Abstract
The growing demand for organ donors to supply the increasing number of patients on kidney waiting lists has led most transplant centers to develop protocols that allow safe use of organs from donors with special clinical situations previously regarded as contraindications. Deceased donors with previous hepatitis B may be a safe resource to increase the donor pool even if there is still controversy among transplantation centers regarding the use of hepatitis B surface antigen-positive donors for renal transplantation. However, when allocated to serology-matched recipients, kidney transplantation from donors with hepatitis B may result in excellent short-term outcome. Many concerns may arise in the long-term outcome, and studies must address the evaluation of the progression of liver disease and the rate of reactivation of liver disease in the recipients. Accurate selection and matching of both donor and recipient and correct post-transplant management are needed to achieve satisfactory long-term outcomes.
MeSH Keywords: Donor Selection, Hepatitis B Core Antigens, Hepatitis B Surface Antigens, Hepatitis B virus, Kidney Transplantation, Tissue Donors
Background
Kidney transplantation is now considered the best replacement therapy for patients with end-stage renal disease (ESRD).
The increasing demand for available organ donors for kidney transplantation and the continuing shortage of organs has led many transplant centers to expand their acceptance criteria by including deceased donors with special clinical situations, such as older donors, non-heart-beating donors, and donors with potentially transmittable infections [1–3].
One strategy focuses on the use of donor kidneys with viral hepatitis, and several organ procurement associations have adopted the policy of accepting deceased donor kidneys with hepatitis B (HBV) or C (HCV) virus infection. Anti-HCV-positive donors can be safely used for transplantation into HCV-positive recipients with no increased rate of post-transplant complications, but with a significant reduction of time on the waiting list for anti-HCV-positive ESRD patients [4–7]. In contrast, organs from donors with active HBV infection (HBsAg-positive) are less frequently accepted due to the high risk of transmission leading to primary infection [8]. In addition, kidney transplant recipients of organs from donors with evidence of HBV Infection (hepatitis B surface antigen-negative/anti-hepatitis B core-positive) may be at higher risk of primary infection following transplantation [8].
Preexisting HBV-infected renal recipients were not at higher risk of kidney failure or death, but they remain at higher risk of liver failure compared to HBV-negative recipients [9]. Before introduction of safe and effective antiviral therapy, most kidney transplant recipients with primary HBV infection rapidly progressed to liver fibrosis and failure and, finally, death.
This review focusses on the current status of kidney transplantation from HBV-positive donors, with a special focus on post-transplant graft and patient outcomes.
Kidney Transplantation From Hepatitis B Core Antibody (HBcAb)-Positive Donors
In endemic areas, the prevalence of HBcAb in the population of deceased organ donors is up to 24% [10,11]. The prevalence of HBV infection is different between countries with low rates (<2%) in Western Europe and the USA, intermediate rates (2–8%) in Mediterranean countries and Japan, and high rates (8–20%) in South Asia and Africa [12]. In about 10% of these individuals, HBV DNA is detected by polymerase chain reaction [13,14]. The use of renal allograft from these potential donors is crucial since excluding all of these donors may result in a significant decline in the global number of available renal allografts [1,15].
Although the presence of HBcAb in the blood of a potential organ donor may indicate an increased risk for HBV transmission to the recipient [16–18], this risk may be more accurately determined by measuring HBV-DNA than HBcAb, but HBV-DNA measurement is not routinely recommended in standard deceased donors. In an interesting study by Cirocco et al. [19], HBV-DNA measured by PCR was negative in all hepatitis B surface antigen (HBsAg)-negative anti-HBc-positive donors. Moreover, in the 9 transplanted kidneys that were tested, the kidney tissue was also negative for HBV-DNA, suggesting that HBcAb-positive HBV-DNA negative donors are unlikely to transmit infection to recipients.
Solid organ transplant recipients of HBcAb-positive organ donors, exposed to immunosuppression, may have a higher risk of developing de novo HBV infection after transplantation.
The risk of developing a de novo HBV infection after transplantation from HBcAb-positive donors may be up to 70% in liver transplant recipients [17,20,21], while the impact of HBcAb-positive donors in renal transplantation appears low to negligible [11,22,23]. However, some authors reported an increased risk of seroconversion or de novo HBV after kidney transplantation from HBcAb-positive deceased donors, particularly in HBcAb-positive recipients [24,25].
Experimental studies demonstrated that hepadna viruses have a strong preference for infecting liver cells rather than extra-hepatic organs [26], and this was confirmed in a clinical setting by Wachs et al. [18], who reported an HBcAb-positive multiorgan donor who transmitted HBV to a liver allograft recipient without apparent transmission to the 2 kidney recipients.
In a series of 356 kidney transplant recipients from HBcAb-positive donors, none of the recipients acquired HBsAg positivity, but 4/10 vaccinated patients seroconverted from HBcAb-negative to HBcAb-positive, without any clinical or biochemical signs of hepatitis [11].
