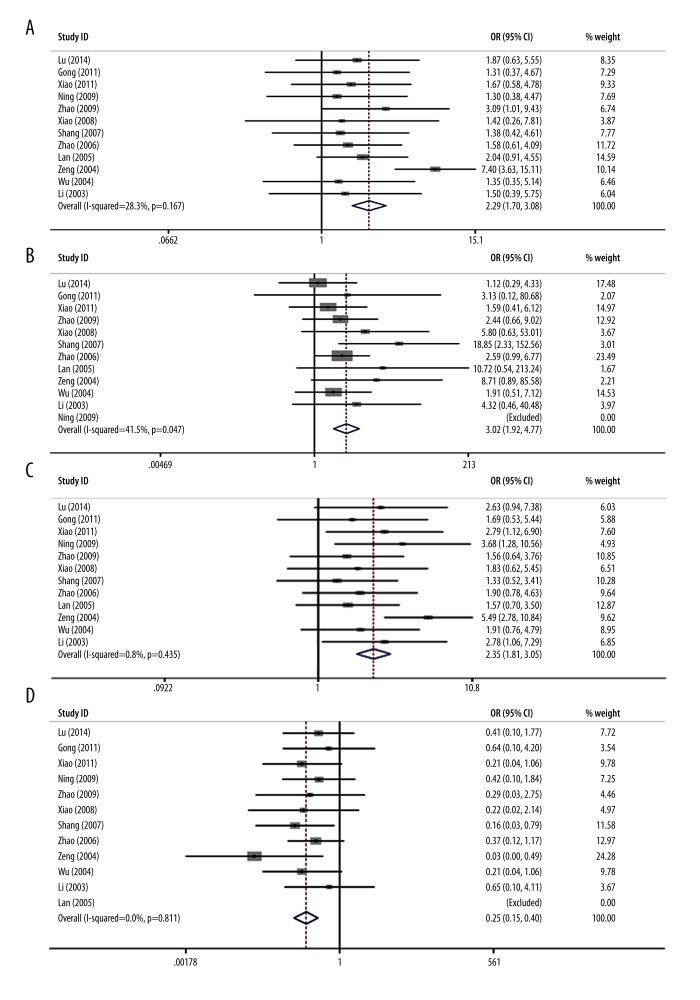
We performed tumor response analysis, and results are shown in Table 2 and Figure 3. Twelve trials were identified with outcome measurements of CR+PR. The results of pooled analysis showed a significant improvement of the tumor response rate in the case group (OR=2.29, 95% CI 1.70–3.08, p=1.1×10−7) compared with the control group (Figure 3A). Specifically, the case group had significant improvement of CR (OR=3.02, 95% CI 1.92–4.77, p=5.0×10−6) in 11 trials (Figure 3B). Similarly, significant improvement of PR was also noticed in the patients receiving combined treatment (OR=2.35, 95% CI 1.81–3.05, p=4.7×10−10) (Figure 3C) in 12 trials. However, patients treated with additive 3DCRT presented a significantly decreased progressive disease rate compared with those receiving TACE alone (PD: OR=0.25, 95% CI 0.15–0.40, p=5.5×10−8) (Figure 3D). All these analyses demonstrated that the combination of TACE and 3DCRT was more effective than TACE alone for the HCC treatment.
Figure 3.
Forest plot of meta-analysis for the comparison of the combined therapy with TACE alone on tumor response. (A) Meta-analysis of CR+PR. (B) Meta-analysis of CR. (C) Meta-analysis of PR. (D) Meta-analysis of PD.
Evaluation of heterogeneity and publication bias
Statistically significant heterogeneity was observed in the 2- and 3-year survival rates, since the I2 indexes were larger than 25%, with P-value less than 0.05 (Table 2). However, no significant heterogeneity was found in the analyses of tumor response (Table 3). Funnel plot and Egger’s test were performed to assess publication bias (Figure 4). No significant publication bias was found based on the funnel plot of 2-year survival rates (P-value of Egger’s test >0.05), but significant publication bias was shown in 1- and 3- year survival rates (P-value of Egger’s test=0.028 and 0.013, respectively (Table 2). For tumor response, the publication bias was found in the cases of CR+PR and CR, with Egger’s test P-value of 0.008 and 0.039, respectively, and the funnel plot for the tumor response is shown in Figure 5.
Table 3.
