Abstract
Background
Several studies have indicated that interleukin (IL)-1β-511 C/T polymorphism may contribute to individual susceptibility to gastric cancer, but the results vary among regions and races. No relevant meta-analysis has been conducted in a Chinese population. Therefore, we performed the current meta-analysis to investigate the possible correlation between IL-1β-511 C/T polymorphism and gastric cancer susceptibility in Chinese subjects.
Material/Methods
PubMed, EmBase, Cochrane Library, Chinese Biology Medicine (CBM), Chinese National Knowledge Infrastructure (CNKI), and Wanfang databases were searched for case-control studies published before 21 January 2015 and investigating a correlation between IL-1β-511 C/T polymorphism and gastric cancer susceptibility. Two investigators independently screened the studies, extracted data, and evaluated the quality of included studies with the Newcastle-Ottawa scale. Meta-analysis was conducted with STATA 12.0.
Results
A total of 27 articles from 28 case-control studies were collected. Meta-analysis showed that IL-1β-511C/T polymorphism was related to increased susceptibility to gastric cancer in Chinese subjects [T vs. C: OR=1.21, 95%CI (1.07–1.37), P<0.01; TT vs. CC: OR=1.41, 95%CI (1.11–1.80), P<0.01; CT vs. CC: OR=1.26, 95% CI (1.05–1.50), P<0.01; TT+CT vs. CC: OR=1.31, 95%CI (1.08–1.58), P<0.01; and TT vs. CT+CC: OR=1.24, 95%CI (1.05–1.47), P<0.01]. Subgroup analysis showed a significant correlation between IL-1β-511C/T polymorphism and susceptibility to gastric cancer in residents of southern China and in patients with intestinal-type gastric cancer, but not in residents of northern China or in patients with diffuse gastric cancer. Moreover, H. pylori-infected subjects carrying T (CT+TT) exhibited a relatively higher risk of GC [OR=2.4, 95% CI (1.2–5.1), P=0.02].
Conclusions
IL-1β-511C/T polymorphism is significantly associated with increased susceptibility to gastric cancer in residents of southern China and in intestinal-type gastric cancer. We also found a synergistic interaction between IL-1β-511C/T polymorphism and H. pylori infection in the development of GC.
MeSH Keywords: China; Interleukin-1beta; Meta-Analysis; Polymorphism, Single-Stranded Conformational; Stomach Neoplasms
Background
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer-related death worldwide [1]. An estimated total of 989 600 new GC cases and 738 000 deaths occurred in 2008, of which more than 70% occurred in developing countries, with the majority from China [1]. GC is a gastrointestinal malignancy caused by environmental and hereditary factors. Helicobacter pylori infection and inflammation are important risk factors of GC [2,3]. IL-1β is an important pro-inflammatory cytokine, mainly produced by inflammatory cells (such as monocytes and macrophages) during the uptake of antigen-antibody complex and antigen presentation, and may expand the inflammatory and immune response [3]. In sustained inflammation due to gastric injury, IL-1β may promote COX-2 and iNOS production to inhibit apoptosis and induce cell injury [4]. Thus, the IL-1β-related cascade has been proposed as an important mechanism underlying the pathogenesis of GC. Moreover, IL-1β is effective in inhibiting the secretion of gastric acid, with potency 6000 times that of H2 receptor antagonists and 100 times that of proton pump inhibitors [5]. The IL-1β-induced inhibition of gastric acid secretion provides a favorable condition for the survival of H. pylori in the gastric mucosa and may further cause atrophy of the gastric mucosa [3], which creates favorable conditions for malignant transformation of gastric epithelial cells [6].
It has been demonstrated that GC susceptibility genes, combined with environmental factors, may play an important role in the development of cancer. In 2000, El-Omar [7] first reported that IL-1β gene polymorphism increased the risk of GC onset, a finding subsequently confirmed by later studies [8–10]. The IL-1β gene is mapped to chromosome 2q14 and has 3 single-nucleotide polymorphisms (SNPs): 31 T/C, 511 C/T, and 3954 C/T. These 3 SNPs are located in the promoter region of chromosome 2q14 and are thought to cause the overexpression of IL-1β. The correlation between SNP 31T/C and GC has been widely accepted [11,12], but few studies have investigated SNP 3954 C/T [13,14]. Thus, this study focusses on the relationship between GC and 511 C/T polymorphism. Xue et al. [15] and Park et al. [16] have examined the correlation between IL-1β gene polymorphism and GC by meta-analysis, but the population of the studies was worldwide and the language was limited to English. China has the largest population worldwide, but weather this polymorphism increases GC susceptibility in Chinese populations remains controversial and no relevant meta-analyses have been performed. Therefore, we conducted a meta-analysis to examine the correlation between IL-1β-511C/T polymorphism and GC susceptibility in Chinese populations.
Material and Methods
This study was conducted according to the PRISMA statement [17].
Inclusion and exclusion criteria
According to the PICOS principles, the inclusion criteria were: 1) case-control studies in which gene frequency or odds ratio and 95% confidence interval (CI) were included; 2) Chinese patients with pathologically proven GC were recruited; 3) IL-1β-511C/T polymorphism was investigated as an exposure factor; 4) the outcome was onset risk for GC, irrespective of death; and 5) controls were recruited from communities or hospitals and were healthy subjects or volunteers without GC symptoms. Exclusion criteria were: 1) studies in which subjects did not meet the inclusion criteria; 2) subjects whose controls were recruited from gastritis or gastric ulcer patients; 3) subjects whose medical information was incomplete or could not be obtained; and 4) subjects whose language was not Chinese or English.
