Abstract
Background information
Focal adhesion kinase (FAK), an essential non-receptor tyrosine kinase, plays pivotal roles in migratory responses, adhesive signaling, and mechanotransduction. FAK-dependent regulation of cell migration involves focal adhesion turnover dynamics as well as actin cytoskeleton polymerization and lamellipodia protrusion. Whereas roles for FAK in migratory and mechanosensing responses have been established, the contributions of FAK to the generation of adhesive forces are not well understood.
Results
Using FAK-null cells expressing wild-type and mutant FAK under an inducible tetracycline promoter, we analyzed the role of FAK in the generation of steady-state adhesive forces using micropatterned substrates and a hydrodynamic adhesion assay. FAK expression reduced steady-state strength by 30% compared to FAK-null cells. FAK expression reduced vinculin localization to focal adhesions by 35% independently from changes in integrin binding and localization of talin and paxillin. RNAi knockdown of vinculin abrogated the FAK-dependent differences in adhesive force. FAK-dependent changes in vinculin localization and adhesive force were confirmed in human primary fibroblasts with FAK knocked down by RNAi. The autophosphorylation Y397 and kinase domain Y576/Y577 sites were differentially required for FAK-mediated adhesive responses.
Conclusions
We demonstrate that FAK reduces steady-state adhesion strength by modulating vinculin recruitment to focal adhesions. These findings provide insights into the role of FAK in mechanical interactions between a cell and the extracellular matrix.
Keywords: fibronectin, integrin, mechanotransduction, extracellular matrix, talin
Introduction
Mechanical interactions between a cell and its environment regulate morphogenesis, tissue homeostasis and remodeling, and pathogenesis (Montell, 2008; Kumar and Weaver, 2009; Wozniak and Chen, 2009). Cell adhesion to extracellular matrices (ECM) provides adhesive forces mediating migratory processes, tissue structure and organization, and mechanotransduction responses (Hynes, 2002; Danen and Sonnenberg, 2003; Ingber, 2003). Adhesion to ECM components, such as fibronectin and laminin, is primarily mediated by the integrin family of heterodimeric αβ receptors (Hynes, 2002). Following activation and ligand binding, integrins rapidly associate with the actin cytoskeleton and cluster together to form focal adhesions, discrete supramolecular complexes that contain structural proteins, such as vinculin, talin, and α-actinin, and signaling molecules, including focal adhesion kinase (FAK), Src, and paxillin (Geiger et al., 2001). Focal adhesions function as structural links, providing strong adhesive forces, and signal transduction elements between the cell and its extracellular environment. These adhesive complexes are dynamic structures that are actively remodeled during cell migration (Ridley et al., 2003; Gupton and Waterman-Storer, 2006). Assembly/disassembly of focal adhesions is regulated by numerous pathways in response to external stimuli, including growth factors and mechanical force (Ridley and Hall, 1992; Greenwood et al., 1998; Riveline et al., 2001). Whereas the biochemical connections in adhesive interactions have been extensively characterized (Zaidel-Bar et al., 2007), the interplay between adhesive structural and molecular composition and adhesive forces remains poorly understood.
FAK, a widely expressed non-receptor protein tyrosine kinase, plays central roles in adhesive interactions by functioning as scaffold for focal adhesion components, including Src, Cas, and paxillin (Hanks et al., 1992; Schaller et al., 1992; Polte and Hanks, 1995; Schaller et al., 1999). FAK functions as an integrator of integrin-mediated signaling to regulate cell migration, survival, cell cycle progression and differentiation (Ilic et al., 1995; Zhao et al., 1998; Owen et al., 1999; Renshaw et al., 1999; Sieg et al., 2000; Webb et al., 2004; Quach et al., 2009; Tomar et al., 2009). FAK expression is essential to development and organogenesis. Deletion of the FAK gene results in early embryonic lethality due to defects in cell migration (Furuta et al., 1995). Tissue-specific knock-out of FAK produces functional defects in angiogenesis/vasculogenesis, branching tubulogenesis, innervation and myelination, cardiac development, and blood-testis barrier function (Shen et al., 2005; Braren et al., 2006; Peng et al., 2008; Watanabe et al., 2008; Forrest et al., 2009; Siu et al., 2009; Wei et al., 2009). FAK has also been implicated in tumor invasion and metastasis (Chan et al., 2009; Shibue and Weinberg, 2009). Finally, FAK has emerged as an important mechanotransducer (Wang et al., 2001; Pirone et al., 2006; Clemente et al., 2007; Leucht et al., 2007; Schober et al., 2007; Young et al., 2009).
FAK-dependent regulation of cell migration involves focal adhesion turnover dynamics (Owen et al., 1999; Wang et al., 2001; Webb et al., 2004). In addition, FAK modulates actin cytoskeleton polymerization and lamellipodia protrusion (Serrels et al., 2007). Whereas roles for FAK in migratory and mechanosensing responses have been established, the contributions of FAK to the generation of adhesive forces are not well understood. We recently demonstrated that FAK promotes integrin activation to enhance the generation of cell-ECM adhesive forces (Michael et al., 2009). These FAK-dependent enhancements in integrin activation and adhesion strengthening only occurred during the early stages of the adhesive process. In the present study, we analyzed steady-state adhesive interactions and demonstrate that FAK reduces steady-state adhesion strength by modulating vinculin recruitment to focal adhesions. These findings establish a multi-faceted role for FAK in the generation of cell-ECM forces.
