Abstract
Inflammatory and apoptotic caspases are central players in inflammation and apoptosis, respectively. However, recent studies have revealed that these caspases have functions beyond their established roles. In addition to mediating cleavage of the inflammasome-associated cytokines interleukin-1β (IL-1β) and IL-18, inflammatory caspases modulate distinct forms of programmed cell death and coordinate cell-autonomous immunity and other fundamental cellular processes. Certain apoptotic caspases assemble structurally diverse and dynamic complexes to direct inflammasome and interferon (IFN) responses to fine tune inflammation. In this Review, we discuss the expanding and interconnected roles of caspases that highlight new aspects of this family of cysteine proteases in innate immunity.
Graphical Abstract
The term caspase was introduced in 1996 to embody two functional roles of this group of enzymes. The “C” refers to cysteine proteases and the “aspase” refers to their ability to cleave after aspartic acid residues 1. Mammalian caspases are broadly divided into two groups based on their functions (Table 1). Inflammatory caspases include caspase 1, 4, 5, 11 and 12. With the exception of caspase 12, all inflammatory caspases participate in the activation of inflammasomes to initiate inflammation and the induction of an inflammatory form of programmed cell death known as pyroptosis 2-4. The human genome contains genes for the inflammatory caspases caspases 1, 4, 5 and 12. The mouse genome, instead, has genes for caspases 1, 11 and 12; caspase 11 being the homologue of human caspases 4 and 5 (Ref. 5).
Table 1.
Classification of caspases
| Caspase | Classification | Initiator or effector | Genomic location |
Domain structure |
||
|---|---|---|---|---|---|---|
| Human | Mouse | Prodomain | Protease domain | |||
| Caspase 1 | Inflammatory | Initiator | 11q23 | 9 A1 |
|
|
| Caspase 2 | Apoptotic | Initiator | 7q34–7q35 | 6 B2.1 |
|
|
| Caspase 3 | Apoptotic | Effector | 4q34 | 8 B1.1 |
|
|
| Caspase 4 | Inflammatory | Initiator | 11q22.2–11q22.3 | Absent |
|
|
| Caspase 5 | Inflammatory | Initiator | 11q22.2–11q22.3 | Absent |
|
|
| Caspase 6 | Apoptotic | Effector | 4q25 | 3 H1 |
|
|
| Caspase 7 | Apoptotic | Effector | 10q25 | 19 D2 |
|
|
| Caspase 8 | Apoptotic | Initiator | 2q33–2q34 | 1 B |
|
|
| Caspase 9 | Apoptotic | Initiator | 1p36.21 | 4 E1 |
|
|
| Caspase 10 | Apoptotic | Initiator | 2q33–2q34 | Absent |
|
|
| Caspase 11 | Inflammatory | Initiator | Absent | 9 A1 |
|
|
| Caspase 12* | Inflammatory | Initiator | 11q22.3 | 9 A1 |
|
|
| Caspase 14 | Keratinocyte differentiation | Effector | 19p13.1 | 10 C1 |
|
|
CARD, caspase activation and recruitment domain; DED, death effector domain.
The human genome encodes 12 caspases. Two isoforms of human caspase 12 have been identified. Caspase 12S is an inactive truncated isoform (not shown), whereas caspase 12L is an active full-length isoform.
Unlike inflammatory caspases, apoptotic caspases initiate and execute an immunologically silent form of programmed cell death known as apoptosis. Members of the apoptotic caspase family include the initiator caspases, caspases 2, 8, 9 and 10, and the effector caspases, caspases 3, 6 and 7 (Table 1). Unlike humans, rodents do not have a gene encoding caspase 10. Caspase 8 and 9 initiate extrinsic and intrinsic apoptosis, respectively 6. The extrinsic pathway of apoptosis is induced by death receptors, including FAS (also known as TNFRSF6, CD95 or APT1), tumour necrosis factor receptor 1 (TNFR1), TNFR2 and TNF-related apoptosis-inducing ligand (TRAIL) death receptors. Signalling via FAS induces formation of the death-inducing signalling complex, which results in the recruitment of caspase 8 (and caspase 10 in human cells) by the adaptor protein FAS-associated via death domain (FADD). Dimerization of caspase 8 triggers the apoptotic cascade7. The engagement of TNFR1 via its cognate ligand TNF promotes the formation of a complex comprised of receptor-interacting serine/threonine-protein kinase 1 (RIPK1), FADD and caspase 8. The RIPK1-FADD-caspase 8 complex initiates apoptosis in the cell when the level of the long isoform of FLICE-like inhibitory protein (FLIPL; FLIP is also known as CFLAR) is low 8-11.
The intrinsic pathway of apoptosis requires the induction of mitochondrial outer membrane permeabilization, which mediates the release of the pro-apoptotic factor cytochrome c for binding by the cytosolic protein apoptotic protease-activating factor 1 (APAF1). APAF1 and caspase 9 assemble a multi-protein complex known as the apoptosome12-16. Activation of initiator caspases via either the extrinsic or intrinsic apoptosis pathway engages subsequent activation of caspase 3, 6 and 7. Caspase 14 can not be classified as either an apoptotic or inflammatory caspase and has a specialized role in the differentiation of keratinocytes 17, 18.
Caspases are expressed by immune cells and non-immune cells and in many tissues and organs. They are produced as inactive zymogens, which consist of a carboxy-terminal protease effector domain comprised of a large subunit and a small subunit (Table 1). Caspases 1, 2, 4, 5, 9, 11 and 12 also harbour an amino-terminal prodomain called a caspase-associated recruitment domain (CARD), whereas caspase 8 and 10 contain death effector domains (DEDs). Effector caspases, caspases 3, 6 and 7, have a short prodomain and exist as inactive homodimers until they are cleaved and activated by initiator caspases19.
The established roles for inflammatory caspases and apoptotic caspases in inflammation and apoptosis, respectively, have been previously reviewed2-4, 6, 11, 20 and are not the focus of this article. However, recent work has shown that inflammatory caspases control fundamental cellular processes independently of their abilities to evoke an inflammatory response. Conversely, apoptotic caspases support the initiation or inhibition of inflammation. Identification of new functionalities associated with caspases pushes the boundaries of the traditional classification of caspases and prompts a new paradigm for understanding their contributions to health and disease. Here, we provide an overview of the converging and newly described functions of inflammatory and apoptotic caspases in the context of inflammation, cell death and immunity.