Literature searching
PubMed, EmBase, Cochrane Library, Chinese Biology Medicine, Chinese National Knowledge Infrastructure, and Wanfang databases were searched for studies, published before January 201, on the relationship between IL-1β-511 C/T polymorphism and GC susceptibility in Chinese populations. The search terms were: gastric cancer, gastric carcinoma, polymorphism, variant, mutation, IL-1β, and interleukin-1β. For example, in PubMed the search strategy was: #1 gastric cancer, #2 gastric carcinoma, #3 interleukin-1β, #4 IL-1β, #5 polymorphism, #6 variant, #7 mutation, #8 (#1 OR #2) AND (#3 OR #4) AND (#5 OR #6 OR #7).
Data extraction
Two investigators independently screened studies and evaluated the study quality. The following information was collected from the studies: first author, publication year, province where the study was conducted, sample size, source of controls, methods for genotyping, Helicobacter pylori infection status, genotype frequency including genotype frequency in different types (Lauren’s classification) of GC, and P value of Hardy-Weinberg equilibrium (HWE). If the P value of HWE was not provided, it was calculated with chi square test according to α=0.05. The collected data were double-checked and any discrepancy was resolved by discussion or by contacting the study authors.
Quality evaluation
The quality of case-control studies was evaluated according to the Newcastle-Ottawa scale (NOS). A total of 8 items are included in the NOS and the maximum score is 9 “stars” [18].
Statistical analysis
Statistical analysis was performed with STATA 12.0. The overall correlation was evaluated with OR and 95% CI of allele model (T vs. C), codominant gene model (TT vs. CC; CT vs. CC), dominant gene model (TT+CT vs. CC), and recessive gene model (TT vs. CT+CC).
The I2 test was used for test of heterogeneity. If there was mild heterogeneity among studies (P≥0.1, I2<50%), the fixed-effects model was used; otherwise, the random-effects model was used (P<0.1, I2≥50%). In addition, subgroup analysis was used to identify the source of heterogeneity on the basis of regions, sources of controls, methods of genotyping, and shift in HWE. In studies in which Lauren’s classification of GC was given, subgroup analysis was done according to the intestinal-type and diffuse-type of GC. To avoid the influence of large bias on the overall effectors, stepwise exclusion was employed for the analysis of sensitivity. Funnel plot and Egger’s test were used to test for publication bias.
Results
Literature searching
The literature search process is shown in Figure 1. The initial literature search identified 864 articles. After removal of duplicate articles, 356 articles remained. After the title and abstract of these articles were screened, we excluded the 131 studies unrelated to IL-1β-511C/T polymorphism, 16 reviews or meta-analyses, and 38 studies that were not from Chinese populations. The full text of each of the remaining articles was reviewed, then we excluded studies without data, studies that were not case-control studies, and studies whose control groups included unhealthy subjects. Finally, 28 case-control studies from 27 articles [19–45] were included for further analysis.
Figure 1.
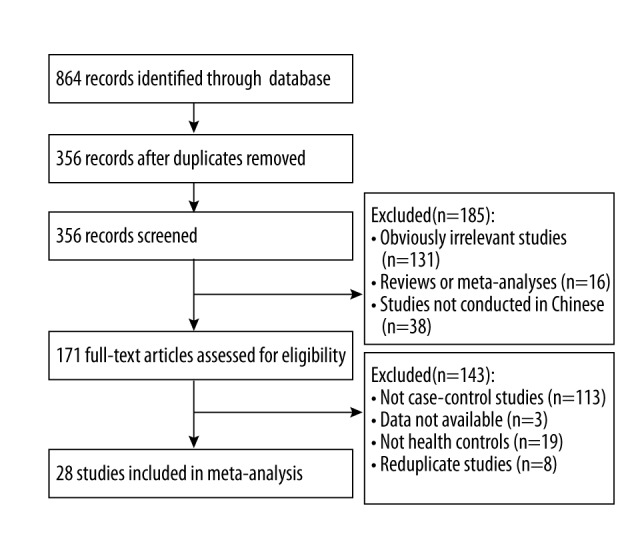
Flow chart of the literature search process.
Characteristics and methodological quality of included studies
The 28 articles were studies conducted with Chinese subjects (including those from Hong Kong, Macao, and Taiwan). Of the 28 studies, 12 were published in English and 16 in Chinese (Table 1). A total of 5136 GC patients and 5332 healthy controls were included in the 28 studies. China was divided into northern and southern China according to the Qinling-Huaihe line. Patients were recruited from northern China in the 28 studies and from southern China in 14 studies. Population-based (PB) controls were recruited in 13 studies and hospital-based (HB) controls in 15. Lauren’s classification was noted in 4 studies. The NOS score of included studies is shown in Table 1.
Table 1.