In their recent review, Mahboobi et al. [15] analyzed the impact of HBcAb status of renal donors on viral transmission to recipients. The meta-analysis of 9 studies collecting a total of 1385 eligible kidney recipients showed that the total rate of seroconversion after renal transplantation was 3.24%; 4 patients seroconverted to HBsAg positive (rate of seroconversion 0.28%), 32 patients developed HBcAb after transplantation, and 2 became seropositive. None of these patients presented clinical signs of hepatitis, higher mortality, or decreased graft survival. In the study by Fong et al. [22], the incidence of anti-HBc conversion in recipients of anti-HBc-positive and anti HBc-negative kidneys was 0.011 and 0.005 per year, respectively. In other retrospective studies, the incidence of seroconversion varied between 0% and 27% [11,13,16,18,22–24,27], but these data must be interpreted cautiously because patients who are immunosuppressed may not develop detectable antibody levels post-transplant [16].
In our recent experience, among 42 kidney transplant recipients of a HBcAb-positive donor, none of the naïve or vaccinated recipients developed HBsAg seroconversion; however, 4 of 28 (14.2%) vaccinated patients seroconverted from HBcAb-negative to HBcAb-positive, but HBV-DNA levels were undetectable in the follow-up, suggesting that prophylaxis in these recipients is probably not warranted [2]. A documented serum anti-HBs level more than 10 IU/mL after vaccination of a naïve patient is considered to confer protective immunity against primary HBV infection and exposure. This anti-HBs level confers immunity even in recipients of HBcAb-positive organs [28–30]. Therefore, kidneys from HBcAb-positive donors are preferably allocated to successfully immunized patients or HbsAg-positive recipients [2,11,22–24].
However, the HBsAb-positive serologic status, from previous exposure or after vaccination, had a variable effect on serologic conversion, and after transplantation with an HBcAb-positive kidney, HBsAb-positive recipients were not completely protected [11,22,26,27]. The rate of seroconversion of kidney recipients of HBcAb-positive kidneys with protective anti-HBs concentration was only 4% as compared with 10% of recipients with no protective levels of HBsAb (<10 IU/mL) [11].
All end-stage renal disease candidates for kidney transplantation should receive hepatitis B vaccine. However, a protective anti-HBs level (>10 IU/mL) after vaccination is achieved in only 60% of peritoneal dialysis patients, 50% of hemodialysis patients, and less of 20% of renal transplant recipients [31].
In HBV-naïve kidney transplant recipients who have not responded to vaccination, chronic infection after transplantation can be prevented by administering hepatitis B immunoglobulin (HBIg) or antiviral therapy with oral nucleo(s)tide analogues in the post-transplant period.
Chung et al. [17] recommended a 7-day high-dose (10 000 units intravenously daily for the first 7 days, then monthly) HBIg or 12-month lamivudine (100 mg every day) prophylaxis in all recipients without evidence of HBV immunity, while Akalin et al. [32] suggested that 1-year prophylaxis with lamivudine alone without HBIg after kidney transplantation from an HBcAb-positive donor may protect against the risk of HBV transmission.
However, implementing anti-HBV prophylaxis in all recipients does not seem reasonable [15] since the risk of transmission of infection through transplantation seems to be very low [33]. Moreover, patients who develop inactive carrier status, as well as patients who become chronic carriers with active HBV replication, should be treated like immunocompetent patients with nucleo(s)tide analogues with high efficacy and low resistance [15–30,33,34].
In the liver transplant setting, prophylaxis with lamivudine and/or HBIg reduces rates of de novo HBV infection from 58% to 11% in HBV non-immune recipients; 18% to 2% in previously vaccinated recipients; and 14% to 3% in isolated HBcAb positive recipients [28], and this benefit is higher when lamivudine is used as monotherapy [28–30,35,36]. Recently, the introduction of effective nucleo(s)tide analogs (NA) entecavir and tenofovir has improved the outcome of patients with chronic hepatitis B and the combination of NA and HBIg is now considered the standard of care for prophylaxis against HBV recurrence after liver transplantation [37], while entecavir has replaced lamivudine as first-line therapy for treatment-naïve recipients in view of the propensity for drug resistance [38].
Translating these experiences in the renal transplantation setting, Veroux et al. [2] applied a prophylaxis with a single dose of 2000 IU of HBIg in all recipients with HBsAb titer <10 IU/mL, and this resulted in a very low incidence of seroconversion when compared with patients who did not receive prophylaxis. All HBcAb-positive patients who underwent prophylaxis did not have HBV reactivation, while 2 patients who did not undergo prophylaxis developed a subclinical reactivation [2].
More recently, Ouseph et al. [14] proposed a different approach – recipients of HBc-positive kidneys start prophylaxis with lamivudine or HBIg; if the recipients have been vaccinated and the titer is protective, HBV-DNA is monitored every 3 months, and prophylaxis is suspended when HBV-DNA is undetectable.