Inflammatory caspases and the inflammasome
Caspase 1 is activated in inflammasomes that are initiated by a member of the nucleotide-binding domain, leucine-rich repeat containing (NLR) or pyrin and HIN domain-containing (PYHIN) family 21. The NLR and PYHIN sensors mediate recognition of microorganism-associated molecular patterns (MAMPs) or damage-associated molecular patterns (DAMPs) (Box 1). To date, five receptors have been established to form an inflammasome, including NLRP1, NLRP3, NLRC4, absent in melanoma 2 (AIM2) and Pyrin (Fig. 1). Furthermore, IFNγ-inducible protein 16 (IFI16), retinoic acid-inducible gene I (RIG-I), NLRP6, NLRP7 and NLRP12 have been suggested to activate caspase 1 (Ref. 4). The caspase 1 inflammasome orchestrates antimicrobial effector functions, tissue repair, tumour suppression, metabolic regulation, autoinflammatory disease and cell survival via membrane biogenesis 22-27.
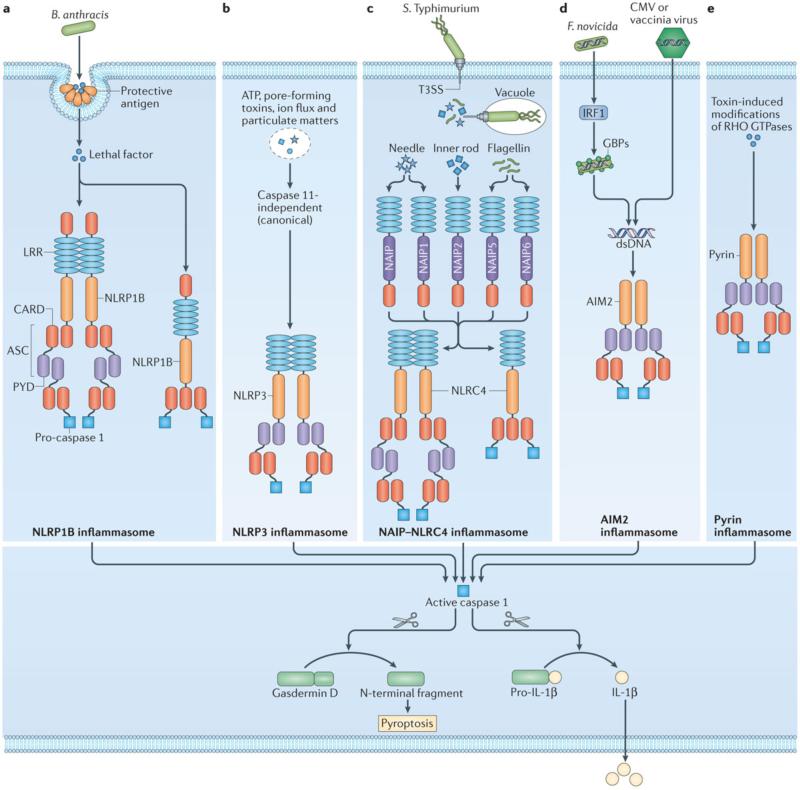
Figure 1. Caspase 1 and the canonical inflammasomes.
a | Release of the Bacillus anthracis anthrax lethal toxin, composed of a protective antigen and a lethal factor, activates the mouse NLRP1B inflammasome by inducing cleavage of NLRP1B 190, 191. b | NLRP3 responds to a plethora of activators and physiological aberrations 141-143. Engagement of the NLRP3 inflammasome independently of caspase 11 is known as the canonical NLRP3 inflammasome pathway. c, Certain pathogenic bacteria such as Salmonella enterica serovar Typhimurium (S. Typhimurium) inject proteins into the host cells via the Type III secretion system (T3SS). These effector proteins are detected by neuronal apoptosis inhibitory proteins (NAIPs) in the cytoplasm, where they engage activation of the NLRC4 inflammasome 150-156. d, The cytosolic bacteria Francisella tularensis subspecies novicida (F. novicida), and the DNA viruses mouse cytomegalovirus (CMV) or vaccinia virus release DNA during infection to activate the AIM2 inflammasome 146-149, 192, 193. The transcription factor interferon-regulatory factor 1 (IRF1) induces expression of interferon-inducible GTPases called guanylate-binding proteins (GBPs) to expose DNA from F. novicida 194, 195. e, Pyrin senses modifications of Rho GTPases caused by Rho-inactivating toxins from bacteria including Vibrio parahaemolyticus, Histophilus somni Clostridium botulinum and Burkholderia cenocepacia 186, 187. Apoptosis-associated speck-like protein containing a CARD (ASC, also known as PYCARD) is an adaptor protein which carries a pyrin domain (PYD) and Caspase activation and recruitment domains (CARD) and is used to bridge the PYD-bearing inflammasome sensors NLRP3, AIM2 and pyrin with the CARD of pro-caspase 1. Inflammasome sensors carrying a CARD, such as NLRP1B or NLRC4, may engage pro-caspase 1 directly by CARD-CARD interactions. In all cases, caspase 1 drives pro-IL-1β and pro-IL-18 processing. Inflammatory caspases cleave gasdermin D, causing pyroptosis which allows the release of matured IL-1β and IL-18 (Refs 47, 62-64). The N-terminal fragment of gasdermin D conveys a signal by caspases 4, 5 and 11 to activate the non-canonical NLRP3 inflammasome.
Recent studies have identified a role for caspases 4, 5 and 11 in inflammasome signalling (Box 2). However, our understanding of the biological functions attributed to caspase 1 and caspase 11 gained from mouse studies will likely be re-evaluated in the years ahead in light of findings showing that conventional Casp1-deficient mouse strains lack caspase 11 expression28, 29. Caspases 4, 5 and 11 have been shown to interact with and activate caspase 1 (Refs 30, 31). Further studies have demonstrated that caspases 4, 5 and 11 activate the NLRP3 inflammasome in response to Gram-negative bacteria and intracellularly-delivered lipopolysaccharide (LPS), placing caspases 4, 5 and 11 as activators of caspase 1 to promote caspase 1-dependent cleavage of IL-1β and IL-18 (Refs 29, 32-41). Direct cleavage of IL-1β and IL-18 by caspase 4 has been proposed 42, 43, but further study is required to confirm this observation. Activation of caspases 4, 5 and 11 also leads to pyroptosis, and the release of IL-1α and the alarmin high-mobility group protein 1 (HMGB1) independently of caspase 1 (Refs 29, 32-39, 44, 45). Emerging evidence now indicates that inflammatory caspases orchestrate distinct functions that do not rely on the effects of IL-1β and IL-18.