Characteristics and quality of included studies.
| Studies | Province | Source of controls | Genotyping | Sample (patients/ controls) | Genotype | HWE value | NOS score | Lauren type | H. pylori infection | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (CC/CT/TT) | Controls (CC/CT/TT) | |||||||||
| He, 2002 [19] | Liaoning | HB | PCR-RFLP | 50/50 | 15/23/12 | 28/19/3 | 0.92 | 5 | No | No |
| Wu, 2003 [20] | Taiwan | HB | PCR-RFLP | 220/230 | 69/106/45 | 61/124/45 | 0.21 | 7 | No | No |
| Chen, 2004 [21] | Taiwan | HB | PCR-RFLP | 142/164 | 24/87/31 | 34/93/37 | 0.65 | 7 | No | Yes |
| Yang, 2004 [22] | Jiangsu | PB | PCR-RFLP | 280/258 | 70/158/52 | 57/136/65 | 0.37 | 7 | No | No |
| Hu, 2004 [23] | Shanxi | PB | PCR-RFLP | 169/86 | 34/97/38 | 19/45/22 | 0.66 | 7 | No | No |
| Zeng, 2005 [24] | Shanxi | HB | PCR-RFLP | 102/102 | 28/46/28 | 25/52/25 | 0.42 | 6 | No | Yes |
| Zeng, 2005 [24] | Guangdong | HB | PCR-RFLP | 104/104 | 24/52/28 | 32/58/14 | 0.13 | 6 | No | Yes |
| Lu, 2005 [25] | Beijing, Shandong | PB | DH. PYLORILC | 250/300 | 72/125/53 | 67/163/70 | 0.13 | 7 | No | No |
| Zhang, 2005 [26] | Gansu | PB | PCR-RFLP | 154/166 | 34/78/42 | 43/71/52 | 0.07 | 6 | No | No |
| Xing, 2006 [27] | Shandong | HB | Taq | 130/142 | 57/33/40 | 68/46/28 | <0.05 | 6 | No | Yes |
| Gao, 2006 [28] | Shandong | HB | PCR-RFLP | 71/65 | 25/23/23 | 39/12/14 | <0.05 | 5 | No | No |
| Zheng, 2007 [29] | Shanghai | HB | PCR-RFLP | 177/298 | 49/83/45 | 77/140/81 | 0.3 | 7 | No | No |
| Wei, 2007 [30] | Henan | PB | PCR-SSCP | 452/218 | 112/290/50 | 100/87/31 | 0.1 | 5 | yes | No |
| Li, 2007 [31] | Hubei | HB | PCR-RFLP | 143/264 | 29/75/39 | 70/137/57 | 0.51 | 6 | No | Yes |
| Zhang, 2007 [32] | Shandong | PB | PCR-RFLP | 214/230 | 55/97/62 | 56/101/73 | 0.08 | 7 | No | No |
| Sun, 2007 [33] | Shandong | HB | Oligochip | 65/55 | 39/12/14 | 25/13/17 | <0.05 | 6 | No | No |
| Feng, 2008 [34] | Henan | PB | PCR-RFLP | 150/154 | 42/54/54 | 91/33/30 | <0.05 | 7 | No | No |
| Jia, 2009 [35] | Shanxi | HB | PCR-RFLP | 106/108 | 13/58/35 | 18/55/35 | 0.65 | 7 | No | No |
| Xiang, 2009 [36] | Chongqing | HB | PCR-RFLP | 35/70 | 9/15/2011 | 18/27/25 | 0.06 | 6 | No | No |
| Chen, 2009 [37] | Guangdong | PB | PCR-RFLP | 563/500 | 143/309/111 | 182/253/65 | 0.11 | 6 | Yes | No |
| Yu, 2009 [38] | Guangdong | PB | PCR-RFLP | 501/500 | 132/269/100 | 182/253/65 | 0.7 | 7 | Yes | No |
| Jiang, 2010 [39] | Hubei | PB | PCR-RFLP | 84/84 | 19/44/21 | 37/38/9 | 0.87 | 7 | No | Yes |
| Li, 2010 [40] | Sichuan | PB | PCR-RFLP | 140/165 | 26/81/33 | 34/94/37 | 0.07 | 7 | No | No |
| Zou, 2011 [41] | Guangdong | HB | MALDI-TOFM | 52/52 | 20/23/9 | 11/28/2013 | 0.57 | 6 | No | No |
| He, 2011 [42] | Jiangsu | HB | PCR-RFLP | 392/508 | 72/196/124 | 148/266/94 | 0.18 | 7 | No | Yes |
| Zhang, 2012 [43] | Jiangsu | HB | PCR-RFLP | 128/127 | 28/61/39 | 37/71/19 | 0.11 | 6 | No | Yes |
| Zhao, 2012 [44] | Qinghai | PB | PCR-RFLP | 197/202 | 31/101/65 | 65/99/38 | 0.98 | 7 | Yes | No |
| Zhang, 2014 [45] | Henan | PB | PCR-RFLP | 65/130 | 12/34/19 | 10/76/44 | <0.05 | 7 | No | No |
HB – hospital-based; PB – population-based; PCR-RFLP – restriction fragment length polymorphism polymerase chain reaction; DHPLC – denaturing high performance liquid chromatography; Taq – Taq polymerase chain reaction; MALDI-TOFMS – matrix-assisted laser desorption ionization time of flight mass spectrometry.
Meta-analysis
Five gene models had obvious heterogeneity; therefore, the random-effects model was used. As shown in Table 2, the onset risk for GC in mutant T allele carriers was 1.21 times that in non-carriers [TT vs. CC: OR=1.41, 95%CI (1.11–1.80); CT vs. CC: OR=1.26, 95% CI (1.05–1.50); TT+CT vs. CC: OR=1.31, 95%CI (1.08–1.58); TT vs. CT+CC: OR=1.24, 95%CI (1.05–1.47)]. Each study was omitted in the allele model for sensitivity analysis and the results showed that the effect value ranged from1.07 to 1.37, and 95% CI was 1.04–1.40, suggesting this meta-analysis is statistically robust and reliable.
Table 2.