If the recipients are not vaccinated and the HBV-DNA is negative, the HBV-DNA is followed monthly, and the recipient is revaccinated with 3 doses.
While most studies reported similar graft and patient survival in kidney transplantation from HBcAb-positive donors compared to those from HBcAb-negative donors [2,11,14,23,24], Fong et al. [22] reported lower 1- and 3-year graft and patient survival in kidney recipients of HBcAb-positive donors compared to those from HBcAb-negative donors. However, the reduction in survival was attributable to donor and recipient factors independent of HBcAb status.
Most of the clinical studies reported an increase in transaminase levels in 0–26% of patients [11,16,18,22–24], but Alkalin et al. [32] suggested that recipients at higher risk for elevated transaminase levels were those who were co-infected with hepatitis C.
In summary, existing data support the use of HBcAb-positive organ donors for kidney transplantation. HBcAb-positive kidneys should be used in successfully vaccinated or in HBsAg-positive patients.
Current evidence does not suggest using extensively prophylaxis for recipients of HBcAb-positive kidneys: however, strict surveillance serologies are mandatory and an HBIg single-shot prophylaxis may reduce the risk of HBV seroconversion or reactivation in all naïve or HBcAb-positive transplant recipients.
Kidney Transplantations From HBsAg-Positive Donors
In geographic areas endemic for HBV infection, HBsAg carrier rates are so high (10–20%) [29] that exclusion of HBsAg donors from the donor pool would significantly reduce the supply of kidney allografts.
It is generally accepted that transplanting an HBsAg-positive allograft into an HBsAg-negative recipient carries a significant risk of novo infection. Although the presence of preexisting acquired immunity after vaccination or after previous HBV infection should protect the recipient from primary de novo HBV infection, most transplant centers do not transplant kidneys from HBsAg-positive donors, and in most countries these donors can be transplanted only in matched HBsAg-positive recipients.
Before introduction of anti-viral treatment, HBsAg-positive kidney transplant recipients had a significantly lower survival compared to HBsAg-negative recipients, and much of the mortality occurred relatively early, mainly due to progressive liver disease [37,38], and HBsAg-positivity was associated with a 2.49-fold higher risk of death after kidney transplantation [39].
Treatment of HBsAg-positive renal transplant recipients with NA confers long-term survival benefit, and rescue therapy with adefovir or entecavir is effective and well tolerated in patients who developed resistance to lamivudine [40].
Antiviral therapy with lamivudine is effective in suppressing HBV DNA and improving transaminase levels [41], and is associated with improved graft survival nearly comparable to HBsAg-negative recipients [42].
Despite reduced mortality as a result of decreased hepatic complications observed in patients treated with lamivudine, liver-related deaths accounts for 40% of all mortalities in HBsAg-positive patients [42]. Prolonged antiviral treatment with lamivudine is associated with progressive increase in drug resistance, with a cumulative probability of developing lamivudine-resistance of 60% at 69 months [42].
The nephrotoxic drug adefovir has been used mainly in patients with lamivudine-resistance. Adefovir as add-on treatment with lamivudine led to 88% undetectable HBV-DNA at 36 months in lamivudine-resistant kidney transplant recipients, and normalization of ALT was achieved in 92.8% of patients after 12 months of treatment [43]. However, evidence of nephrotoxicity was reported in up to 50% of kidney transplant recipients, requiring in most cases treatment discontinuation [42].
Entecavir has been recently introduced as part of antiviral therapy of HBsAg-positive kidney transplant recipients, due to its high resistance barrier and favorable safety profile [42,44]. Entecavir is associated with a more potent response than lamivudine and the tolerability profile is favorable but, when used in lamivudine-resistant kidney allograft recipients, the virological response could be variable and relatively slower compared with treatment-naïve patients [42,44].
There are limited data on the use of tenofovir in kidney transplant recipients, and there are concerns about its potential nephrotoxicity [45,46].
Although antiviral treatment should be continued lifelong after kidney transplantation due to the risk of viral reactivation and progression of liver disease, a recent study reported a successful withdrawal of antiviral treatment in 4 HbsAg-positive renal transplant recipients with complete suppression of HBV infection having received antivirals for 14.3 months. They remained HBV-DNA negative for a median of 60.5 months [47].
Most studies investigating use of HBsAg-positive kidney donors have shown that the policy of transplanting such kidneys to HBsAg-positive recipients with natural immunity is reasonable and seems to be safe [48–52].
An interesting study comparing patient survival among 24 HBsAg-positive recipients of kidneys from HBsAg-positive donors with that among 42 HBsAg-positive recipients of kidneys from HBsAg-negative donors [53] reported no statistically significant differences between the 2 groups in number of episodes of hepatitis. However, recipients of kidneys from HBsAg-negative deceased donors had significantly higher 1- and 5-year survival rates than recipients of kidneys from HBsAg-positive donors.