Results
FAK reduces steady-state adhesion strength
To examine the role of FAK in adhesive force responses, we used FAK-null cells engineered for tetracycline-regulated expression of wild-type and mutant FAK (Tet-FAK cells) (Owen et al., 1999; Michael et al., 2009). Tet-FAK cells were maintained in the off-condition (FAK−) and FAK expression was induced at 48 hours prior to any experiment to ensure steady-state FAK levels (FAK+). We previously demonstrated that FAK is expressed at high levels in the absence of tetracycline, while FAK expression is repressed in the presence of tetracycline (Michael et al., 2009). This inducible behavior in response to tetracycline is specific for FAK as no differences in expression levels were detected between these two culture conditions for other proteins, including Pyk2, vinculin, talin, and β1 integrin. We analyzed two independent clones with equivalent results and present results for one clone (clone 46 for wild-type FAK).
Tet-FAK cells were cultured overnight on fibronectin-coated micropatterned substrates (5µm diameter circles, 75βm center-to-center spacing) to ensure equivalent adhesive area and cell shape between FAK+ and FAK− conditions. Tet-FAK cells readily adhere and remain constrained to the micropatterned area as single cells, consistent with previous analyses with other cell types (Gallant et al., 2005).
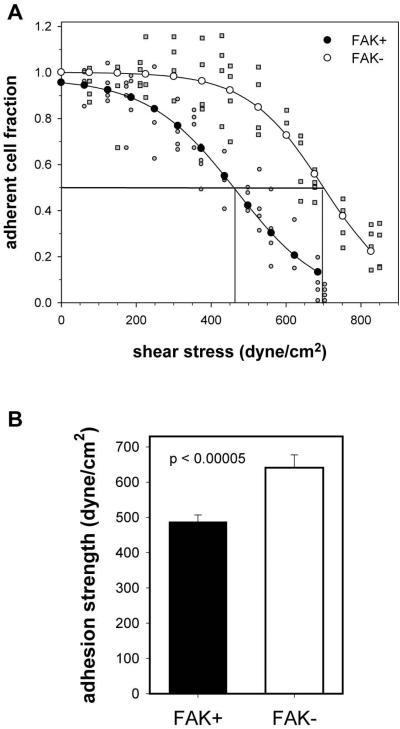
We measured the steady-state levels of adhesion strength for FAK+ and FAK− cells at 24 hours using a hydrodynamic adhesion assay that provides direct and sensitive population-based measurements of adhesive force (Gallant et al., 2005). We previously demonstrated that steady-state adhesion is reached by 4 hours in this cellular system (Michael et al., 2009). For this adhesion assay, coverslips containing micropattered cells are placed and spun on a rotating disk submerged in buffer at prescribed speeds. The disk rotation generates a well-defined 3-D fluid flow that applies a controlled hydrodynamic force to adherent cells. The hydrodynamic force increases linearly with radial position along the surface of the coverslip, such that cells at the center of the substrate experience negligible forces whereas the applied detachment force increases toward the outer edge of the disk, resulting in decreasing cell numbers. In this manner, a linear range of forces is applied to a large cell population and adhesive strength measurements are obtained for > 6,000 cells in a single experiment. After spinning, adherent cells are fixed and stained, and cell numbers at different radial positions are quantified using a motorized microscope stage and image analysis system. The fraction of adherent cells (f) is calculated by dividing the number of cells in each field by the number of cells at the center of the coverslip, where negligible forces are applied. The detachment profile (cell adherent fraction vs. shear stress τ [stress = force/area]) is then fit to a sigmoid curve to obtain the shear stress for 50% detachment. The shear stress for 50% detachment (τ50) represents the mean adhesive force. Figure 1A shows typical detachment profiles (gray circles (FAK+) and squares (FAK−) represent cell densities at a specific radial position and fitted points (filled circles (FAK+), empty circles (FAK−)) and sigmoid fit. The right-ward shift in the detachment profile for the FAK− cells compared to the FAK+ condition indicates a higher adhesive force. The τ50 values over several independent experiments were averaged (Fig. 1B). FAK− cells exhibited a 33% increase in adhesion strength compared to FAK+ cells (p < 0.00005). This finding indicates that FAK reduces steady-state adhesive forces.
Figure 1.
FAK modulates steady state levels of adhesive force. (A) Adhesion profiles showing the fraction of adherent cells as a function of applied shear stress for FAK-expressing (FAK+) and FAK-null (FAK−) cells. Experimental values (FAK+, gray circles; FAK-, gray squares) were fit to a sigmoid curve (fitted values: FAK+, filled circles; FAK− empty circles) to obtain the shear stress for 50% detachment, indicated by vertical lines. (B) Mean adhesion strength values showing increased adhesive force for FAK− cells (average ± standard deviation).