Results of meta-analysis for the IL-1β-511C/T polymorphism and GC risk.
| Subgroup | Studies | T vs. C | TT vs. CC | CT vs. CC | (TT+CT) vs. CC | TT vs. (CC+CT) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | I2 (%) | OR (95% CI) | P | I2 (%) | OR (95% CI) | P | I2 (%) | OR (95% CI) | P | I2 (%) | OR (95% CI) | P | I2 (%) | ||
| Total | 28 | 1.21 (1.07–1.37) | 0.003 | 78.7 | 1.41 (1.11–1.80) | 0.005 | 75.1 | 1.26 (1.05–1.50) | 0.013 | 68.8 | 1.31 (1.08–1.58) | 0.005 | 75.3 | 1.24 (1.05–1.47) | 0.013 | 65.6 |
| Region | 28 | |||||||||||||||
| North | 13 | 1.27 (0.99–1.55) | 0.060 | 82.7 | 1.42 (0.93–.170) | 0.103 | 77.1 | 1.25 (0.88–1.74) | 0.161 | 72.1 | 1.32 (0.94–1.84) | 0.107 | 80 | 1.22 (0.83–2.20) | 0.118 | 58.1 |
| South | 15 | 1.58 (1.11–2.24) | 0.015 | 74.5 | 1.45 (1.07–2.00) | 0.016 | 73 | 1.28 (1.03–1.59) | 0.029 | 66.0 | 1.32 (1.05–1.65) | 0.016 | 71.4 | 1.26 (0.99–1.60) | 0.062 | 69.4 |
| Source of controls | 28 | |||||||||||||||
| Population based | 13 | 1.31 (1.07–1.60) | 0.008 | 84.7 | 1.57 (1.06–2.32) | 0.023 | 82 | 1.48 (1.12–1.97) | 0.006 | 77.9 | 1.52 (1.13–2.05) | 0.005 | 82.3 | 1.24 (0.99–1.55) | 0.108 | 73.7 |
| Hospital based | 15 | 1.13 (0.96–1.32) | 0.132 | 69.2 | 1.38 (1.02–1.86) | 0.084 | 67.6 | 1.09 (0.94–1.26) | 0.245 | 32.4 | 1.14 (0.92–1.41) | 0.234 | 59.9 | 1.28 (1.03–1.60) | 0.054 | 57.1 |
| Genotyping | 28 | |||||||||||||||
| PCR-RFLP | 24 | 1.24 (1.08–1.42) | 0.002 | 78.6 | 1.56 (1.20–2.02) | 0.002 | 75.4 | 1.32 (1.19–1.46) | 0 | 54.6 | 1.35 (1.12–1.63) | 0.002 | 70.5 | 1.29 (1.08–1.55) | 0.006 | 65.6 |
| Other | 4 | 1.04 (0.72–1.51) | 0.819 | 81.8 | 1.00 (0.57–1.75 ) | 0.992 | 68.8 | 1.30 (1.04–1.64) | 0.024 | 91.6 | 1.03 (0.49–2.19) | 0.934 | 90.4 | 0.97 (0.63–1.47) | 0.871 | 56.6 |
| HWE | 28 | |||||||||||||||
| Yes | 23 | 1.19 (1.05–1.34) | 0.005 | 74.6 | 1.41 (1.10–1.81) | 0.006 | 73.3 | 1.26 (1.06–1.50) | 0.009 | 62.8 | 1.30 (1.08–1.58) | 0.004 | 74.3 | 1.22 (1.07–1.46) | 0.037 | 66.6 |
| No | 5 | 1.30 (0.74–2.27) | 0.366 | 89.1 | 1.33 (0.58–3.08) | 0.502 | 84.1 | 1.185 (0.50–2.81) | 0.700 | 85.3 | 1.25 (0.55–2.82) | 0.594 | 34.1 | 1.35 (0.83–2.20) | 0.226 | 64.8 |
| Lauen type | 4 | |||||||||||||||
| Intestinal | 4 | 1.58 (1.40–1.79) | 0.00 | 0 | 2.71 (2.08–3.52) | 0 | 0 | 1.81 (1.29–2.53) | 0.001 | 62 | 1.96 (1.51–2.54) | 0 | 42 | 1.83 (1.39–2.41) | 0 | 21.1 |
| Diffuse | 4 | 1.09 (0.9–1.32) | 0.374 | 27.9 | 0.95 (0.44–2.02) | 0.883 | 61.1 | 1.02 (0.86–1.54) | 0.231 | 56.2 | 1.43 (0.74–1.60) | 0.428 | 62 | 0.73 (0.35–1.52) | 0.395 | 67 |
Subgroup analysis
Patients were divided into the southern China group and northern China group. Four gene models proved that IL-1β-511C/T polymorphism increased the susceptibility of GC in patients in southern China. However, no significant correlation between this polymorphism and GC susceptibility was found in patients in northern China in any gene model (Table 2).