Pyroptosis and other effector functions
Inflammatory caspases orchestrate physiological functions that are independent of their ability to induce the secretion of IL-1β and IL-18. Studies have shown that wild-type mice and mice lacking IL-1β and IL-18 are sensitive to LPS-induced endotoxaemia or Escherichia coli-induced septic shock, whereas mice lacking only caspase 11 or both caspase 1 and caspase 11 are relatively resistant31, 46-48. The contribution of caspases 1 and 11 to LPS-induced endotoxaemia was revisited recently using mice with a genomic deletion of caspase 1 alone. Kayagaki and colleagues29 microinjected a bacterial artificial chromosome transgene encoding caspase 11 into Casp1−/−Casp11−/− mouse embryos to re-establish caspase 11 expression in this mouse line so that it only lacks caspase 1 expression (referred to as Casp1−/−Casp11Tg). This study found that caspase 11 largely drives LPS-induced mortality, but caspase 1 contributes in part to this process29. In agreement, a mutant mouse strain with a gene encoding human caspase 4 introduced into its genome has increased sensitivity to LPS-induced endotoxaemia 49. Interestingly, antibody-mediated neutralization of the caspase 11-associated alarmin HMGB1 largely protects mice from LPS-induced mortality 47. In another mouse model of lethal systemic inflammation, activation of caspase 1 via the NLRC4 inflammasome by means of systemic delivery of cytosolic flagellin causes the production of inflammatory mediators called eicosanoids, which drive inflammation and mortality independently of IL-1β and IL-18 (Ref. 50). In keratinocytes, caspase 1 can mediate the export of proteins that are unrelated to inflammation, including proangiogenic fibroblast growth factor 2 (Ref 51).
A well-known effect that is mediated by inflammatory caspases is pyroptosis35, 36, 52-55 (Box 3). Although the molecular signatures between caspase 1- and caspase 11-induced cell death are likely to differ, recent studies have revealed that pyroptosis triggered by either caspase is an effective antimicrobial defence against certain intracellular pathogens even in the absence of IL-1β and IL-18 release43, 53, 56-58. Caspase 1-induced pyroptosis releases intracellular bacteria from infected macrophages for uptake by neutrophils, whereby bacteria are killed through a mechanism requiring reactive oxygen species (ROS) 56. Indeed, neutrophils are resistant to pyroptosis in response to some inflammasome activators 59, which makes them a suitable cell type to facilitate the clearance of infectious agents that normally replicate in macrophages. Similarly, caspase 11-dependent pyroptosis is protective against infection by cytosolic bacteria, and during intraperitoneal infection by the pathogenic bacteria Burkholderia thailandensis, the effect is largely independent of IL-1β and IL-18 (Ref 53). Epithelial cells infected by Salmonella enterica serovar Typhimurium (S. Typhimurium) also undergo caspase 1-dependent pyroptosis mediated by the NAIP-NLRC4 inflammasome, leading to physical extrusion of infected cells from the gut epithelium to reduce overall intraepithelial bacterial burden57. A similar study demonstrated that caspase 4-dependent pyroptosis controls the amount of S. Typhimurium in human intestinal cell monolayers 43. In another infection model, the marked susceptibility of mice lacking caspases 1 and 11 to subcutaneous infection by Francisella tularensis subspeciesnovicida (F. novicida) is not fully recapitulated in wild-type mice injected with neutralizing antibodies to IL-1β and IL-18 (Ref 60), which highlights a critical role for pyroptosis in the control of F. novicida infection in addition to IL-1β and IL-18 release. However, a detrimental role for caspase 1-dependent pyroptosis has been observed during HIV-1 infection, whereby pyroptosis depletes quiescent lymphoid CD4+ T cells and contributes to chronic inflammation and immunodeficiency 61.
Biochemical analyses have revealed that caspases 4, 5 and 11 directly cleave the substrate gasdermin D, yielding an N-terminal fragment of gasdermin D that drives pyroptosis and activation of the non-canonical NLRP3 inflammasome62, 63 (Fig. 2). Caspase 1 also cleaves gasdermin D to mediate pyroptosis by canonical inflammasomes62-64 (Fig. 1). However, caspase 1-dependent gasdermin D-independent pyroptosis can be observed following prolonged inflammasome activation, which raises the possibility that other pro-pyroptotic factors exist 62. Importantly, mice with a deletion of the gene encoding gasdermin D are less susceptible to LPS-induced mortality compared with wild-type mice 62, providing further support for a role of pyroptosis in the pathogenesis of endotoxaemia.
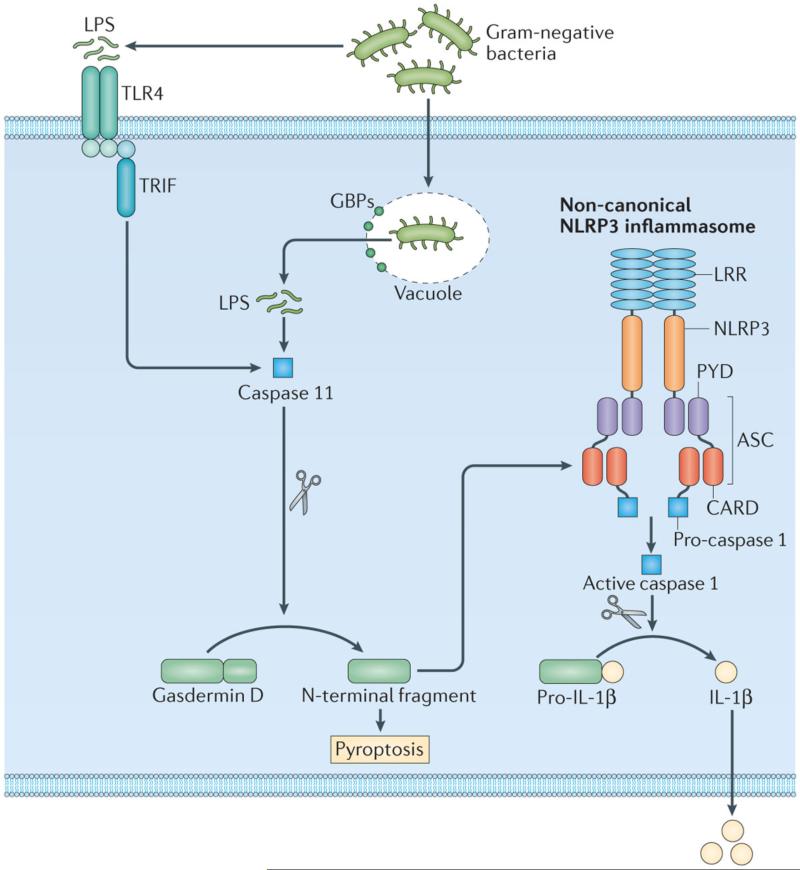
Figure 2. Inflammatory caspases and the non-canonical NLRP3 inflammasome.