In a recent study, we reported 5 successful kidney transplantations from HBsAg-positive donors into HBsAg-positive recipients, with no post-transplant elevation of transaminases and a significant reduction of waiting time for transplantation [49].
Recent observations suggest the opportunity to transplant HBsAg-positive kidneys into HBsAb-positive recipients, and recipients were given hepatitis B immunoglobulin at the time of and after transplantation. Lamivudine was also recommended for these recipients [15,37,38,50].
Asuman et al. [52] compared clinical and biochemical parameters of kidney transplant recipients of HBsAg-positive living donors (group 1, 111 patients) with a group of recipients of HBsAg-negative donors (group 2, 2057 patients). Living kidney transplantation from HBsAg-positive/HBV DNA-negative donor was performed only if recipients displayed an HBsAb titer >10 mIU/mL. Since all recipients were naturally or vaccine-induced HBsAb-positive at the time of transplantation, they received neither HBIg nor lamivudine. This study demonstrated that 1-, 2-, and 4-year serum creatinine levels, glomerular filtration rates, and liver function test results were similar between the 2 groups without de novo hepatitis B virus infection throughout the study period. Moreover, graft and patient survivals were similar between the 2 groups. Another study [53] reported no de novo HBV infection in 35 HBV-immune (anti-HBs >10 IU/mL) patients who underwent kidney transplantation from HBsAg-positive, HBV DNA-negative living donors.
Although these studies may be criticized because an HBsAb titer >10 IU/L may be not completely protective against the risk of viral transmission, HBsAg-positive living donors may be an attractive source for expanding the donor pool in those countries where HBV is endemic and the rate of deceased donor organ donation is low or insufficient.
In their large series, Jiang et al. [54] compared the clinical outcomes in 373 HBsAb-positive kidney transplant recipients receiving a kidney from either HBsAg-positive donors (n=65) or HBsAg-negative donors. Recipients of HBsAg-positive grafts received a prophylaxis with 400 IU of HBIg on the day of surgery and again 1 month after transplantation, if the donor had undetectable serum HBV DNA; if the donor had detectable HBV DNA, the recipient received weekly HBIg for 12 weeks and daily lamivudine 100 mg for 6 months.
There were no differences in graft or patient survival between the 2 groups, and none of the 7 recipients receiving a graft from an HBV DNA-positive donor displayed evidence of HBV infection 20 months after transplantation.
In a recent study, Yilmaz et al. [55] compared the long-term outcomes in 26 kidney transplant recipients receiving a kidney from HBsAg-positive, HBV-DNA positive and HBcAb positive donors with 52 kidney transplant recipients of HBsAg-negative, HBcAb-negative donors. All recipients were HBsAg-negative and HbsAb levels were similar and protective in both groups (452±384 vs. 448±431, p=0.724), and recipients of HBsAg-positive donors were treated with lamivudine. The rate of acute hepatitis was significantly higher in recipients of HBsAg-positive donors (11% vs. 0%, p=0.012), and all of the patients who developed an acute hepatitis had a HBsAb protective titer after vaccination, while none of the patients who had acquired natural immunity against HBsAg developed acute hepatitis, suggesting that vaccination may not completely protect these patients against the risk of viral transmission.
Despite a higher incidence of acute hepatitis, kidney transplant recipients of HBsAg-positive, HBV-DNA positive donors had similar graft and patient survival rates, acute rejection rates, and post-transplant complications compared to recipients of HBsAg-negative kidneys.
Taken together, these studies confirmed that protective immunity to HBV (acquired through previous infection or vaccination) prevents chronic HBV infection in recipients of HBsAg-positive kidneys.
Transplantation of HBsAg-positive kidneys into HBV-naïve recipients may result in primary infection of the recipient, who may achieve long-term HBV suppression with NA antiviral drugs (entecavir and tenofovir), which prevents progression to cirrhosis and liver failure [8,37,38]. However, these patients remain at higher risk for hepatocellular carcinoma [8].
Although some authors reported the possibility of fulminant hepatic failure after kidney transplantation from an HBsAg-positive donor into an HBsAg-negative immunized recipients [56], in a recent study, Chancharoenthana et al. [30] compared outcomes of kidney transplantation between HBsAg-negative recipients with anti-HBs titer above 100 mIU/mL undergoing transplantation from HBsAg-positive donors without HBV viremia (n=43) and HBsAg-negative donors (n=86). After a mean follow-up of 58.2 months, there were no significant differences in graft and patients survivals, and no HBV-infective markers (HBsAg, HBcAb, HBeAg, HBV DNA) were detected in the HBsAg-positive donor group. Moreover, recipients of HBsAg-positive donors with no prophylaxis (n=20) had similar outcomes with those treated with lamivudine alone (n=21) or lamivudine in combination with HBIg (n=2). This study, therefore, suggested that kidney transplantation from HbsAg-positive donors into HbsAg-negative recipients with non-protective anti-HBs titer provide excellent graft and patient survival without evidence of HBV transmission [30].