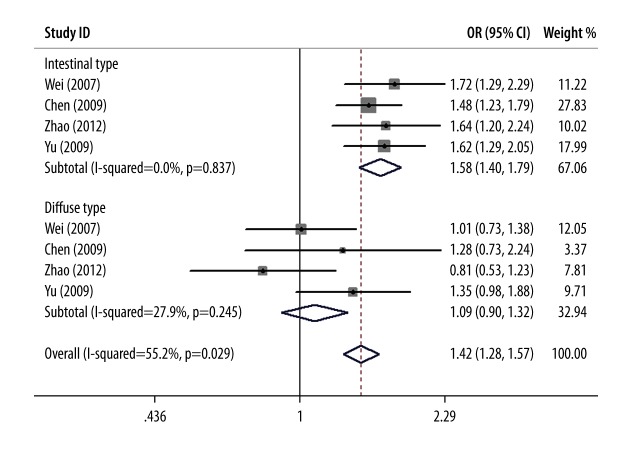
Lauren’s classification was mentioned in 4 studies [30,37,38,44]. The test for heterogeneity showed little heterogeneity among studies (I2=0, P=0.837), and the fixed-effects model was used. Meta-analysis showed that, in the intestinal-type of GC, the onset risk for GC in mutant T allele carriers was 1.58 times that in non-carriers (OR=1.58, 95%CI: 1.40~1.79, P<0.01) and consistent results were found in the dominant gene model, co-dominant gene model, and recessive gene model, but in diffuse-type GC, no correlation was observed in any allele model (Table 2). A forest plot of subgroup meta-analysis according to Lauren’s classification in T vs. C gene model is shown in Figure 2.
Figure 2.
Forest plot of subgroup meta-analysis according to Lauren’s classification in T vs. C gene model.
Subgroup analysis was also performed according to the source of controls, methods of genotyping, and shift in HWE, and results are shown in Table 2
Interactions with H. pylori infection, IL-1β-31 polymorphism for GC
Interaction between H. pylori infection and IL-1β-511 polymorphism for GC was investigated in 7 studies [21,24,27,31,39,42,43]. As in shown in Table 3, H. pylori infection was associated with a trend towards an increased risk of GC, with a pooled OR of 1.9 (95% CI: 1.1–3.5, P=0.03) in non-carriers of T allele. In subjects not infected with H. pylori, the carriage of T (CT+TT) was not associated with an increased susceptibility to GC, with a pooled OR of 1.3 (95% CI: 1.0–1.70, P=0.75). However, H. pylori-infected subjects with carriage of T (CT+TT) exhibited a relatively higher risk of GC, with an OR of 2.4 (95% CI: 1.2-5.1, P=0.02). These data demonstrate a synergistic interaction between IL-1β-511 and H. pylori infection in the occurrence or development of GC.
Table 3.
Combined risks of IL-1β-511 polymorphism and H. pylori infection for GC.
| IL-1β-511 polymorphism | H. pylori infection | OR (95% CI) | P value |
|---|---|---|---|
| C homozygote | (−) | 1 | – |
| T carrier | (−) | 1.3 (1.0–1.7) | 0.75 |
| C homozygote | (+) | 1.9 (1.1–3.5) | 0.03 |
| T carrier | (+) | 2.4 (1.2–5.1) | 0.02 |
Within the included studies, the possible interaction between IL-1β-511 and IL-1β-31 polymorphisms in the development of GC was examined only in Zeng’s research [24], but no linkage disequilibrium was found.
Publication bias
The Begg’s funnel plots of 5 gene models were symmetrical (Figure 3). Egger’s test also showed no publication bias (Egger value was 0.694, 0.509, 0.426, 0.381, and 0.310 for T vs. C, TT vs. CC, CT vs. CC, TT+CT vs. CC, and TT vs. CT+CC, respectively) in our research.
Figure 3.
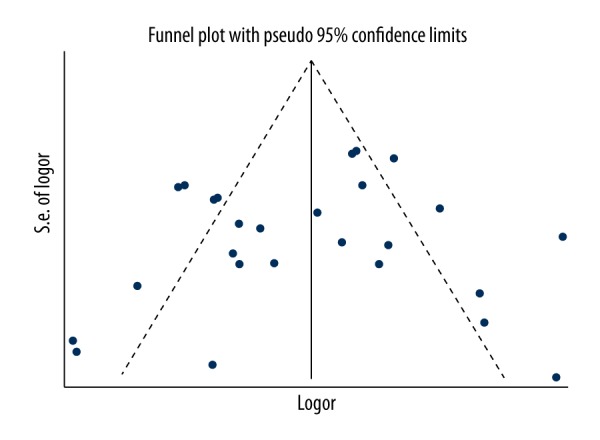
Funnel plot of publication bias on the basis of T vs. C gene model.
Discussion
Since El-Omar first reported that the IL-1β-511 gene allele T carriers was associated with a trend towards an increased onset risk of GC in whites [7], several subsequent studies have reported that this association varies among different regions, races, and cultural habits [31,44,46]. The relationship between IL-1β 511C/T polymorphisms and GC susceptibility in Chinese populations had been investigated in numerous case-control studies, but conflicting results were reported [36].We conducted a meta-analysis to investigate this possible correlation in Chinese populations by using 5 gene models. A total of 28 case-control studies were recruited from 27 relevant studies, and this meta-analysis showed that IL-1β 511C/T polymorphism significantly increased GC susceptibility in Chinese subjects, a finding which is consistent with those of Yu [38] and Zhao [44]. Subgroup analysis showed that IL-1β 511C/T polymorphism increased the onset risk for GC in Chinese subjects in southern China, but not in northern China. According to Lauren’s classification, IL-1β 511C/T polymorphism increased the onset risk for intestinal-type GC, but not for diffuse-type GC.