FAK modulates steady-state adhesive force via vinculin recruitment to the adhesive interface
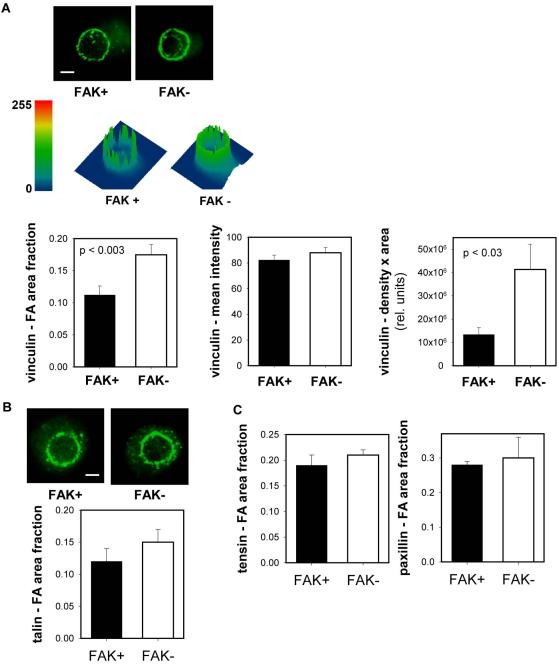
The reduction in steady-state adhesion strength for FAK-expressing cells could arise from (i) a decrease in the number of integrin/ECM bonds, (ii) modified position/distribution of bonds, (iii) reduction in the coupling of integrin/ECM bonds to the cytoskeleton (e.g., focal adhesion assembly), or (iv) change in the conformation state of the bound integrins. We previously demonstrated that integrin α5β1 binding to fibronectin provided the dominant adhesion mechanism in this cellular system (Michael et al., 2009). Biochemical analyses of integrin binding revealed no differences in the numbers of bound integrin for FAK+ and FAK− cells at steady state, and no differences in integrin localization within the adhesive interface were observed by immunostaining (Michael et al., 2009). In addition, we previously demonstrated that there were no differences in the activation state of β1 between FAK+ and FAK− conditions at steady-state (Michael et al., 2009). We therefore postulated that the differences in steady-state adhesive force arise from differences in focal adhesion assembly. We have demonstrated that focal adhesion assembly, independently from integrin binding, contributes significantly to adhesion strength (Gallant et al., 2005). Immunostaining for vinculin demonstrated localization of this cytoskeletal protein around the periphery of the micropatterned contact area for both FAK+ and FAK− cells (Fig. 2A). We conducted a comprehensive analysis of vinculin localization focusing on fractional area, intensity (density), and the product of intensity and area (Fig. 2A). FAK+ cells exhibited a significant (35%) reduction in the adhesive area occupied by vinculin compared to FAK− cells. There are no differences in mean intensity between FAK+ and FAK− cells. The differences in the density & area product between FAK+ and FAK− are attributed to differences in focal adhesion area. Differences in recruitment to the adhesive area were specific for vinculin as no differences in staining were detected between FAK+ and FAK− cells for talin, tensin, and paxillin (Fig. 2B,C).
Figure 2.
FAK modulates vinculin localization to the adhesive interface independently from changes in integrin binding. (A) Immunostaining for vinculin recruitment to micropatterned adhesive area showing differences in vinculin-containing focal adhesion area (scale bar, 2 µm). Surface plots displaying vinculin staining intensity (height) and distribution within micropatterned area. FAK+ cells exhibited reduced vinculin-positive area compared to FAK− cells, while vinculin intensity was equivalent for both cell types. Quantification of vinculin-occupied adhesive area, intensity, and area-intensity product. (B,C) Immunostaining (talin) and quantification of focal adhesion components recruited to micropatterned adhesive area showing no differences between FAK+ and FAK− cells.