Despite these surprising results, this practice is not advocated in most transplant centers, and transplantation of a kidney from an HbsAg-positive donor into a HBV-naïve recipient should be considered only in urgent situations where the benefit of kidney transplantation outweigh the risk of acquired HBV infection.
Conclusions
HBV-infected organ donors may be a relevant organ source for kidney transplantation. Kidneys from HBcAb-positive organ donors may be transplanted safely in successfully vaccinated or in HBsAg-positive patients with an anti-HBs titer >10 IU/L; these organ could be allocated even to HBsAb-negative recipients with a very low risk of transmission. In these patients, an antiviral prophylaxis with lamivudine or, alternatively, with entecavir, may be warranted.
Kidney transplantation from HBsAg-positive donor kidneys may be safely allocated in HBsAg-positive recipients. All transplant recipients should undergo prophylaxis with lamivudine or, in case of failure, with adefovir or entecavir, which can confer long-term survival benefits.
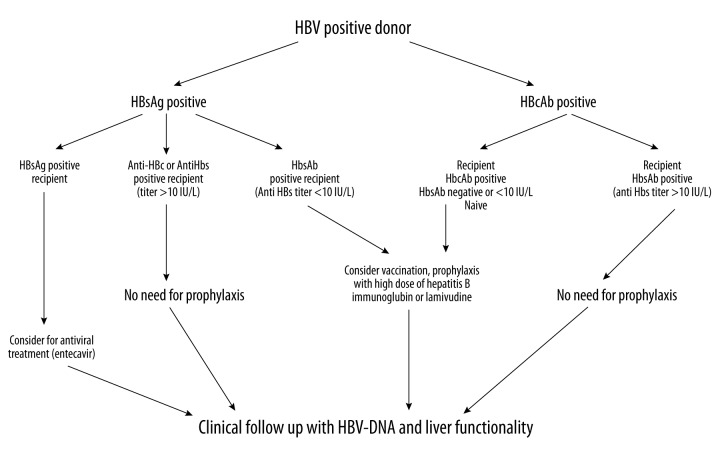
Kidney transplantation in HBsAb-positive recipients with an anti-HBs titer of at least >10 IU/L could be performed safely with post-transplant HBV monitoring and, eventually, with the use of antivirals to prevent the risk of hepatitis B progression. Kidney transplantation from HBsAg-positive donors to HBV-naïve recipients, although some promising results have been reported, needs further data to be extensively adopted, and should be limited to urgent life-threatening conditions (Figure 1).
Figure 1.
Kidney transplantation from anti-HBc positive donors can be performed safely without the need for prophylaxis in recipients with HBV immunity (>10 IU/mL). In patients with low titer of anti-HBs (<10 IU/mL), a pre-transplant vaccine booster should be administered and, if successful, no prophylaxis is needed. In patients not responding to vaccine, consider prophylaxis with hepatitis B Immunoglobulin or lamivudine. HBsAg-positive recipients receiving a kidney graft from an HBcAb-positive donor should receive prophylaxis with entecavir or tenofovir. Kidney transplantation from HbsAg-positive donors should be limited to HbsAg-positive recipients (consider for prophylaxis with entecavir) or successfully immunized recipients (anti-HBs titer >10 IU/mL, no need for prophylaxis). In anti-HBc-negative/anti-HBs-negative (<10 IU/mL) recipients, administer vaccine boosters pretransplant. If unsuccessful, lamivudine should be administered from time of transplant. If anti-HBs titer increases >10 IU/mL, no post-transplant prophylaxis is required. Transplant into naïve recipients should be avoided or limited to those recipients in whom the potential benefit of receiving an HBsAg-positive kidney outweigh the risk of post-transplant de novo hepatitis [from ref. 1, 2, 8, 11, 14, 15, 22, 23, 29, 30, 37, 38, 46]. HBV – hepatitis B virus; HBsAg – hepatitis B surface antigen; HBcAb – hepatitis B core antibody; HBsAb – hepatitis B antibody.
Footnotes
Source of support: Supported by Italian Ministry of Health (Project code PE-2011-02350135) and University of Catania (FIR-2014)
Conflicts of interest
All the Authors have no conflicts of interest to declare.