Meta-analysis of entire database for tumor response.
| Tumor response | No. of studies | Analysis method | Heterogeneity | OR | Publication bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | p-value# | Overall | Lower | Upper | p-value* | Begg | Egger | |||
| CR+PR | 12 | Fixed | 28.3 | 0.167 | 2.29 | 1.70 | 3.08 | 1.1×10−7 | 0.193 | 0.008 |
| CR | 11 | Fixed | 0.0 | 0.580 | 3.02 | 1.92 | 4.77 | 5.0×10−6 | 0.350 | 0.039 |
| PR | 12 | Fixed | 0.8 | 0.435 | 2.35 | 1.81 | 3.05 | 4.7×10−10 | 0.837 | 0.151 |
| PD | 11 | Fixed | 0.0 | 0.811 | 0.25 | 0.15 | 0.40 | 5.5×10−8 | 0.586 | 0.165 |
P-value from heterogeneity test;
P-value from OR test.
Figure 4.
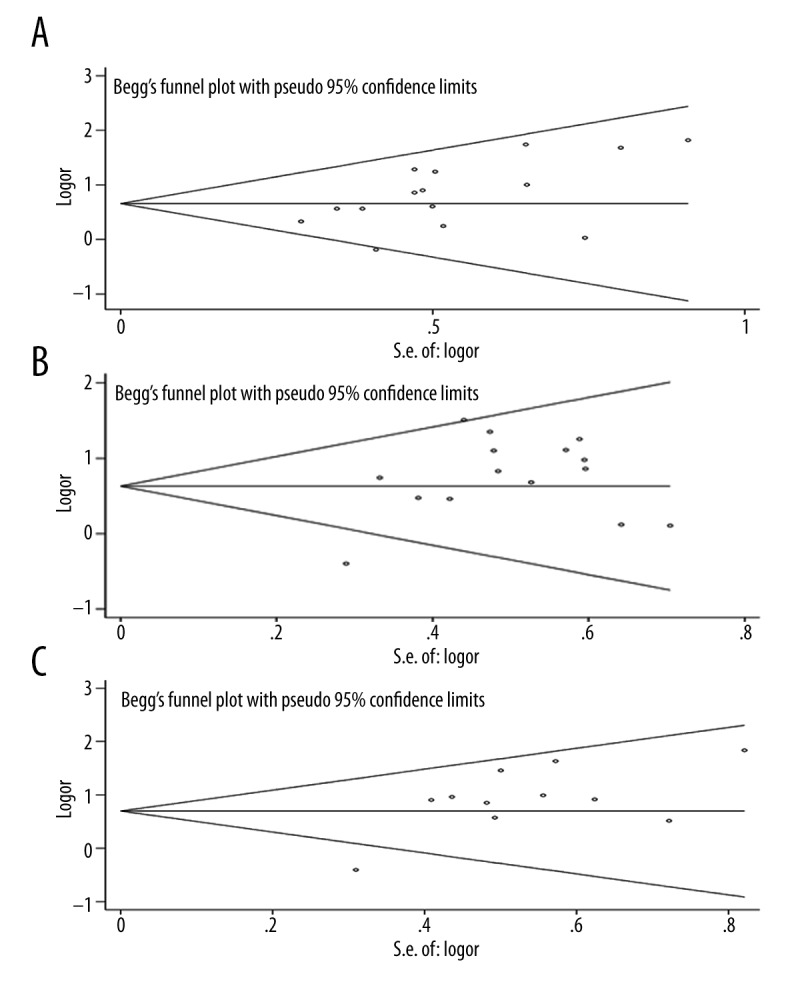
Begg’s funnel plot of meta-analysis for the comparison of the combined therapy with TACE alone in terms of overall survival rates. (A) One-year survival. (B) Two-year survival. (C) Three-year survival.
Figure 5.