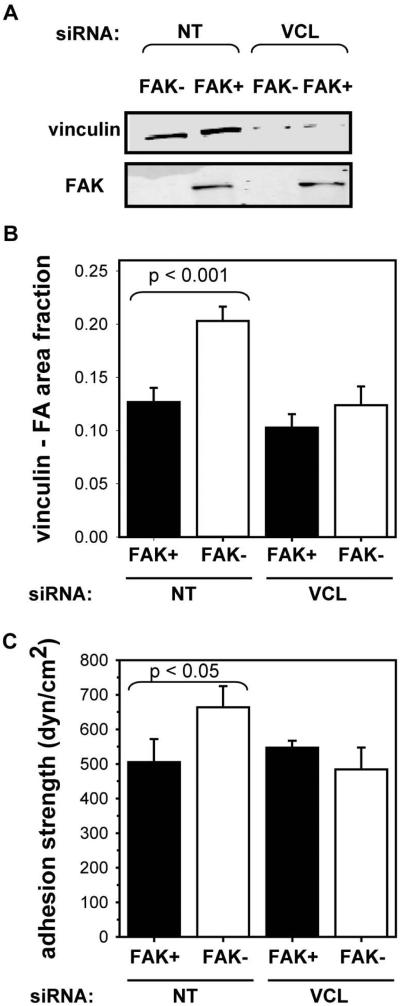
Recruitment of focal adhesion components to integrin clusters is expected to increase adhesive force by efficiently distributing mechanical loads among bound integrins. In fact, we previously demonstrated that vinculin recruitment to focal adhesions enhances adhesion strengthening by 30% in fibroblasts (Gallant et al., 2005). We therefore carried out RNAi knock-down experiments to determine the contributions of vinculin to steady-state adhesion strength in FAK+ and FAK− cells. Tet-FAK cells were co-transfected with a GFP plasmid and siRNA pooled duplexes for either vinculin (VCL) or non-targeting control sequence (NT) via nucleofection. After 24 h, cells were sorted for GFP expression and cultured in the appropriate tetracycline condition for 48 h prior to cell adhesion analyses. siRNA VCL knock-down reduces vinculin levels by 80% in FAK+ and FAK− cells (Fig. 3A); the non-targeting control sequence has no effects on vinculin levels. Immunostaining analyses demonstrated that vinculin knock-down eliminates the differences in vinculin localization between FAK+ and FAK− cells at 24 h (Fig. 3B), whereas significant differences in vinculin localization are still evident between FAK+ and FAK− cells for the non-targeting control siRNA. Notably, vinculin knock-down abrogates differences in steady-state adhesion strength between FAK+ and FAK− cells (Fig. 3C). Interestingly, no significant differences in vinculin recruitment or adhesion strength were observed between siRNA-treated and control FAK+ cells. We attribute this finding to residual vinculin localization to focal adhesions since only 15-20% of the total vinculin pool localizes to focal adhesions (Gallant et al., 2005). Vinculin exhibits a high affinity for localization to focal adhesions and other investigators have experienced difficulties in completely eliminating vinculin localization from focal adhesions via RNAi approaches (S.W. Craig, personal communication). Taken together, these results demonstrate that FAK regulates steady-state adhesive force by modulating vinculin localization to focal adhesions.
Figure 3.
FAK regulates steady-state adhesive force by modulating vinculin localization to focal adhesions. (A) RNAi knock-down (VCL = vinculin; NT = non-targeting control) reduced vinculin levels by 80% in FAK+ and FAK−. (B) Vinculin knock-down eliminated differences in steady-state vinculin recruitment to the adhesive area between FAK+ and FAK− cells. (C) Vinculin knock-down eliminated differences in steady-state adhesion strength between FAK+ and FAK− cells.
Role of FAK Y397 and Y576/Y577 on adhesive force
We also examined the role of tyrosine phosphorylation sites in FAK in adhesive force using Tet-FAK cells expressing FAK mutants. FAK Y397 is an autophosphorylation site that promotes interaction with SH2 domain-containing signaling proteins including Src, PLC-γ, PI3K and Shc (Hanks et al., 2003). FAK Y576 and Y577 are in the activation loop of the kinase domain, and mutation of these tyrosines reduces FAK catalytic activity (Calalb et al., 1995). We used Tet-FAK cells expressing the mutants FAK-F397 and FAK-F576/F577 previously described (Owen et al., 1999). Results are presented for FAK-F397 clone 18 and FAK− F576/F577 clone 16. Western blotting for FAK previously confirmed induction of FAK mutants in these Tet-FAK cells (Owen et al., 1999; Michael et al., 2009).
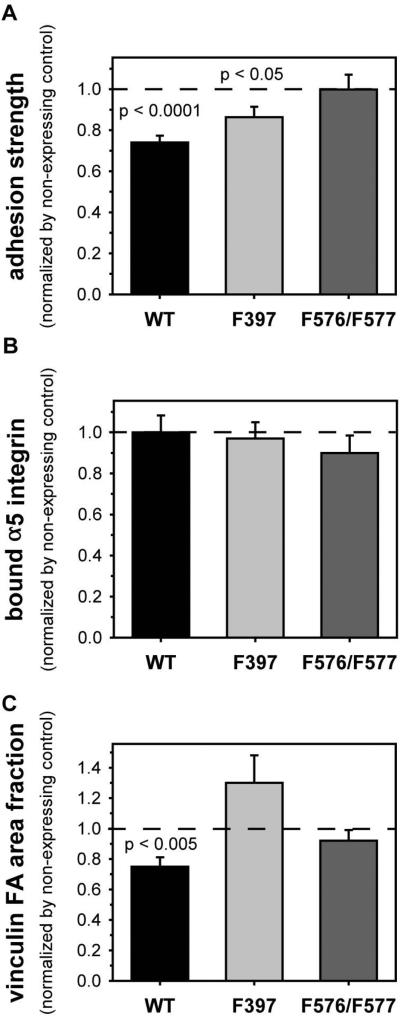
An advantage of the tetracycline-inducible FAK expression system is the ability to directly compare effects of expressed proteins within the same cells, thereby avoiding limitations associated with clonal variability. Therefore, results for cells expressing FAK mutants were normalized to the values for the matched non-expressing control cells. Steady-state levels of adhesion strength exhibited differences among wild-type and mutant FAK (Fig. 4A). Compared to the 30% reduction in adhesion strength for wild-type FAK, expression of FAK-F397 resulted in a partial (14%) reduction in steady-state adhesive force compared to its matched non-expressing control. Expression of FAK-F576/F577 did not alter steady-state levels of adhesive force compared to its non-expressing control, indicating that phosphorylation of these residues in the catalytic loop is required for the effects of FAK on steady-state adhesive force. No significant differences were observed in steady-state levels of bound integrins between FAK mutants and their respective non-expressing controls (Fig. 4B), indicating that differences in integrin binding did not contribute to the differences in adhesion strength. In contrast, significant differences in vinculin recruitment to focal adhesions were observed among FAK variants (Fig. 4C). Whereas expression of wild-type FAK reduced vinculin localization to focal adhesions compared to its matched non-expressing control, expression of either FAK-F397 or FAK-F576/F577 had no significant effects in vinculin recruitment (p > 0.05). These results indicate that the Y397 and Y576/577 sites play distinct roles in FAK function, but both sites are critical to FAK function in adhesive responses.