References
- 1.Veroux M, Corona D, Veroux P. Kidney transplantation from donors with hepatitis. In: Veroux M, Veroux P, editors. Kidney Transplantation: Challenging the future UAE. Bentham Science Publisher; 2012. pp. 71–84. [Google Scholar]
- 2.Veroux M, Corona D, Ekser B, et al. Kidney transplantation from hepatitis B virus core antibody-positive donors: prophylaxis with hepatitis B immunoglobulin. Transplant Proc. 2011;43:967–70. doi: 10.1016/j.transproceed.2011.01.155. [DOI] [PubMed] [Google Scholar]
- 3.Veroux P, Veroux M, Sparacino V, et al. Kidney transplantation from donors with viral B and C hepatitis. Transplant Proc. 2006;38:996–98. doi: 10.1016/j.transproceed.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 4.Veroux P, Veroux M, Puliatti C, et al. Kidney transplantation from hepatitis C virus-positive donors into hepatitis C virus-positive recipients: a safe way to expand the donor pool. Transplant Proc. 2005;37:2571–73. doi: 10.1016/j.transproceed.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 5.Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. 2015;11:172–82. doi: 10.1038/nrneph.2015.5. [DOI] [PubMed] [Google Scholar]
- 6.Fabrizi F, Messa P, Martin P. Current status of renal transplantation from HCV-positive donors. Int J Artif Organs. 2009;32:251–61. doi: 10.1177/039139880903200502. [DOI] [PubMed] [Google Scholar]
- 7.Veroux M, Corona D, Sinagra N, et al. Kidney transplantation from donors with hepatitis C infection. World J Gastroenterol. 2014;20:2801–9. doi: 10.3748/wjg.v20.i11.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilmore HL, Gane EJ. Hepatitis B positive donors in renal transplantation: Increasing the deceased donor pool. Transplantation. 2012;94:2005–10. doi: 10.1097/TP.0b013e31824e3db4. [DOI] [PubMed] [Google Scholar]
- 9.Reddy PN, Sampaio MS, Kuo HT, et al. Impact of pre-existing hepatitis B infection on the outcomes of kidney transplant recipients in the United States. Clin J Am Soc Nephrol. 2011;6:1481–87. doi: 10.2215/CJN.09201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KH, Wai CT, Lim SG, et al. Risk of de novo hepatitis B from antibody to hepatitis B core antigen-positive donors in liver transplantation in Singapore. Liver Transplant. 2001;7:469–70. doi: 10.1002/lt.500070514. [DOI] [PubMed] [Google Scholar]
- 11.De Feo TM, Grossi P, Poli F, et al. Kidney transplantation from anti-HBC+ donors: Results from a retrospective Italian study. Transplantation. 2006;81:76–80. doi: 10.1097/01.tp.0000189930.89031.1b. [DOI] [PubMed] [Google Scholar]
- 12.Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;57:395–403. doi: 10.1016/s1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 13.Grob P, Jilg W, Bornhak H, et al. Serological pattern “Anti-HBc alone”: Report on a qorkshop. J Med Virol. 2000;62:450–55. doi: 10.1002/1096-9071(200012)62:4<450::aid-jmv9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Ouseph R, Eng M, Ravindra K, et al. Review of the use of hepatitis B core antibody-positive kidney donors. Transplant Rev. 2010;24:167–71. doi: 10.1016/j.trre.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Mahboobi N, Tabatabaei SV, Blum HE, Alavian SM. Renal grafts from anti-hepatitis B core-positive donors: a quantitative review of the literature. Transpl Infect Dis. 2012;14:445–51. doi: 10.1111/j.1399-3062.2012.00782.x. [DOI] [PubMed] [Google Scholar]
- 16.Fytili P, Ciesek S, Manns MP, et al. Anti-HBc seroconversion after transplantation of Anti-HBc positive nonliver organs to anti-HBc negative recipients. Transplantation. 2006;81:808–9. doi: 10.1097/01.tp.0000198585.51562.57. [DOI] [PubMed] [Google Scholar]
- 17.Chung RT, Feng S, Delmonica FL. Approach to the management of allograft recipients following the detection of hepatitis B virus in the prospective organ donor. Am J Transplant. 2001;1:185–91. [PubMed] [Google Scholar]
- 18.Wachs ME, Amend WJ, Ascher NL, et al. The risk of transmission of hepatitis B from HBsAg(−), HBcAb (+), HbIgM (−) organ donors. Transplantation. 1995;59:230–34. [PubMed] [Google Scholar]
- 19.Cirocco R, Zucker K, Contreras N, et al. The presence of hepatitis B core antibody does not preclude kidney donation: lack of viral DNA in the serum and biopsies of core antibody-positive donors and clinical follow-up. Transplantation. 1997;63:1702–3. doi: 10.1097/00007890-199706150-00030. [DOI] [PubMed] [Google Scholar]
- 20.Donataccio D, Roggen F, De Reyck C, et al. Use of AntiHBc positive allografts in adult liver transplantation: toward a safer way to expand the donor pool. Transplant Int. 2006;19:38–43. doi: 10.1111/j.1432-2277.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 21.Takemura N, Suguwara Y, Tamura S, Makuuchi M. Liver transplantation using hepatitis B core antibody – positive grafts: Review and university of Tokyo experience. Dig Dis. 2007;52:2472–77. doi: 10.1007/s10620-006-9656-5. [DOI] [PubMed] [Google Scholar]