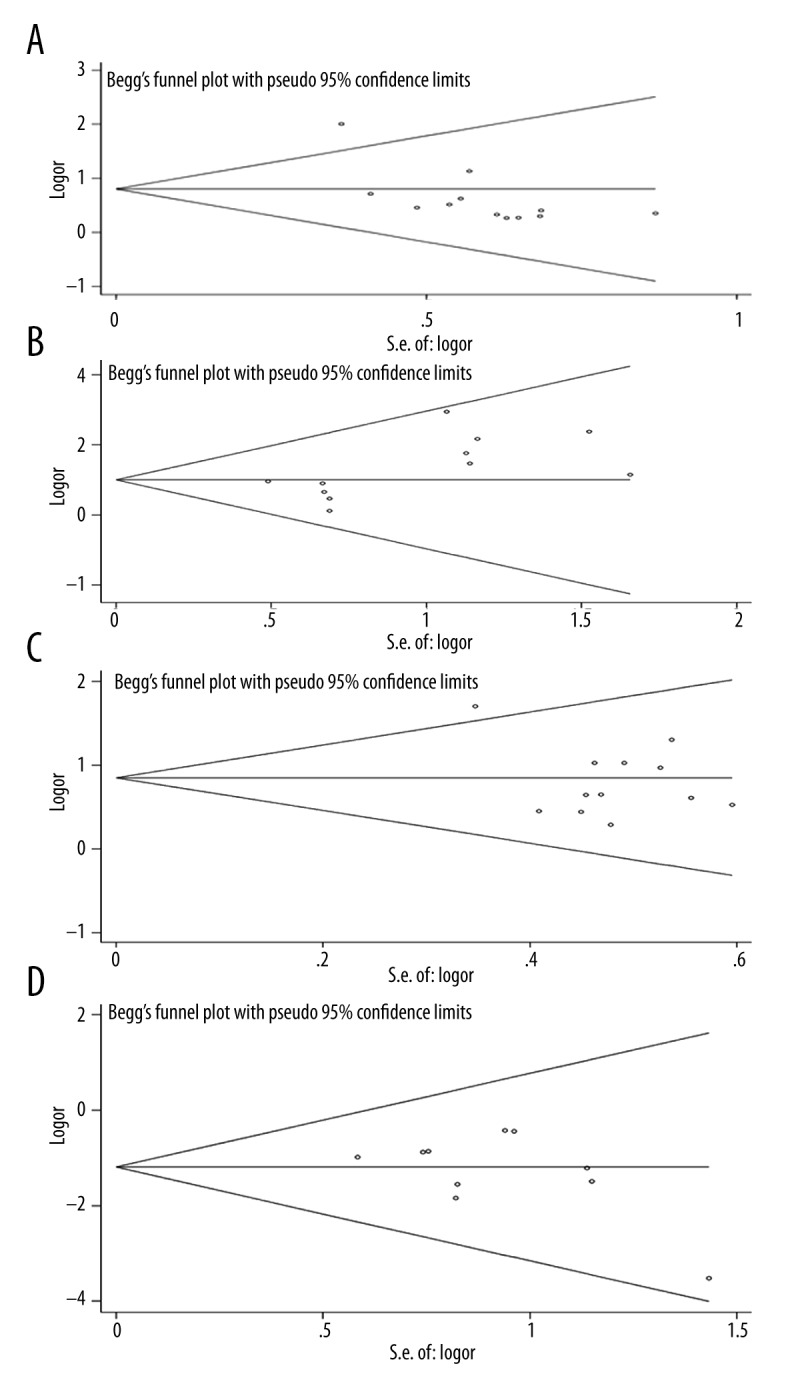
Begg’s funnel plot of meta-analysis for the comparison of the combined therapy with TACE alone on tumor response. (A) CR+PR. (B) CR. (C) PR. (D) PD.
Discussion
In this study we performed a meta-analysis based on the published data to compare the overall survival rates and tumor responses for patients who received the combination therapy of TACE and 3DCRT or TACE alone. TACE is the most commonly offered treatment, which is performed clinically by delivering a highly concentrated dose of chemotherapeutic drugs, including doxorubicin, cisplatin, or mitomycin, into the tumor through cannulating a feeding artery to reduce systemic toxicity [28,29]. In addition to TACE and the hepatectomy mentioned above, which are frequently utilized to treat HCC, radiotherapy is also an option for HCC treatment. Although radiotherapy has been used as an effective therapy for HCC for over 40 years, liver toxicity from the ionizing radiation limits its use [30]. The newly developed technology, 3DCRT, significantly and accurately minimizes the normal tissues exposed to irradiation during the treatment in combination with radiotherapy, although the application of radiotherapy still largely depends on the individual tolerance of the liver to radiation-induced damage [9]. The combined treatment, 3DCRT plus TACE, was thus designed and used clinically for patients with unresectable HCC, as well as those who are sensitive to irradiation. However, there has been less research on whether the introduction of 3DCRT into TACE is able to substantially increase the overall survival rate of HCC patients.