Figure 4.
FAK-mediated adhesive force requires phosphorylation of FAK activation sites. Results for expressed FAK proteins are normalized to values for the FAK-null matched controls. (A) Adhesion strength is differentially modulated by phosphorylation of Y397 and Y576/Y577. (B) Inactivating mutations to Y397 and Y576/Y577 do not alter steady-state integrin binding levels. (C) Recruitment of vinculin to focal adhesions requires phosphorylation of Y397 and Y576/Y577.
FAK knock-down in human fibroblasts increases adhesive force and vinculin recruitment
We next examined the effects of FAK expression on adhesion strength and vinculin recruitment in primary fibroblasts to rule out any artifacts associated with the Tet-FAK cells. Human dermal fibroblasts were transduced with FAK siRNA-puromycin or control puromycin retrovirus, and puromycin-resistant cells were selected. We previously showed that fibroblasts transduced with FAK siRNA retrovirus exhibited a 95% reduction in FAK expression compared to control cells, whereas vinculin and Pyk2 levels remained unchanged (Michael et al., 2009).
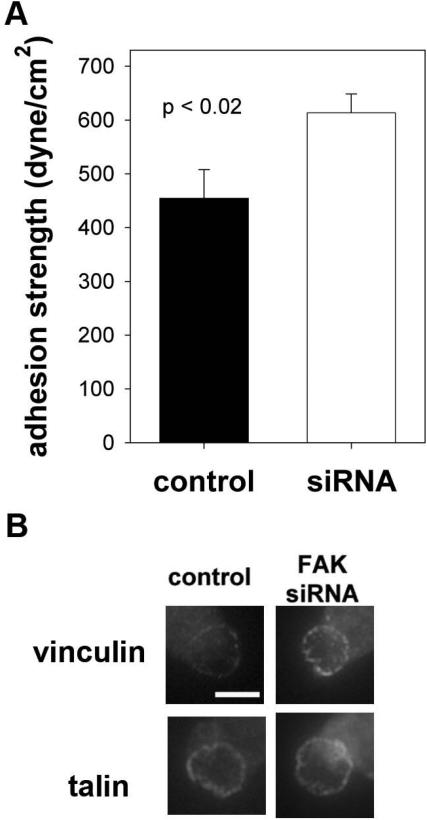
Measurements of adhesion strength for micropatterned cells showed 35% higher levels for fibroblasts with knocked-down FAK expression compared to control cells (Fig. 5A). This relative increase in adhesion strength is in excellent agreement with the results for the Tet-FAK cells. Furthermore, consistent with our observations for Tet-FAK cells, FAK knock-down enhances vinculin, but not talin, localization to the adhesive area (Fig. 5B).
Figure 5.
FAK knock-down in human dermal fibroblasts modulates adhesive force. Human dermal fibroblasts were transduced with FAK siRNA-puro or control retrovirus and puromycin-resistant cells were selected. (A) Knock-down of FAK increases cell adhesion strength. (B) FAK knock-down enhances vinculin, but not talin, recruitment to adhesive interface.