LPS released by Gram-negative bacteria activates TLR4 and its adaptor TIR-domain-containing adaptor protein inducing IFNβ (TRIF), inducing caspase 11 expression via type I interferon signalling. Cytoplasmic LPS which enters the cytoplasm owing to rupture of the pathogen-containing vacuole by guanylate-binding proteins (GBPs) binds mouse caspase 11 and activate the non-canonical NLRP3 inflammasome 29, 32-41, 73. Cytoplasmic LPS also binds human caspase 4 and 5. ASC, apoptosis-associated speck-like protein containing a CARD (also known as PYCARD).
In addition to infectious diseases, caspases 1 and 11 orchestrate protective responses against colorectal cancer independently of IL-1β and IL-18 in a context-specific manner. Activation of caspase 1 and release of IL-18 via the NLRP3 inflammasome contribute to protection of colorectal tumorigenesis and subsequent growth of metastasised cancer cells in mice 65-67. By contrast, activation of the NLRC4 inflammasome prevents cancer through a mechanism that suppresses overt proliferation of colonic crypt cells 68. How NLRC4 and NLRP3 differentially coordinate caspase 1 functions to achieve IL-18-dependent and -independent effects during colorectal tumorigenesis is largely unknown. Recent studies have also demonstrated a requirement for caspase 11 in mediating resistance against dextran sulfate sodium (DSS)-induced intestinal inflammation in mice69-71. Whether IL-1β and IL-18 are contributing factors in this case is contentious, with both similar and reduced levels of IL-1β and IL-18 being reported in caspase 11-deficient mice treated with DSS compared with treated wild-type mice 70, 71. Differences in gut microbiota harboured by mice housed in different facilities and differences in experimental conditions could account for these discrepancies. Nevertheless, there is clear evidence to indicate that caspase 1- and caspase 11-mediated cellular and pathological effects might occur independently of concomitant release of IL-1β and IL-18.
Inflammatory caspases and LPS sensing
Although the membrane-bound Toll-like receptor 4 (TLR4) has been recognized as the bona fide LPS sensor for nearly two decades 72, recent studies have identified an exquisite role for caspase 11 in the recognition of LPS (Table 1). Caspase 11 induces the release of IL-1α, IL-1β and IL-18 and pyroptosis when LPS is transfected or electroporated into the cytoplasm or when Gram-negative bacteria expose their LPS in the cytoplasm 35, 36, 53 (Fig. 2). Similarly, caspase 4 drives IL-1α and IL-1β secretion and cell death in human monocyte-derived macrophages, monocytes, keratinocytes or epithelial cell lines in response to transfection of LPS37-39, 73. In addition, the vacuolar-restricted pathogens S. Typhimurium and Legionella pneumophila have been shown to activate caspases 4 and 11 (Refs 33, 37, 53, 73, 74), probably owing to aberrant entry of a small proportion of bacteria into the cytoplasm following rupture of the pathogen-containing vacuole caused by guanylate-binding proteins 54, 75.
The presence of a CARD in caspase 11 suggests that a CARD-bearing protein might act as an upstream receptor that directly binds LPS. However, a search for a potential binding partner failed to identify interaction between any CARD-bearing proteins with LPS 73. Instead, caspases 4, 5 and 11 were found to directly bind LPS and its lipid A moiety 73. Specifically, the full-length 43 kDa form, but not the 38 kDa form, of caspase 11 is precipitated by LPS 73. Unlabelled LPS competes with biotinylated LPS for caspase 11 binding, whereas biotinylated muramyl dipeptide (MDP) and Pam3CSK4 do not precipitate caspase 11. The surprising observation that certain members of the inflammatory caspase family are sensors of cytosolic LPS clearly broadens the traditional scope of caspase functions.
Enigmatic roles of caspase 12
Caspase 12 is a member of the inflammatory caspase family and is expressed in many organs 76, 77. An early study indicated that caspase 12 is localized to the endoplasmic reticulum (ER) and is activated by ER stress signals, but not by other apoptotic stimuli 78. The human genome encodes either or both the full-length and truncated isoforms of caspase 12, whereas the mouse and rat genomes encode only a full-length caspase 12 (Refs 79, 80). The gene that encodes full-length human caspase 12 protein (referred to as caspase 12-L or the ancestral or active form) spans 8 exons. However, the inactive truncated form of human caspase 12 consists of only the prodomain (referred to as C caspase 12-S or the derived or inactive form) owing to a TGA stop codon at amino acid position 125 in exon 4 of the gene. Analysis of the global human population revealed that only 28% of the sub-Saharan African populations and less than 1% in those outside of Africa carry one or more alleles for full-length caspase 12 protein 81. Further studies revealed that between 1.7 to 12.5% of individuals in certain regions of Middle East, South Asia and East Asia carry at least one allele encoding full-length caspase 12 protein82, 83. Homozygosity for full-length caspase 12 is rare in humans and is found in only 2% of individuals with an African ancestry 80, 84.
The role of caspase 12 in the immune system and the reason for its loss of function in populations outside of Africa are unclear. Several human and mouse studies suggested that caspase 12 could have a role in the negative regulation of inflammation and caspase 1 activity, as well as to confer resistance to bacterial sepsis and infection 80, 85. This is exemplified by a study showing whole blood derived from individuals of African descent harbouring full-length caspase 12 produce less IL-1β and granulocyte/macrophage colony-stimulating factor when stimulated with LPS compared with individuals harbouring a truncated version of caspase 12 (Ref 80). By contrast, another study found no correlation between caspase 12 variants and cytokine production84. There is some evidence to suggest that homozygosity for the full-length caspase 12 protein is linked to lower baseline joint narrowing scores in African-Americans with rheumatoid arthritis86. No association between a particular variant of caspase 12 and susceptibility to sepsis induced by the fungal pathogen Candida albicans and to community-acquired pneumonia was found 87, 88. There is also limited evidence to suggest malaria contributed to the persistence of full-length caspase 12 in African populations89. The reason for the apparent discrepancy between these studies is unclear. Nevertheless, it has been proposed that the loss of caspase 12 function in many human populations is indicative of positive selection, an event which probably occurred within the past 100,000 years 81. Consistent with this idea, a loss of caspase 12 function in Europe has been predicted to predate animal domestication and be unrelated to zoonotic infections from livestock 90.
Biochemical analyses have found that rat caspase 12 cleaves itself and does not induce the cleavage of caspases 1, 3, 4, 5, 8 and 9, the apoptotic substrates poly(ADP-ribose) polymerases (PARP) and inhibitor of caspase-activated DNase (ICAD), pro-IL-1β, pro-IL-18 and amyloid-β precursor proteins 91. However, the full-length or catalytically inactive form of rat caspase 12 interacts with caspase 1 and partially with caspase 5, but not with caspase 9 (Ref 85), and prevents caspase 1 activity and the release of IL-1β in macrophages stimulated with TLR ligands 85, 91. The inhibitory activity of rodent caspase 12 does not appear to be restricted to caspase 1. Overexpression of rat caspase 12 in HEK293T cells dampens the activation of nuclear factor-κB (NF-κB) signalling induced by MDP 92. Caspase 12 displaces the E3 ubiquitin ligase TNFR-associated factor 6 (TRAF6) in the NOD2-RIPK2 complex and interacts with the kinase RIPK2 (Ref 92). The biochemical functions exhibited by rodent caspase 12 raise the possibility that it might be an anti-inflammatory caspase. However, further studies are required to fully elucidate the immunological repertoire of this underappreciated caspase.