In spite of the heterogeneity and publication bias, the present study shows that the strategy of combination therapy exhibited distinctly higher survival rates than the monotherapy, which is consistent with a previous study [27]. Specifically, in our study, improvements of survival rates were observed at 1 year (OR=1.95, 95% CI 1.54–2.47), 2 years (OR=1.87c, 95% CI 1.49–2.34), and 3 years (OR=2.00, 95% CI 1.52–2.64) after treatment with TACE+3DCRT for 654 patients. The increased survival rates were also supported by more positive tumor responses in the combination therapy. The assessment of tumor response demonstrated that combined treatment was able to increase the tumor response rate (CR+PR: OR=2.29, 95% CI 1.70–3.08), and simultaneously decrease the rate of further development of tumor tissue (PD: OR=0.25, 95% CI 0.15–0.40). Thus, the 2 clinical efficacies indicated a better outcome with the combination therapy. Importantly, heterogeneity of 2-year survival rate was moderate (I2=41.5%), and the I2 index of 3-year survival rate was over 50% (51.8%). The value of I2 index between 25% and 75% usually cannot be directly interpreted as significant heterogeneity. In this scenario, P-value is used to assess heterogeneity because it yields more information (e.g., sample size, case-control proportion, and, more importantly, data from examination). Here, since the P-values for 2- and 3-year survival rates are 0.047 and 0.023, respectively, and both of them are larger than 0.01, we assume the heterogeneity is acceptable.
There are several limitations that may affect the reliability of the conclusions regarding the positive effects of TACE plus 3DCRT on patients in these trials. Firstly, the data in this study were restricted to the population of China, although the restriction was unexpected prior to the analysis; therefore, the results are probably applicable with ethnic specificity. Secondly, publication bias was shown in 1- and 3- year survival rates (P-value of Egger’s test was 0.028 and 0.013, respectively) and the tumor responses CR+PR and CR (P-value of Egger’s test was 0.008 and 0.039, respectively). Lastly, factors such as age, sex, genetic background, and tumor size were not considered during the analysis. Given these limitations, a well-designed meta-analysis with large sample size among different ethnicities needs to be performed. The results of our study should be interpreted with caution.
Conclusions
Our meta-analysis of 17 studies including total 1652 patients suggests survival and tumor response advantages of combined therapy of TACE plus 3DCT over TACE alone. We believe our study may help further HCC treatment research.
Footnotes
Source of support: Departmental sources
References
- 1.Xu H, He Y-T, Zhu J-Q. Liver cancer mortality trends during the last 30 years in Hebei province: Comparison results from provincial death surveys conducted in the 1970’s, 1980’s, 1990’s and 2004–2005. Asian Pac J Cancer Prev. 2012;13(5):1895–99. doi: 10.7314/apjcp.2012.13.5.1895. [DOI] [PubMed] [Google Scholar]
- 2.Heinzow HS, Brockmann JG, Köhler M, et al. Liver transplantation versus supraselective transarterial chemoembolization in palliative patients with hepatocellular carcinoma exceeding the Milan Criteria – is it time for a more individual approach? Ann Transplant. 2013;18:515–24. doi: 10.12659/AOT.884018. [DOI] [PubMed] [Google Scholar]
- 3.Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62(2):440–51. doi: 10.1002/hep.27745. [DOI] [PubMed] [Google Scholar]
- 4.Murata S, Mine T, Sugihara F, et al. Interventional treatment for unresectable hepatocellular carcinoma. World J Gastroenterol. 2014;20(37):13453–65. doi: 10.3748/wjg.v20.i37.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng YC, Chen TW, Fan HL, et al. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014;19:309–16. doi: 10.12659/AOT.890505. [DOI] [PubMed] [Google Scholar]
- 6.Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253(3):453–69. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 7.Yu YQ, Xu DB, Zhou XD, et al. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer. 1993;71(1):62–65. doi: 10.1002/1097-0142(19930101)71:1<62::aid-cncr2820710111>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Tse RV, Guha C, Dawson LA. Conformal radiotherapy for hepatocellular carcinoma. Crit Rev Oncol Hematol. 2008;67(2):113–23. doi: 10.1016/j.critrevonc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Merle P, Mornex F, Trepo C. Innovative therapy for hepatocellular carcinoma: three-dimensional high-dose photon radiotherapy. Cancer Lett. 2009;286(1):129–33. doi: 10.1016/j.canlet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Liu MZ, Wang XS, Cai L, et al. [External radiation and combined transcatheter arterial chemoembolization for unresectable primary liver cancer]. Ai Zheng. 2005;24(1):82–86. [in Chinese] [PubMed] [Google Scholar]