Discussion
We demonstrate that FAK reduces steady-state adhesive force via vinculin localization to focal adhesions. Vinculin localization to the adhesive interface most likely modulates adhesive force by altering the local distribution of forces at the adhesive interface (Gallant et al., 2005; Gallant and Garcia, 2007). This work provides new insights into the role of FAK in the generation of adhesive forces involved in mechanical interactions between a cell and its environment. We recently demonstrated that during the early stages of adhesion FAK enhances the adhesion strengthening rate by upregulating integrin activation to enhance integrin binding (Michael et al., 2009). These FAK-dependent changes in integrin activation and binding and adhesive force generation were only observed in the early stages (<1-2 hour) of the adhesion process. In the present study, we demonstrate that, as the adhesive process reaches steady-state, FAK has an opposite effect in adhesive forces by reducing adhesion strength. This down-regulation in adhesive force arises from a separate mechanisms involving vinculin localization to focal adhesions. Once localized to focal adhesions, vinculin is proposed to be the primary functional link between focal adhesions and the actin cytoskeleton (Humphries et al, 2007). Taken together, these results indicate that FAK plays a multi-faceted, time-dependent role in adhesive force generation. These time-dependent, mechanism-distinct roles for FAK in adhesive force generation may be related to the biochemically distinct stages in cell adhesion to fibronectin recently established by Sheetz and colleagues (Zhang et al., 2008). Whereas we demonstrate significant contributions for FAK in adhesive force generation, previous studies with deformable substrates did not reveal a role for FAK in the development of traction forces (Wang et al., 2001; Pirone et al., 2006). We attribute these seemly contradictory observations to fundamental differences among cellular forces. We propose that mechanical interactions between a cell and its environment involve diverse force components (e.g., traction/propulsive, contractile, tensile, adhesive). Importantly, these factors are not equal and should therefore be considered as distinct entities that together comprise cellular forces.
Analysis of FAK phosphorylation mutants demonstrated important roles for the Y397 autophosphorylation site and the Y576/Y577 sites in the activation loop of the kinase domain. The Y576/Y577 sites in the activation loop were necessary for FAK-dependent modulation of adhesive force. Mutation of the Y397 autophosphorylation site partially blocked FAK-mediated adhesive responses, indicating that other residues in FAK, such as Y576/Y577, contribute to adhesive force responses independently from Y397. Migratory and spreading activities exhibit a strong dependence on Y397 (Owen et al., 1999; Sieg et al., 2000; Wang et al., 2001; Webb et al., 2004), and mutation of this residue often results in complete blocking of these responses. While Y576/Y577 are important for maximal FAK kinase activity and Y397 autophosphorylation (Owen et al., 1999), mutation of these sites does not completely block these activities and has a less significant impact on cell migration and long-term spreading in comparison to Y397 mutation (Owen et al., 1999). Our findings reveal that the FAK Y576/Y577 sites can impact adhesive force generation through a mechanism that is unrelated to Y397 phosphorylation. These results emphasize the lack of direct correspondence between adhesion strength and cell migration/spreading assays. The critical role of the Y576/Y577 sites in adhesive force generation could indicate that an exogenous FAK substrate(s), whose phosphorylation is dependent on FAK activation loop phosphorylation, is important for this process.
The mechanism by which FAK regulates the steady-state levels of vinculin localization to the adhesive area remains unknown. We performed immunoprecipitation experiments of vinexin E, a binding partner of activated vinculin (Chen et al., 2005), with FAK but showed no interaction (data not shown), indicating that vinexin E is not a direct link between FAK and vinculin activation. We postulate that effectors responsible for focal adhesion disassembly during migration (Webb et al., 2004) may be involved in the FAK-dependent reduction in steady-state adhesion strength. Interestingly, recent studies with fluorescence recovery after photobleaching suggest that vinculin exchange dynamics within focal adhesions are directly coupled to phosphorylation of tyrosine 1065 (Möhl et al, 2009). Specifically, phosphorylation of vinculin decreases as focal adhesions mature and stabilize. It is possible that the loss of FAK reduces phosphorylation of vinculin, and thus modifies the dynamics of vinculin in focal adhesions. Moreover, talin represents an attractive potential effector since talin binding to vinculin influences vinculin activation (Cohen et al., 2006). Talin has also been implicated in formation of the ECM-cytoskeleton linkage, focal adhesion formation, and substrate traction (Jiang et al., 2003; Zhang et al., 2008). Additional analyses beyond the scope of the present work are required to establish the mechanistic links between FAK and its effectors in adhesive force generation.
Materials and Methods
Cells and reagents
Tet-FAK cells were maintained as described previously (Owen et al., 1999). Primary human dermal fibroblasts were kindly provided by A.P. Kowalczyk (Emory Univ.). The FAK siRNA construct has been described (Benlimame et al., 2005). Retrovirus packaging and transductions were performed as described previously (Byers et al., 2002). Monoclonal antibodies against vinculin (clone V284, Upstate), talin (clone 8d4, Sigma), paxillin (clone Z035, Zymed), and tensin (clone 5, BD Biosciences) were used for immunostaining. Antibodies against vinculin (clone V284, Upstate) and FAK (06-543 polyclonal, Upstate) were used for Western blotting.
Micropatterned substrates
Micropatterned substrates were generated by microcontact printing of self-assembled monolayers of alkenethiols on gold (Gallant et al., 2005). Arrays of CH3-terminated alkanethiol (HS-(CH2)11-CH3, Sigma) circles were stamped onto Au-coated glass coverslips using a PDMS stamp (Sylgard 184/186 Elastomer kit). The remaining exposed areas were functionalized with a tri(ethylene glycol)-terminated alkanethiol (HS-(CH2)11-(CH2CH2O)3-OH, ProChimia Surfaces). Patterned substrates were coated with human plasma fibronectin (20 µg/ml), blocked with 1% heat-denatured BSA, and incubated in PBS. This process results in an array of fibronectincoated circular islands 5 µm in diameter and spaced 75 µm apart to promote single cell attachment to each island.