Caspase 8 and inflammation
Caspase 8 is recognized for its role in apoptosis. However, ongoing investigations have uncovered essential functions for this protease in the regulation of inflammation (Fig. 3). Caspase 8 is required for the activation of NF-kB signalling 93-100 and for direct or indirect cleavage of pro-IL-1β or pro-IL-18 together with or independently of the caspase 1 inflammasome 95, 96, 101-119. By contrast, there is also evidence suggesting that caspase 8 contributes to inhibition of inflammation, including its ability to block inflammasome activities120, IFN responses 121, 122 and a type of inflammatory programmed cell death called necroptosis 123, 124 (Fig. 3). We discuss below the diverse characteristics of caspase 8 in the context of inflammation.
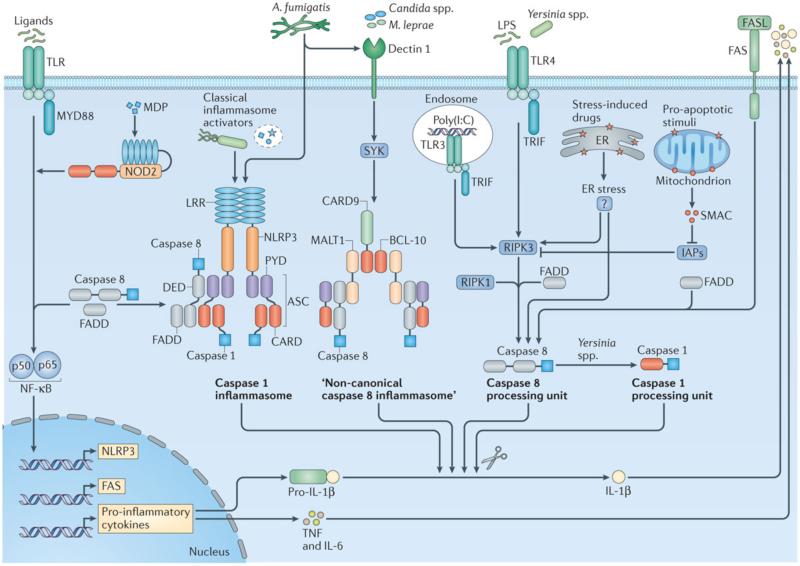
Figure 3. Caspase 8 mediates initiation of NF-κB signalling and maturation of IL-1β.
Recognition of ligands by Toll-like receptors (TLRs) leads to signalling via the adaptor MyD88 and subsequent NF-κB activation. Caspase 8 and its adaptor Fas-associated death domain (FADD) contribute to this pathway, inducing transcription of genes encoding pro-inflammatory cytokines, some of which such as pro-IL-1β and pro-IL-18 require further proteolytic processing 93-100. Caspase 8 or FADD is also recruited to the NLRC4, NLRP3 and AIM2 inflammasome complexes activated by classical inflammasome activators (Salmonella enterica serovar Typhimurium, LPS plus ATP or nigericin, Citrobacter rodentium, Escherichia coli, Francisella novicida, dsDNA, or Aspergillus fumigatus), in which caspase 8 either activates caspase 1, leading to caspase 1-dependent processing of pro-IL-1β and pro-IL-18, and/or modulates cell death 95, 96, 111, 112, 117. However, in the absence of caspase 1, caspase 8 may induce apoptosis in the inflammasome speck 112, 113. Caspase 8 also assembles a range of structurally diverse complexes to directly mediate processing of pro-IL-1β independently of caspase 1. Candida species, A. fumigatus and Mycobacterium leprae infection trigger dectin 1 and its adaptor SYK to engage a so-called “non-canonical caspase 8 inflammasome” complex that is composed of CARD9, BCL-10, MALT1, ASC and caspase 8. Caspase 8 directly processes pro-IL-1β in the absence of caspase 1 and does not require internationalization of the pathogen 104. In response to TLR3 or TLR4 activators, the adaptor TIR-domain-containing adaptor protein inducing IFNβ (TRIF) promotes assembly of a caspase 8 processing unit for direct cleavage of IL-1β in a manner that may require the kinases RIPK1 and RIPK3, and the adaptor FADD 101, 105-108. For example, TLR4-TRIF signalling via LPS and a yet-unidentified signal released as a result of stress in the endoplasmic reticulum (ER) engage caspase 8-dependent processing of IL-1β with or without RIPK3 (Ref 108). LPS-induced TLR4-TRIF signalling and release of second mitochondrial-derived activator of caspases (SMAC; also known as DIABLO) owing to exposure to proapoptotic signals delivered by doxorubicin, staurosporine or cycloheximide activate caspase 8-dependent processing of IL-1β 106, 107. Here, SMAC or SMAC mimetics suppress inhibitor of apoptosis proteins (IAPs) from blocking RIPK3. During Yersinia infection, RIPK1 and FADD are essential in mediating caspase 8 activation via TRIF- and RIPK3-dependent and -independent mechanisms, whereby activation of caspase 8 ultimately drives caspase 1-mediated processing of IL-1β 115, 116. Signalling via TLRs induces upregulation of FAS (also known as CD95 and TNFRSF6). Interaction between FAS and FASL licenses caspase 8-dependent IL-1β release via FADD, independently of RIPK3 or caspase 1 (Ref 109, 110).
Caspase 8 modulates NF-κ B signalling
Caspase 8 can regulate NF-κB activation. Studies have identified in two patients a homozygous arginine-to-tryptophan mutation at the p18 subunit of caspase 8 which impairs activation and apoptosis of T cells, B cells and natural killer (NK) cells 93. These patients have normal levels of total T and B cells, but a reduced proportion of CD4+ T cells 93. They also suffer a range of clinical symptoms, including splenomegaly, lymphadenopathy, eczema, airway conditions, susceptibility to recurrent herpes simplex virus infection and pneumonia, and show a poor response to immunization 93. Further examination of the lymphocyte and NK cell populations revealed defective activation of NF-κB 98. Caspase 8 is also required for NF-κB signalling and production of pro-inflammatory cytokines in mouse macrophages and B cells (FIG. 3) 94-97. Mouse macrophages lacking caspase 8 and the kinase RIPK3 infected with S. Typhimurium, Citrobacter rodentium, E. coli or stimulated with LPS, Pam3Cys4, poly(I:C) or MDP failed to induce robust expression of pro-IL-1β, IL-6 and TNF compared with wild-type macrophages or macrophages lacking RIPK3 (Refs 95-97). Additional studies confirmed that the caspase 8 adaptor FADD is required for NF-κB activation in response to a range of stimuli 96, 97, 99, 102. It is worthy to note that genomic deletion of caspase 8 or FADD results in embryonic lethality in mice 125-128, a phenotype that is rescued by genomic deletion of Ripk3 (Ref 129-131). Therefore, studies have used macrophages deficient in both caspase 8 and RIPK3 or FADD and RIPK3. B cells derived from mice lacking caspase 8 only in the B cell population exhibit impaired phosphorylation and nuclear translocation of the p65 subunit of NF-κB and expression of genes encoding IL-6, TNF, IFNβ and CXCL10 in response to LPS stimulation 94.