- 22.Fong TL, Bunnapradist S, Jordan SC, Cho YW. Impact of hepatitis B core antibody status on outcomes of cadaveric renal transplantation: analysis of United Network of Organ Sharing Database between 1994 and 1999. Transplantation. 2002;73:85–89. doi: 10.1097/00007890-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 23.Veroux M, Puliatti C, Gagliano M, et al. Use of hepatitis B core antibody-positive donor kidneys in hepatitis B surface antibody-positive and -negative recipients. Transplant Proc. 2005;37:2574–75. doi: 10.1016/j.transproceed.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 24.Madayag RM, Johnson LB, Bartlett ST, et al. Use of renal allografts from donors positive for hepatitis B core antibody confers minimal risk for subsequent development of clinical hepatitis B virus disease. Transplantation. 1997;64:1781–86. doi: 10.1097/00007890-199712270-00027. [DOI] [PubMed] [Google Scholar]
- 25.Knöll A, Pietrzyk M, Loss M, et al. Solid-organ transplantation in HbsAg-negative patients with antibodies to HBV core antigen: Low risk of HBV reactivation. Transplantation. 2005;79:1631–33. doi: 10.1097/01.tp.0000163468.80223.74. [DOI] [PubMed] [Google Scholar]
- 26.Ganem D, Prince AM. Hepatitis B virus infection – natural history and clinical consequences. N Engl J Med. 2004;350:1118–29. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 27.Satterthwaite R, Ozgu I, Shidban H, et al. Risks of transplanting kidney from hepatitis B surface antigen-negative, hepatitis B surface antibody-negative, hepatitis B core antibody-positive donors. Transplantation. 1997;64:432–35. doi: 10.1097/00007890-199708150-00011. [DOI] [PubMed] [Google Scholar]
- 28.Skagen CL, Jou JH, Said A. Risk of the novo hepatitis in liver recipients from hepatitis-B core antibody-positive grafts – a systematic analysis. Clin Transplant. 2011;25:E243–49. doi: 10.1111/j.1399-0012.2011.01409.x. [DOI] [PubMed] [Google Scholar]
- 29.Huprikar S, Danziger-Isakov L, Ahn J, et al. Solid organ transplantation from hepatitis B virus-positive donors: Consensus guidelines for recipient management. Am J Transplant. 2015;15:1162–72. doi: 10.1111/ajt.13187. [DOI] [PubMed] [Google Scholar]
- 30.Chancharoenthana W, Townamchai N, Pongpirul K, et al. The outcomes of kidney transplantation in hepatitis B surface antigen (HBsAg)-negative recipients receiving graft from HBsAg-positive donors: a retrospective, propensity score-matched study. Am J Transplant. 2014;14:2814–20. doi: 10.1111/ajt.12921. [DOI] [PubMed] [Google Scholar]
- 31.Kurz P, Kohler H, Meurer S, et al. Impaired cellular immune response in chronic renal failure. Evidence for T-cell defect. Kidney Int. 1986;29:1209–14. doi: 10.1038/ki.1986.129. [DOI] [PubMed] [Google Scholar]
- 32.Akalin E, Ames S, Sehgal V, et al. Safety of using hepatitis B virus core antibody or surface antigen-positive donors in kidney or pancreas transplantation. Clin Transplant. 2005;19:364–66. doi: 10.1111/j.1399-0012.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 33.Lonze BE, Dagher NN, LIu M, et al. Outcomes of renal transplants from centers for disease control and prevention high-risk donors with prospective recipient viral testing: a single-center experience. Arch Surg. 2011;146:1261–66. doi: 10.1001/archsurg.2011.267. [DOI] [PubMed] [Google Scholar]
- 34.Marzano A. Management of HBV infection during immunosuppressive treatment. Mediterr J Hematol Infect Dis. 2009;1:e2009025. doi: 10.4084/MJHID.2009.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saab S, Waterman B, Chi AC, Tong MJ. Comparison of different immunoprophylaxis regimens after liver transplantation with hepatitis B core AT donors: A systematic review. Liver Transplant. 2010;16:300–7. doi: 10.1002/lt.21998. [DOI] [PubMed] [Google Scholar]
- 36.Krasnodębski M, Grat M, Masior L, et al. Differential impact of risk factors for tumor recurrence in hepatitis B and hepatitis C virus-infected patients undergoing liver transplantation for hepatocellular carcinoma. Ann Transplant. 2015;20:70–75. doi: 10.12659/AOT.892395. [DOI] [PubMed] [Google Scholar]