- 11.Shim SJ, Seong J, Han KH, et al. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25(6):1189–96. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 12.Zeng ZC, Tang ZY, Fan J, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10(5):307–16. doi: 10.1097/00130404-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Meihong Z, Fengping L, Qi’an J, et al. Three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for inoperable primary liver cancer. Chin J Radiat Oncol. 2006;15:39–41. [Google Scholar]
- 14.Lan D, Gong X, Wei X. The efficacy analysis of transcatheter hepatic arterial chemoembolization combined with radiotherapy for primary liver cancer. Chin J Radiat Oncol. 2005;14:152–53. [Google Scholar]
- 15.Li Y, Ying Y, Haibo Z, et al. Threedimensional conformal radiation combined with transarterial chemoembolization for unresectable primary liver cancer. Chin J Radiat Onco. 2003;12:30–32. [Google Scholar]
- 16.Shang Y, et al. Prospective randomized clinical study of transcatheter arterial chemoembolization, combined with three-dimensional conformal radiotherapy for primary liver cancer: An analysis of 40 cases. World Chin J Digestology. 2007;15:3140–42. [Google Scholar]
- 17.Wu DH, Zhi FZ, Chen LH. Evaluating the efficacy of transcatheter arterial chemoembolization combined with hypofractionated 3-dimensional conformal radiotherapy for hrpstocellular carcinoma. Chin J Dig. 2004;24:353–59. [Google Scholar]
- 18.Chung YL, Jian JJ, Cheng SH, et al. Sublethal irradiation induces vascular endothelial growth factor and promotes growth of hepatoma cells: implications for radiotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006;12(9):2706–15. doi: 10.1158/1078-0432.CCR-05-2721. [DOI] [PubMed] [Google Scholar]
- 19.Lu DH, Fei ZL, Zhou JP, et al. A comparison between three-dimensional conformal radiotherapy combined with interventional treatment and interventional treatment alone for hepatocellular carcinoma with portal vein tumour thrombosis. J Med Imaging Radiat Oncol. 2015;59(1):109–14. doi: 10.1111/1754-9485.12207. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XB, Wang JH, Yan ZP, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115(6):1245–52. doi: 10.1002/cncr.24139. [DOI] [PubMed] [Google Scholar]
- 21.Chia-Hsien Cheng J, Chuang VP, Cheng SH, et al. Unresectable hepatocellular carcinoma treated with radiotherapy and/or chemoembolization. Int J Cancer. 2001;96:243–52. doi: 10.1002/ijc.1022. [DOI] [PubMed] [Google Scholar]
- 22.Qiao-ying G, Jin-gao L, Gong-li K. Effects of transcatheter arterial chemoembolization (TACE) verse TACE combined with three dimensional radiation (3-DCRT) in the treatment of patients with primary hepatocellular carcinoma. Practical Journal of Cancer. 2011;26(6):634–36. [Google Scholar]
- 23.Sihai N, et al. Three-dimensional conforntal radiotherapy combined with transcatheterarterial chemoembolization for hepatocellular carcinome. Sichuan Medical Journal. 2009;30(12):1896–98. [Google Scholar]
- 24.Zemin X, et al. Transcatheter arterial chemoembolization combined with 3-dimensional conformal radiotherapy for patients with unresectable primary hepatic carcinoma. Chinese Journal of Clinical Oncology. 2008;35(1):18–21. [Google Scholar]
- 25.Peng X, et al. Effect comparison of TACE plus 3DCRT and TACE alone in the treatment of primary hepatic cancer. Journal of Basic and Clinical Oncology. 2011;24(6):506–7. [Google Scholar]
- 26.Zhao C, Ren Y, Wen S, et al. Analysis of the curative effect of interventional therapy plus 3 dimensional conformal radiation therapies for hepatic cell carcinoma. West China Medical Journal. 2009;24(7):1686–89. [Google Scholar]
- 27.Meng MB, Cui YL, Lu Y, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2009;92(2):184–94. doi: 10.1016/j.radonc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Wiggermann P, Sieron D, Brosche C, et al. Transarterial chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE) Med Sci Monit. 2011;17(4):189–95. doi: 10.12659/MSM.881714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258(2):627–34. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 30.Zou LQ, Zhang BL, Chang Q, et al. 3D conformal radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2014;20(45):17227–34. doi: 10.3748/wjg.v20.i45.17227. [DOI] [PMC free article] [PubMed] [Google Scholar]