To date, there is no consensus on the molecular mechanism underpinning how caspase 8 mediates NF-κB signalling. One report found that full-length caspase 8 and its catalytic activity are crucial for driving NF-κB activities 98, whereas another showed that the DEDs of caspase 8 are sufficient 132. Further studies have reported that the catalytic activity of caspase 8 is dispensable or even serves to reduce NF-κB signalling99, 132. A dual and opposing role for caspase 8 has also been proposed, whereby pro-caspase 8 contributes to TNFR1-induced activation of NF-κB, whereas activated caspase 8 engages cleavage of NF-κB-inducing kinase (NIK; also known as MAP3K14) to prevent TNFR1-induced NF-κB activation 99. Further investigation demonstrated that caspase 8 targets and cleaves FLIPL, which generates an N-terminal p43 fragment and a C-terminal p12 fragment 100. The p43 fragment of FLIPL, TRAF2 and caspase 8 form a tripartite complex and subsequently activates the NF-κB signalling pathway 100. Despite a lack of clarity with respect to the specific mechanism involved, it is clear that caspase 8 is essential for the activation of NF-kB signalling that leads to the production of a range of pro-inflammatory cytokines.
Caspase 8 mediates direct cleavage of IL-1β
Another pathway by which caspase 8 contributes to inflammation is the conversion of pro-IL-1β to its mature bioactive form. Recombinant caspase 8 directly cleaves human and mouse pro-IL-1β at the Asp117 site – the same site targeted by caspase 1 – whereas caspases 3, 7 and 9 or a catalytically inactive mutant of caspase 8 fail to do so 101-103. Depending on the stimuli received by the cell, caspase 8 assembles macromolecular complexes of varying complexity to mediate the cleavage of pro-IL-1β (Fig. 3). Upon recognition of certain fungal and mycobacterial infection in human dendritic cells (DCs), dectin 1 and its adaptor SYK promote the formation of a CARD9–BCL-10–MALT1 (mucosa-associated-lymphoid-tissue lymphoma-translocation protein 1) complex that drives the transcription of the gene encoding IL-1β. This complex further recruits ASC and caspase 8 to form a so-called “non-canonical caspase 8 inflammasome”, whereby caspase 8 directly mediates the processing of pro-IL-1β in the absence of caspase 1 (Ref 104). By definition, this complex cannot be classified as an inflammasome as it does not contain an inflammatory caspase.
Direct cleavage of pro-IL-1β by caspase 8 in macrophages has been observed under conditions in which inhibitor of apoptosis proteins (IAPs) are blocked. Genetic deletion of the IAPs XIAP, cIAP1 and cIAP2 or inhibition of these IAPs by mimetics of second mitochondrial-derived activator of caspases (SMAC; also known as DIABLO) in macrophages results in LPS-induced secretion of IL-1β through a mechanism that depends on both caspase 8 and the caspase 1-NLRP3 inflammasome102. When XIAP, cIAP1 and cIAP2 are inhibited, both caspase 1- and caspase 8-dependent pathways of IL-1β secretion are licensed by a common upstream kinase RIPK3, which is supressed by IAPs 102. The complex interplay between IAPs was further revealed by studies showing that bone marrow-derived macrophages (BMDMs) or mice with a deletion of the genes encoding cIAP1 or cIAP2 have an impaired ability to activate the NLRP3 inflammasome 133, whereas deletion of the gene encoding XIAP promotes LPS-induced secretion of IL-1β in a RIPK3-dependent manner134. A later study further confirmed that RIPK3 is required for LPS-induced caspase 1- or caspase 8-dependent secretion of IL-1β in mouse bone marrow-derived DCs (BMDCs) 105. The caspase 8-containing pro-IL-1β processing complex is reported to also contain RIPK1, RIPK3 and FADD and requires signalling via the TLR adaptor TIR-domain-containing adaptor protein inducing IFNβ (TRIF)105. This finding is consistent with a previous study showing that the C-terminal receptor-interacting protein homotypic interaction motif (RHIM) of TRIF is essential for driving caspase 8-dependent IL-1β processing101.
A role for caspases 1 and 8 in the release of IL-1β has also been described in the context of ER stress106-108, 135-137. The NLRP3 inflammasome responds to ER stress and induces caspase 1-dependent release of IL-1β in human and mouse cells 135, 137. A further study reported that ER stress induced by an attenuated Brucella abortus strain leads to NLRP3-dependent activation of caspase 2 (Ref 136). Caspase 2 then mediates activation of the BH3-only protein BID and release of mitochondrial DNA, which leads to the activation of caspase 1 and secretion of IL-1β 136. Additional studies found that proapoptotic chemotherapeutic agents and drugs that induce stress in the ER promote caspase 8-dependent, caspase 1-independent secretion of IL-1β in LPS-primed BMDCs or BMDMs106-108. Furthermore, TRIF is required to mediate IL-1β release in this process106, 108. The amount of cIAP1 is decreased in cells treated with LPS and the proapoptotic chemotherapeutic agent doxorubicin 106, which may imply a role for cIAP1 as a suppressor of the caspase 8-dependent IL-1β-processing unit 102. It has, therefore, been proposed that proapoptotic stimuli dysregulate mitochondrial functions and induce the release of SMAC from the mitochondria. SMAC then binds and inhibits IAPs, and thus, prevents both IAP-mediated degradation of RIPK3 and inhibition of caspase 8-dependent processing of IL-1β102, 106 (Fig. 3). Whether ER stress is directly causing reduced levels of IAPs leading to the secretion of IL-1β is unknown. However, RIPK3 has been shown to be partially involved in this TRIF–caspase 8-dependent pathway 108. Engagement of FAS ligand (FASL; also known as TNFSF6 and CD95) by FAS is another pathway by which caspase 8 is activated to cleave IL-1β and IL-18 independently of caspase 1 (Refs 109, 110). In this context, TLR-induced FAS expression drives caspase 8- and FADD-mediated release of IL-1β and IL-18 (Ref 109). The observations that RIPK3 is dispensable, and priming by TLR7 – which uses the adaptor MYD88 rather than TRIF – sufficiently induce FAS expression and FASL-mediated IL-1β production 109, indicate that the FAS-regulated caspase 8 complex likely differs in composition to the aforementioned TRIF-dependent caspase 8-RIPK3-RIPK1 complex. The precise architecture of these caspase 8-containing units remains to be defined (Fig. 3). Inhibition of inflammation and the inflammasome by caspase 8 have also been observed and are further discussed below.