Adhesion strength assay
Adhesion strength was measured using our spinning disk system (García et al., 1998; Gallant et al., 2005). Micropatterned substrates with adherent cells were spun in PBS + 2 mM dextrose for 5 min at a constant speed. The applied shear stress (τ) is given by the formula τ = 0.8r(ρεω3)1/2, where r is the radial position and ρ and µ are the fluid density and viscosity, respectively. In some experiments, the spinning buffer was supplemented with 5% dextran to increase fluid viscosity. After spinning, cells were fixed in 3.7% formaldehyde, permeabilized in 1% Triton X-100, stained with ethidium homodimer-1 (Invitrogen), and counted at specific radial positions using a 10X objective lens in a Nikon TE300 microscope equipped with a Ludl motorized stage, Spot-RT camera, and Image-Pro analysis system. Sixty-one fields (80–100 cells/field before spinning) were analyzed and cell counts were normalized to the number of cell counts at the center of the disk. The fraction of adherent cells (f) was then fit to a sigmoid curve f = 1/(1 + exp[b(τ-τ50)]), where τ50 is the shear stress for 50% detachment and b is the inflection slope. τ50 characterizes the mean adhesion strength for a population of cells.
Focal adhesion assembly
For staining of focal adhesion components, cells were permeabilized in cytoskeleton-stabilizing buffer (0.5% Triton X-100 + 50 mM NaCl + 150 mM sucrose + 3 mM MgCl2 + 20 µg/ml aprotinin + 1 µg/ml leupeptin + 1 mM PMSF + 50 mM Tris, pH 6) for 10 min, fixed in 3.7% formaldehyde for 5 min, blocked in 5% fetal bovine serum, and incubated with primary antibodies against focal adhesion components followed by AlexaFluor-labeled secondary antibodies (Invitrogen). Images were captured using a Nikon 100X objective (1.3 NA) and Spot RT Camera/Software. Equal exposure times and gains were used for all image acquisition. Focal adhesion area fractions and intensities were quantified using calibrated image analysis software (ImagePro 4.5, Media Cybernetics). Briefly, a circular region of interest was defined around the micropatterned, circular adhesive area which encompassed a single cell. For each region of interest, fluorescence intensity and area values were quantified following thresholding to remove background fluorescence. Threshold values for background fluorescence were determined from images obtained from negative control samples stained with irrelevant primary antibody.
RNAi knock-down of vinculin
siRNA pooled sequences encoding for mouse vinculin (M-060130-00-0010) and a non-targeting (D-001210-01) negative control were purchased from Dharmacon. Sense sequences for the 4 pooled vinculin siRNAs were: CGAGAUCAUUCGUGUGUUAUU, GCCAAUAAAUCGACAGUGGUU, GGGAUUACCUCAUUGACGGUU, AAGAUGAGUGCUGAAAUUAUU. FAK-inducible cells were transfected using a Nucleofector II (Amaxa). For each sample, 2 million cells were resuspended in 100 µl of nucleofector solution MEF 2 with 1000 nM siRNA and 2.5 µg of pMAX GFP plasmid and cotransfected using program T-20 (60% transfection efficiency). Twenty-four hours after transfection, cells were FACS-sorted for GFP expression and cultured in appropriate tetracycline condition for 48 h. The spinning disk assay, Western blot, and immunostaining were performed 72 h after initial transfection.
Statistical analyses
Non-linear regression analysis was performed using SigmaPlot 2001 software (SPSS). Analysis of variance (ANOVA) statistical analyses were performed using SYSTAT 11 software.
Acknowledgements
This work was supported by NIH [R01-GM065918 and R01-GM049882] and a NSF Graduate Fellowship (KEM). The authors thank A.P. Kowalczyk (Emory Univ.) for human dermal fibroblasts, S.W. Craig (Johns Hopkins Univ.) and C. Zhu (Georgia Tech) for helpful discussions. The FAK siRNA-puro construct was kindly provided by M.A. Alaoui-Jamali (McGill Univ.).