The incidence of GC was influenced by regional differences. East Asians have relatively higher morbidity of GC, especially in China, Japan, and Korea, accounting for 60% of GC cases worldwide [47]. The number of GC cases in China accounted for 40% in these 3 countries, although the incidence is lower than in Japan and Korea. Epidemiological statistics also showed the incidence of GC was not entirely consistent in different regions [48]. The Qinling-Huaihe line is the geographical boundary between northern and southern China, with differences in hereditary background, climate, and diet and other lifestyle factors [49]. In this study, we investigated the association between IL-1β-511C/T polymorphism and onset risk for GC in Chinese subjects in southern and northern China. Our results showed that IL-1β-511C/T polymorphism significantly increased GC susceptibility of Chinese in southern China, but not in northern China. This finding implies that the hereditary background is different between GC patients in these 2 regions.
In 1965, Lauren classified GC into the intestinal type and diffuse type according to the pathological features [50]. This analysis showed that IL-1β-511C/T polymorphism significantly increased the onset risk for intestinal-type GC, but had no influence on the onset risk of diffuse-type GC. Intestinal-type GC was generally accompanied by high or moderate differentiation and has a relatively good prognosis, but diffuse-type GC was the opposite [51]. The IL-1β-511C/T gene was correlated with occurrence of intestinal-type GC, but determining whether detection of its mutation would help to assess the prognosis of GC needs epidemiological studies.
The potential mechanisms of tumorigenesis are inexplicable as a result of the involvement of multiple risk factors, including the complicated gene-environment and gene-gene interactions [52]. H. pylori infection is regarded as the major cause of GC among environmental factors [2]. In this meta-analysis, we found the susceptibility to GC was significantly increased for T carriers of IL-1β-511C/T polymorphism with H. pylori infection, suggesting that a synergistic interaction between IL-1β-511 and H. pylori infection exists in the development of GC, which is consistent with results reported by Li [31].
Gastric epithelial cells were damaged by inflammation and immune response induced by vacuole toxin and ammonia generated by H. pylori. As a pro-inflammatory factor produced in response to various stimuli, IL-1β plays an important role in the promotion of inflammatory response and expansion of immune responses. Moreover, the overexpression of IL-1β can inhibit gastric acid secretion, thus providing a more favorable environment for the survival and growth of H. pylori, which could further cause atrophy and damage of the gastric mucosa [6]. There 2 mechanisms may explain the conclusion that IL-1β-511C/T polymorphism and its expression contribute to host susceptibility to GC in subjects with H. pylori infection.
Cox first reported the linkage disequilibrium in the interleukin-1 gene cluster and synergistic effects between IL-1β-511 and IL-1β-31polymorphism in 212 white subjects [53]. However, in Zeng’s study [24] no evidence of linkage disequilibrium was found between the 2 SNPs in both high prevalence and low prevalence regions, suggesting that the 2 SNPs were independent risk factors for GC in both areas.
We acknowledge that our study has limitations. 1) The sample size of studies included was not large. 2) Some models have considerable heterogeneity. 3) There were differences in patients and controls among studies, and the sources of controls were inconsistent among studies; in several studies, some controls were recruited from hospitals but others from the general population, thus introducing the possibility of selection bias. 4) Only 4 studies stratified patients according to Lauren’s classification, and subgroup analysis for pathological types other than intestinal-type GC and diffuse-type GC could not be conducted. Because of these factors, the universality of our conclusions is limited. Multicenter, randomized, controlled studies with larger sample sizes are needed to confirm the relationship between IL-1β-511C/T polymorphism and GC susceptibility. Confirmation of this relationship may provide new ideas and clues for the prevention and therapy of GC and accelerate realization of its clinical value.
Conclusions
This meta-analysis indicated that IL-1β-511C/T polymorphism was significantly associated with increased susceptibility to GC in Chinese populations, mainly in residents of southern China and in intestinal-type GC. We found a synergistic interaction between IL-1β-511C/T polymorphism and H. pylori infection in the development of GC. However, further studies are needed to clarify the specific mechanism of the IL-1β-511C/T polymorphisms in the etiology of gastric cancer.
Footnotes
Source of support: This research was supported (in part) by the Fujian Province Natural Science Foundation Youth Innovation Project of China (2009D003) and Natural Science Foundation for the Youth (81201892) without commercial or not-for-profit sectors
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kiga K, Mimuro H, Suzuki M, et al. Epigenetic silencing of miR-210 increases the proliferation of gastric epithelium during chronic Helicobacter pylori infection. Nat Commun. 2014;5:4497. doi: 10.1038/ncomms5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou L, Lin S, Ding S, et al. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chin Med J (Engl) 2014;127(8):1454–58. [PubMed] [Google Scholar]