Caspase 8 mediates caspase 1-dependent processing of IL-1β
More recent studies have revealed a requirement for caspase 8 in activating caspase 1 within the inflammasome complex to generate bioactive IL-1β138, 139. Confocal microscopy studies have identified the presence of caspase 8 within the ASC or caspase 1 inflammasome speck in macrophages or DCs infected with S. Typhimurium 95, 111, F. novicida 112, C. rodentium 96 and A. fumigatus 117 (Box 1). Super resolution and confocal microscopy studies have further identified bioactive caspase 1 and caspase 8 molecules being bound by a concentric ring of endogenous NLRC4 or NLRP3 and ASC in macrophages infected with S. Typhimurium 111.
In response to canonical inflammasome activators, caspase 1 is still required for the processing of pro-IL-1β and pro-IL-18 (Refs 95, 96, 117, 140-156). The role of caspase 8 in this context is to activate caspase 1 or modulate pyroptosis, rather than to participate directly in the processing of pro-IL-1β and pro-IL-18 (Fig. 3). Indeed, recombinant caspase 8 cleaves caspase 1 in vitro 96, allowing caspase 1 to be fully activated to induce the cleavage of IL-1β and IL-18 in response to inflammasome activators LPS and ATP, C. rodentium, Y. pestis, Y. pseudotuberculosis and A. fumigatus 96, 115-117. During Yersinia infection, activation of caspase 1 by caspase 8 also requires RIPK1 and FADD, with a partial contribution from TRIF and RIPK3 (Refs 115, 116). In response to β-glucans and heat-killed C. albicans, both caspases 1 and 8 regulate IL-1β release independently of one another 118. Consistent with this concept, prolonged stimulation with LPS and nigericin or infection with S. Typhimurium in the absence of caspase 1 activates a delayed pathway leading to caspase 8-dependent processing of IL-1β 95, 114. Caspase 1 and caspase 8 also synergistically contribute to the production of IL-1β that drives the development of osteomyelitis 119. Mouse neutrophils carrying a missense mutation in the gene encoding proline-serine-threonine phosphatase interacting protein 2 (PSTPIP2) induce more robust cleavage of IL-1β and release exuberant amounts of IL-1β relative to wild-type neutrophils, which is the basis for the development of spontaneous bone inflammation and destruction. However, genomic deletion of genes encoding caspases 1 and 8 prevents excessive production of IL-1β and the development of PSTPIP2-mediated osteomyelitis 119.
Precisely how caspases 1 and 8 operate synergistically and independently of one another and the nature of the activators that dictate this relationship remain to be fully elucidated. Interestingly, caspase 8-dependent activation of the NLRP3 inflammasome is regulated by RIPK3 and the necroptosis effector MLKL only after prolonged stimulation157. This would, in part, reflect the compositional flexibility and dynamics of the caspase 8-containing complex, which is observed when different contextual cues are sensed by the cell at different times. Moreover, the growing evidence supporting a role for caspase 8 in the initiation of inflammation reinforces the perspective that the function of this caspase is clearly not confined to apoptosis.
Inhibition of inflammation and the inflammasome
In addition to its pro-inflammatory function, caspase 8 has anti-inflammatory roles. Studies have found that mice lacking caspase 8 in hepatocytes or DCs develop chronic inflammatory or autoimmune diseases158, 159. Heighten inflammation in mice lacking caspase 8 in DCs could be prevented by deletion of the TLR adaptor MYD88, suggesting a role for caspase 8 in supressing TLR signalling159. In the skin, loss of caspase 8 expression promotes IL-1α secretion and transcriptional activation of NF-κB-responsive genes during wound healing160.
One of the abilities of caspase 8 to modulate inflammation includes inhibition of inflammasome activity (Fig. 4). Mouse DCs that lack caspase 8 results in assembly of the NLRP3 inflammasome and release IL-1β in response to LPS or Pam3CSK4 even without an inflammasome activator120. LPS-induced IL-1β production in DCs lacking caspase 8 requires RIPK1, RIPK3, the effector MLKL and the serine/threonine protein phosphatase PGAM5. This study suggested that LPS stimulation of DCs promotes an association of caspase 8 with FADD to inhibit the assembly of a RIPK1-RIPK3 complex and its downstream effector functions, leading to activation of the NLRP3 inflammasome120. It has also been proposed that caspase 8 and FADD bind NLRP3 to directly inhibit the inflammasome 120. It remains unclear in what context caspase 8 acts as a negative regulator of inflammation and whether this function is cell-type specific.
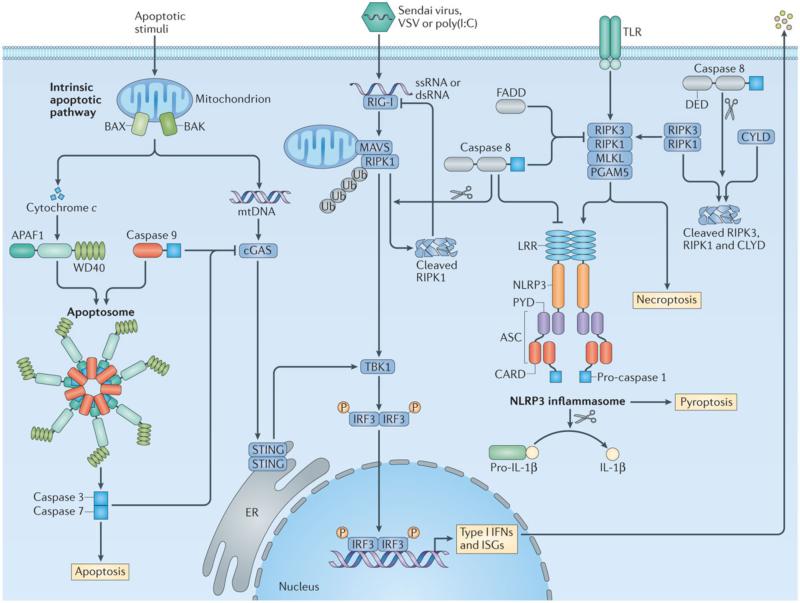
Figure 4. Inhibition of inflammation by caspase 8 and other apoptotic caspases.