Abbreviations used
- ECM
extracellular matrix
- FAK
focal adhesion kinase
References
- Benlimame N, He Q, Jie S, Xiao D, Xu YJ, Loignon M, Schlaepfer DD, Alaoui-Jamali Jamali.M. FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion. J. Cell Biol. 2005;171:505–516. doi: 10.1083/jcb.200504124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J. Cell Biol. 2006;172:151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers BA, Pavlath GK, Murphy TJ, Karsenty G, García AJ. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblast-specific transcription factor Runx2/Cbfal. J. Bone Miner. Res. 2002;17:1931–1944. doi: 10.1359/jbmr.2002.17.11.1931. [DOI] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J. Cell Biol. 2009;185:357–370. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cohen DM, Choudhury DM, Kioka N, Craig SW. Spatial distribution and functional significance of activated vinculin in living cells. J. Cell Biol. 2005;169:459–470. doi: 10.1083/jcb.200410100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- Clemente CF, Tornatore TF, Theizen TH, Deckmann AC, Pereira TC, Lopes-Cendes I, Souza JR, Franchini KG. Targeting focal adhesion kinase with small interfering RNA prevents and reverses load-induced cardiac hypertrophy in mice. Circ. Res. 2007;101:1339–1348. doi: 10.1161/CIRCRESAHA.107.160978. [DOI] [PubMed] [Google Scholar]
- Cohen DM, Kutscher B, Chen H, Murphy DB, Craig SW. A conformational switch in vinculin drives formation and dynamics of a talin-vinculin complex at focal adhesions. J. Biol. Chem. 2006;281:16006–16015. doi: 10.1074/jbc.M600738200. [DOI] [PubMed] [Google Scholar]
- Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J. Pathol. 2003;201:632–641. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- Forrest AD, Beggs HE, Reichardt LF, Dupree JL, Colello RJ, Fuss B. Focal adhesion kinase (FAK): A regulator of CNS myelination. J. Neurosci. Res. 2009 doi: 10.1002/jnr.22022. 10.1002/jnr.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- Gallant ND, García AJ. Model of integrin-mediated cell adhesion strengthening. J. Biomech. 2007;40:1301–1309. doi: 10.1016/j.jbiomech.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Gallant ND, Michael KE, García AJ. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell. 2005;16:4329–4340. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García AJ, Huber F, Boettiger D. Force required to break α5β1 integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. J. Biol. Chem. 1998;273:10988–10993. doi: 10.1074/jbc.273.18.10988. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell. Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Greenwood JA, Pallero MA, Theibert AB, Murphy-Ullrich JE. Thrombospondin signaling of focal adhesion disassembly requires activation of phosphoinositide 3-kinase. J. Biol. Chem. 1998;273:1755–1763. doi: 10.1074/jbc.273.3.1755. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Ryzhova L, Shin NY, Brábek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front. Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–337. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht P, Kim JB, Currey JA, Brunski J, Helms JA. FAK-Mediated mechanotransduction in skeletal regeneration. PLoS ONE. 2007;2:e390. doi: 10.1371/journal.pone.0000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo Trifilo.J., Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J. Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael KE, Dumbauld DW, Burns KL, Hanks SK, García AJ. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol. Biol. Cell. 2009;20:2508–2519. doi: 10.1091/mbc.E08-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhl C, Kirchgessner N, Schäfer C, pper K, Born S, Diez G, Goldmann WH, Merkel R, Hoffmann B. Becoming stable and strong: the interplay between vinculin exchange dynamics and adhesion strength during adhesion site maturation. Cell Motil. Cytoskeleton. 2009;66:350–364. doi: 10.1002/cm.20375. [DOI] [PubMed] [Google Scholar]
- Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- Owen JD, Ruest PJ, Fry DW, Hanks SK. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Wu X, Druso JE, Wei H, Park AY, Kraus MS, Alcaraz A, Chen J, Chien S, Cerione RA, Guan JL. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6638–6643. doi: 10.1073/pnas.0802319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirone DM, Liu WF, Ruiz SA, Gao L, Raghavan S, Lemmon CA, Romer LH, Chen CS. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol. 2006;174:277–288. doi: 10.1083/jcb.200510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA. Focal Adhesion Kinase Signaling Regulates the Expression of Caveolin 3 and beta1 Integrin, Genes Essential for Normal Myoblast Fusion. Mol. Biol. Cell. 2009 doi: 10.1091/mbc.E09-02-0175. 10.1091/mbc.E09-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Price LS, Schwartz MA. Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J. Cell Biol. 1999;147:611–618. doi: 10.1083/jcb.147.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Parsons JT. Complex formation with focal adhesion kinase: A mechanism to regulate activity and subcellular localization of Src kinases. Mol. Biol. Cell. 1999;10:3489–3505. doi: 10.1091/mbc.10.10.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 1994;14:16801–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE, Reichardt LF, Fuchs E. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 2007;176:667–680. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 2007;9:1046–1056. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- Shen TL, Park AY, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan JL. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 2005;169:941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA. Integrin {beta}1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. U.S.A. 2009 doi: 10.1073/pnas.0904227106. 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Siu ER, Wong EW, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J. Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Hanks SK, Wang YY. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe F, Miyazaki T, Takeuchi T, Fukaya M, Nomura T, Noguchi S, Mori H, Sakimura K, Watanabe M, Mishina M. Effects of FAK ablation on cerebellar foliation, Bergmann glia positioning and climbing fiber territory on Purkinje cells. Eur. J. Neurosci. 2008;27:836–854. doi: 10.1111/j.1460-9568.2008.06069.x. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Wei WC, Kopec AK, Tang MJ. Requirement of focal adhesion kinase in branching tubulogenesis. J. Biomed. Sci. 2009;16:5. doi: 10.1186/1423-0127-16-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell. Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol. Biol. Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SR, Gerard-O'Riley R, Kim JB, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J. Bone Miner. Res. 2009;24:411–424. doi: 10.1359/JBMR.081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 2008;10:1062–1068. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J. Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]