- 4.Lakhanpal M, Yadav DS, Devi TR, et al. Association of interleukin-1beta-511 C/T polymorphism with tobacco-associated cancer in northeast India: a study on oral and gastric cancer. Cancer Genet. 2014;207(1–2):1–11. doi: 10.1016/j.cancergen.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Oger P, Dessaux Y, Petit A, et al. Validity, sensitivity and resolution limit of the PCR-RFLP analysis of the rrs (16S rRNA gene) as a tool to identify soil-borne and plant-associated bacterial populations. Genet Sel Evol. 1998;30:1–22. [Google Scholar]
- 6.Ragini S, Antara K, Mohan K, et al. Mucosal IgA & IL-1β in Helicobacter pylori Infection. Indian J Clin Biochem. 2013;28(1):19–23. doi: 10.1007/s12291-012-0262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 8.Santos JC, Ladeira MSP, Pedrazzoli J, et al. Relationship of IL-1 and TNF-α polymorphisms with Helicobacter pylori in gastric diseases in a Brazilian population. Braz J Med Biol Res. 2012;45(9):811–17. doi: 10.1590/S0100-879X2012007500099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu B, Zou HY, Yang YH, et al. Interleukin-1B-31 gene polymorphism in Hakka gastric cancer patients in Guangdong, China. Genet Mol Res. 2014;13(3):5873–79. doi: 10.4238/2014.August.7.2. [DOI] [PubMed] [Google Scholar]
- 10.Van der Ploeg AH, Kumpf O, Seelow E, et al. The course of gastric cancer following surgery is associated with genetic variations of the interleukin-1 receptor antagonist and interleukin-1β. Gastric Cancer. 2015;18(1):77–83. doi: 10.1007/s10120-014-0349-z. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi A, Ohmiya N, Shirai K, et al. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005;14:2487–93. doi: 10.1158/1055-9965.EPI-05-0326. [DOI] [PubMed] [Google Scholar]
- 12.Tatemichi M, Sawa T, Gilibert I, et al. Increased risk of intestinal type of gastric adenocarcinoma in Japanese women associated with long forms of CCTTT pentanucleotide repeat in the inducible nitric oxide synthase promoter. Cancer Lett. 2005;217:197–202. doi: 10.1016/j.canlet.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Wen YY, Pan XF, Loh M, et al. Association of the IL-1B +3954 C/T polymorphism with the risk of gastric cancer in a population in Western China. Eur J Cancer Prev. 2014;23(1):35–42. doi: 10.1097/CEJ.0b013e3283656380. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Zheng H, Zhou Y, et al. Association of IL-1beta gene polymorphism with cachexia from locally advanced gastric cancer. BMC Cancer. 2007;7:45. doi: 10.1186/1471-2407-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue H, Lin B, Ni P, et al. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J Gastroenterol Hepatol. 2010;2512(10):1604–17. doi: 10.1111/j.1440-1746.2010.06428.x. [DOI] [PubMed] [Google Scholar]
- 16.Park MJ, Hyun MH, Yang JP, et al. Effects of the interleukin-1β-511 C/T gene polymorphism on the risk of gastric cancer in the context of the relationship between race and H. pylori infection: A meta-analysis of 20,000 subjects. Mol Biol Rep. 2015;42(1):119–34. doi: 10.1007/s11033-014-3748-7. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statementJ. J Clin Epidemiol. 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. 200. [DOI] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O’Connell D, et al. New Castle-Ottawa Quallty Assessment Scale – case control studies [EB/OL] 2015. Apr 15, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19.He XM, Fu YB, Jiang L, et al. Relationship between interleukin-1B and interleukin-1 receptor antagonist gene polymorphisms and susceptibility to gastric cancer. Zhonghua Yi Xue Za Zhi. 2002;82(10):40–43. [PubMed] [Google Scholar]
- 20.Wu MS, Wu CY, Chen CJ, et al. Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int J Cancer. 2003;104(5):617–23. doi: 10.1002/ijc.10987. [DOI] [PubMed] [Google Scholar]
- 21.Chen A, Li CN, Hsu PI, et al. Risks of interleukin-1 genetic polymorphisms and Helicobacter pylori infection in the development of gastric cancer. Aliment Pharmacolther. 2004;20(2):203–11. doi: 10.1111/j.1365-2036.2004.01826.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Hu Z, Xu Y, et al. Interleukin-1B gene promoter variants are associated with an increased risk of gastric cancer in a Chinese population. Cancer Lett. 2004;215(2):191–98. doi: 10.1016/j.canlet.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Hu S, Song QB, Yu D, et al. Association of interleukin-1 gene polymorphism with gastric cancer in a high-risk area of China. Di Yi Jun Yi Da Xue Xue Bao. 2004;24(10):1171–73. [PubMed] [Google Scholar]
- 24.Zeng ZR, Hu PJ, Hu S, et al. Association of interleukin 1B gene polymorphism and gastric cancers in high and low prevalence regions in China. Gut. 2003;52(12):1684–89. doi: 10.1136/gut.52.12.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu W, Pan K, Zhang L, et al. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor α and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26(3):631–36. doi: 10.1093/carcin/bgh349. [DOI] [PubMed] [Google Scholar]
- 26.Zhang WH, Wang XL, Zhou J, et al. Association of interleukin-1B (IL-1B) gene polymorphisms with risk of gastric cancer in Chinese population. Cytokine. 2005;30(6):378–81. doi: 10.1016/j.cyto.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Xing PX, Zeng QD, GAO W, et al. Relationship between IL-1 gene polymorphisns and gastric adenocarcinoma. Chin J Curr Adv Gen Surg. 2006;9(6):359–62. [Google Scholar]
- 28.Gao W, Cui X, Hu AL, et al. Relationship between Interleukin-1B and Interleuk in-1 receptor antagonist gene polymorphisms and susceptibility to gastric cancer. Journal of Jiangsu University (Medicine Edition) 2006;16(4):339–41. [Google Scholar]