- 37.Cholongitas E, Tziomalos K, Pipili C. Management of patients with hepatitis B in special populations. World J Gastroenterol. 2015;21:1738–48. doi: 10.3748/wjg.v21.i6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yap DY, Chan TM. Evolution of hepatitis B management in kidney transplantation. World J Gastroenterol. 2014;20:468–74. doi: 10.3748/wjg.v20.i2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabrizi F, Martin P, Dixit V, et al. HBsAg seropositive status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005;5:2913–21. doi: 10.1111/j.1600-6143.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 40.Yap DY, Tang CS, Yung S, et al. Long-term outcome of renal transplant recipients with chronic hepatitis B infection: impact of antiviral treatments. Transplantation. 2010;90:325–30. doi: 10.1097/TP.0b013e3181e5b811. [DOI] [PubMed] [Google Scholar]
- 41.Fabrizi F, Dulai G, Dixit V, et al. Lamivudine for the treatment of hepatitis B virus-related liver disease after renal transplantation: meta-analysis of clinical trials. Transplantation. 2004;77:859–64. doi: 10.1097/01.tp.0000116448.97841.6d. [DOI] [PubMed] [Google Scholar]
- 42.Yap DY, Tang CS, Yung S, et al. Long-term outcome of renal transplant recipients with chronic hepatitis B infection- impact of antiviral treatments. Transplantation. 2010;90:325–30. doi: 10.1097/TP.0b013e3181e5b811. [DOI] [PubMed] [Google Scholar]
- 43.Lai HW, Chang CC, Chen TH, et al. Safety and efficacy of adefovir fro lamivudine-resistant hepatitis B virus infection in renal transplant recipients. J Formos Med Assoc. 2012;111:439–44. doi: 10.1016/j.jfma.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Yap DY, Yung S, Tang CS, et al. Entecavir treatment in kidney transplant recipients infected with hepatitis B. Clin Transplant. 2014;28:1010–15. doi: 10.1111/ctr.12410. [DOI] [PubMed] [Google Scholar]
- 45.Krummel T, Parvez-Braun L, Frantzen L, et al. Tenofovir-induced acute renal failure in an HIV patient with normal renal function. Nephrol Dial Transplant. 2005;20:473–74. doi: 10.1093/ndt/gfh640. [DOI] [PubMed] [Google Scholar]
- 46.Duadè M, Rostaing L, Saunè K, et al. Tenofovir therapy in hepatitis B virus-positive solid-organ transplant recipients. Transplantation. 2011;91:916–20. doi: 10.1097/TP.0b013e3182100f59. [DOI] [PubMed] [Google Scholar]
- 47.Cho JH, Lim JH, Park GY, et al. Successful withdrawal of antiviral treatment in kidney transplant recipients with chronic hepatitis B infection. Transplant Infect Dis. 2014;16:295–303. doi: 10.1111/tid.12202. [DOI] [PubMed] [Google Scholar]
- 48.Kim JA, Huh W, Lee KW, et al. Cadaveric renal transplantation in hepatitis B antigen-positive recipients using hepatitis B antigen-positive donor organs with lamivudine treatment. Transplant Proc. 2004;36:1434–37. doi: 10.1016/j.transproceed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Veroux M, Puliatti C, Macarone M, et al. Kidney transplantation from hepatitis B surface antigen-positive donors into hepatitis B surface antigen-positive recipients: Preliminary findings. Transplant Proc. 2005;37:2467–68. doi: 10.1016/j.transproceed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto M, Yoshimura N, Nakai I, et al. Kidney transplantation from a hepatitis B surface antigen-positive donor to a HBsAg-negative recipient. Transplant Proc. 1999;31:2869. doi: 10.1016/s0041-1345(99)00595-3. [DOI] [PubMed] [Google Scholar]
- 51.Lai MK, Chang SC, Chueh CC, et al. Kidney transplantation from hepatitis B surface antigen (HbsAg)-positive donors: changes of relative HBV genomic copy number after transplantation. Transplant Proc. 1996;28:1518–19. [PubMed] [Google Scholar]
- 52.Asuman Yavuz H, Tekin S, Yuksel Y, et al. Donors with hepatitis B surface antigen positivity. Transpl Proc. 2015;47:1312–14. doi: 10.1016/j.transproceed.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Tuncer M, Tekin S, Yucetin L, et al. Hepatitis B surface antigen positivity is not a contraindication for a living kidney donation. Transplant Proc. 2012;44:1628–29. doi: 10.1016/j.transproceed.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 54.Jiang H, Wu J, Zhang X, et al. kidney transplantation from hepatitis B surface antigen positive donors into hepatitis B surface antibody positive recipients: a prospective nonrandomized controlled study from a single centre. Am J Transplant. 2009;9:1583–88. doi: 10.1111/j.1600-6143.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 55.Yilmaz VT, Ulger BV, Aliosmanoglu I, et al. Assessment of long-term outcomes in HbsAg-negative renal transplant recipients transplanted from HbsAg-positive donors. Ann Transplant. 2015;20:390–96. doi: 10.12659/AOT.894073. [DOI] [PubMed] [Google Scholar]
- 56.Magiorkinis E, Paraskevis D, Pavlopoulou ID, et al. Renal Transplantation from hepatitis B surface antigen (HBsAg)-positive donors to HBsAg-negative recipients: a case of post-transplant fulminant hepatitis associated with an extensively mutated hepatitis B virus strain and review of current literature. Transplant Infect Dis. 2013;15:393–99. doi: 10.1111/tid.12094. [DOI] [PubMed] [Google Scholar]