- 29.Zheng LZ, Cai W, Chen WS, et al. Association between functional genetic polymorphisms of IL-1B and IL-1 RN and susceptibility To gastric cancers. Journal of Shanghai Jiaotong University (Medical Science) 2007;27(4):428–32. [Google Scholar]
- 30.Wei M. Study on correlation between interlenkin-1B polymorphisms and increased risk of gastric carcinoma. Chin J Cancer Prev Treat. 2007;14(4):258–60. [Google Scholar]
- 31.Li C, Xia HH, Xie W, et al. Association between interleukin-1 gene polymorphisms and Helicobacter pylori infection in gastric carcinogenesis in a Chinese population. J Gastroenterol Hepatol. 2007;22(2):234–39. doi: 10.1111/j.1440-1746.2006.04379.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Zheng H, Zhou Y, et al. Association of IL-1beta gene polymorphism with cachexia from locally advanced gastric cancer. BMC Cancer. 2007;7:45. doi: 10.1186/1471-2407-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun H, Wang Y, Ma X, et al. A method of oligochip for single nucleotide polymorphism genotyping in the promoter region of the interleukin-1 beta gene and its clinical application. Oligonucleotides. 2007;17(3):336–44. doi: 10.1089/oli.2007.0071. [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, Zhang J, Dai L, et al. Inflammatory cytokine gene polymorphisms in gastric cancer cases’ and controls’ family members from Chinese areas at high cancer prevalence. Cancer Lett. 2008;270(2):250–59. doi: 10.1016/j.canlet.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Jia A, Gong J, Li YC, et al. Correlation of polymorphisms of the IL-1 promoter region and tumor necrosis factor alpha gene with susceptibility of non-cardiac gastric cancer in a Han nationality of Shaanxi Chinese population. Journal of Xi’an Jiaotong University (Medical Sciences) 2009;30(1):70–73. 127. [Google Scholar]
- 36.Xiang Y, Yang ZB, Chen P, et al. Relationship between IL-1B and TNF-α gene polymorphisms and susceptibilities to gastric ulcer and cancer. Chin J Biologicals. 2009;22(100):1010–14. [Google Scholar]
- 37.Chen B, Zeng ZR, WU XQ, et al. Association between interleukin-1B-511 polymorphism and gastric cancer of different clinical and pathological characteristics. Journal of Sun Yat-Sen University (Medical Sciene) 2009;30(3):331–35. 347. [Google Scholar]
- 38.Yu J, Zeng Z, Wang S, et al. IL-1B-511 polymorphism is associated with increased risk of certain subtypes of gastric cancer in Chinese: A case-control study. Am J Gastroenterol. 2010;105(3):557–64. doi: 10.1038/ajg.2009.644. [DOI] [PubMed] [Google Scholar]
- 39.Jiang DL, Chen YP, Dai Y. Association of interleukin-1B gene polymorphism and H. pylori infection and susceptibility to gastric cancer. Acta Med Univ Sci Technol Huazhong. 2010;39(5):695–97. [Google Scholar]
- 40.Li K, Yang J, Chen ZR. Relationships among interleukin-1beta,interleukin-1 receptor antagonist gene polymorphism and susceptibility to gastric cancer. Sichuan Da Xue Xue Bao Yi Xue Bao. 2010;41(6):1039–43. [PubMed] [Google Scholar]
- 41.Zu HY, Qiu B, Yang QH, et al. Relationship between IL-1B-511 gene polymorphism and susceptibility to gastric cancer among ke populationin in Meizhou, Guangdong. Guangdong Medical Journal. 2011;32(23):3074–76. [Google Scholar]
- 42.He BS, Pan YQ, Xu YF, et al. Polymorphisms in interleukin-1B (IL-1B) and interleukin 1receptor antagonist (IL-1RN) genes associate with gastric cancer risk in the Chinese population. Dig Dis Sci. 2011;56(7):2017–23. doi: 10.1007/s10620-010-1557-y. [DOI] [PubMed] [Google Scholar]
- 43.Zhang QS, Pan YQ, Xu YQ, et al. Association between IL-1 gene polymorphism with Helicobacter pylori infection and gastric cancer. Chin J Clin Lab Sci. 2012;30(3):207–9. [Google Scholar]
- 44.Zhao JD, Geng PL, Li ZQ, et al. Associations between interleukin-1 polymorphisms and gastric cancers among three ethnicities. World J Gastroenterol. 2012;18(47):7093–99. doi: 10.3748/wjg.v18.i47.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang JX, Duan GC, Guo AY, et al. Study on the relationship of IL-1B gene polymorphism and Helicobacter pylori infection with gastric cancer. Medical Innovation of China. 2014;11(27):1–3. [Google Scholar]
- 46.Persson C, Engstrand L, Nyrén O, et al. interleukin-1 beta polymorphisms and risk of gastric cancer in Sweden. Scand J Gastroenterol. 2009;44(3):339–45. doi: 10.1080/00365520802556015. [DOI] [PubMed] [Google Scholar]
- 47.Sung JJ, Ng EK, Lin JT, et al. Digestive cancer management in Asia: position statements: a report on GI Oncology Summit in 2011. J Gastroenterol Hepatol. 2012;27(9):1417–22. doi: 10.1111/j.1440-1746.2012.07194.x. [DOI] [PubMed] [Google Scholar]
- 48.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12(1):17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jie F. Geography (textbook) [M] People Education Press. 2013:5–6. [Google Scholar]
- 50.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 51.Yang J, Wu Z, Li L, et al. Research progress of Lauren classification for gastric cancer. Zhong Nan Da Xue Bao Yi Xue Ban. 2015;40(8):934–40. doi: 10.11817/j.issn.1672-7347.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Pharoah PD, Dunning AM, Ponder BA, et al. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004;4(11):850–60. doi: 10.1038/nrc1476. [DOI] [PubMed] [Google Scholar]
- 53.Cox A, Camp NJ, Nicklin MJ, et al. An analysis of linkage disequilibrium in the interleukin-1 gene cluster, using a novel grouping method formultiallelic markers. Am J Hum Genet. 1998;62(5):1180–88. doi: 10.1086/301817. [DOI] [PMC free article] [PubMed] [Google Scholar]