In the intrinsic pathway of apoptosis, mitochondrial DNA (mtDNA) is released owing to mitochondrial outer membrane permeabilization mediated by the pro-apoptotic effector proteins BAX (BCL-2-associated X protein) and BAK (BCL-2 antagonist/killer). Released mtDNA binds to the DNA sensor cGAS (cGAMP synthase), which signals through its adaptor STING, TBK1 and IRF3 to activate type I interferon and interferon-stimulated genes (ISGs) 167, 168. Cytochrome c is also released by mitochondria, which is sensed by the sensor APAF1 (apoptotic protease-activating factor 1). APAF1 and caspase 9 assembles the apoptosome to activate effector caspases 3 and 7 (Refs 12-16). During apoptosis, caspases 3, 7 and 9 prevent signal transduction by the cGAS-STING pathway leading to type I interferon production through an as-yet-undefined mechanism 167, 168. The RNA sensor RIG-I recognizes the dsRNA viruses Sendai virus and vesicular stomatitis virus (VSV) or the synthetic dsRNA ligand poly(I:C). RIG-I interacts with the adaptor mitochondrial antiviral signalling protein (MAVS) at the mitochondria, where the kinase RIPK1 is recruited and undergoes K63-linked polyubiquitylation at site Lys-377. Mitochondria-associated polyubiquitylated RIPK1 induces activation of antiviral responses via TBK1 and IRF3. Following activation of IRF3, polyubiquitylated RIPK1 is cleaved by caspase 8, whereby cleaved RIPK1 inhibits RIG-I signalling 121. Caspase 8 also suppresses activation of the NLRP3 inflammasome 121. Upon stimulation of Toll-like receptor 2 (TLR2) or TLR4 in dendritic cells, caspase 8 and FADD inhibit the ripoptosome composed of the kinases RIPK3 and RIPK1. In the absence of caspase 8 or FADD, the ripoptosome signals through the effector MLKL and the mitochondrial serine/threonine-protein phosphatase PGAM5 to activate the NLRP3 inflammasome 121. Caspase 8 and FADD have also been proposed to directly interact and inhibit NLRP3. There is also evidence to suggest that caspase 8 mediates cleavage of RIPK3, RIPK1 and the deubiquitylating enzyme CYLD as a mechanism to inhibit necroptosis and inflammation 161-163.
Inhibition of necroptosis
An indirect way in which caspase 8 inhibits inflammatory responses is through its ability to inhibit necroptosis – an inflammatory form of programmed cell death mediated by RIPK1 and RIPK3 independently of caspases20, 123, 124. In addition to its ability to bind and inhibit RIPK1-RIPK3-mediated inflammasome activation 120, caspase 8 cleaves RIPK1, RIPK3 and the deubiquitylating enzyme CYLD, which is responsible for removing ubiquitin chains from RIPK1, and direct its function towards necroptosis161-163 (Fig. 4). Indeed, spontaneous inflammation that develops in the absence of caspase 8 is prevented by inhibition of necroptosis164, 165. Mice with a conditional deletion of the gene encoding caspase 8 in the intestinal epithelium exhibit spontaneous ileitis, loss of Paneth and goblet cells, elevated infiltration of granulocytes and CD4+ T cells into the lamina propria, and increased expression of inflammatory markers 164. Importantly, inhibition of necroptosis by necrostatin 1 largely prevents TNF-induced lethality and small intestinal tissue destruction in these mice 164. Another report also found that caspase 8 prevents RIPK3-mediated necroptosis, death of enterocytes and immune cell infiltration of the colon 165. These findings are further supported by a study showing that mice lacking FADD in the intestinal epithelium are hypersusceptible to necrosis of epithelial cells, loss of Paneth cells and develop colitis – defects that are largely prevented by genomic deletion of the gene encoding RIPK3 (Ref 166). The ability of caspase 8 to provide a checkpoint to suppress necroptosis-induced inflammation adds another layer of functional complexity to this enigmatic protease.
Apoptotic caspases inhibit type I IFNs
Apoptotic caspases have a major role in suppressing type I IFN responses. Defective caspase 8 functions have been shown to lead to inflammatory skin disease owing to increased IFN activity 122. In this study, a mouse strain was generated to carry a set of wild-type caspase 8 alleles and a set of enzymatically-defective caspase 8 alleles122. This was achieved by microinjecting fertilized oocytes from wild-type mice with a bacterial artificial chromosome carrying a set of alleles encoding caspase 8 lacking its enzymatic activity. Mice harbouring a set of wild-type caspase 8 alleles develop normally in the presence of a second set of alleles encoding caspase 8 lacking its enzymatic activity 122. However, deletion of one of the two wild-type alleles encoding caspase 8 results in the development of a chronic inflammatory skin disorder as well as infiltration of leukocytes into the lungs and pancreas 122. This same study also found that mice lacking caspase 8 specifically in keratinocytes also develop cutaneous inflammation. The disease pathogenesis was not a result of TNF, IL-1 or TLR signalling, but to increased activation of the transcription factor IFN-regulatory factor 3 (IRF3) and TANK-binding kinase 1 (TBK1) in the epidermis 122. Furthermore, keratinocytes lacking caspase 8 express elevated levels of IFNβ and IFN-inducible proteins following exposure to transfected double stranded DNA compared with transfected wild-type keratinocytes 122. Increased expression of IRF3 and IRF7 in BMDCs lacking caspase 8 has also been observed 159.
More recent studies further highlight a relationship between caspase 8 and other apoptotic caspases and type I IFNs. Recognition of RNA viruses by the cytosolic sensor RIG-I triggers signalling via the adaptor mitochondrial antiviral signalling protein (MAVS; also known as IPS1, VISA and CARDIF) at the mitochondria. RIPK1 is recruited to the mitochondria where it is polyubiquitylated to drive IRF3-dependent production of type I IFNs 121. Importantly, caspase 8 cleaves polyubiquitylated RIPK1, converting RIPK1 into an inhibitor of RIG-I 121 (Fig. 4). In the absence of caspase 8, fibroblastoid cells or hepatocytes infected with the RNA viruses Sendai virus and vesicular stomatitis virus or stimulated with the synthetic double stranded RNA poly(I:C) display enhanced activation of IRF3 (Ref 121).
The intrinsic pathway of apoptosis regulated by caspase 9 must also maintain immunological silence. Released mitochondrial DNA owing to mitochondrial outer membrane permeabilization activates the DNA sensor cyclic GMP–AMP synthase (cGAS) and its adaptor stimulator of IFN genes (STING) that induce type I IFNs and IFN-stimulated genes for antiviral defence167, 168. However, caspases 3, 7 and 9 inhibit the induction of type I IFN responses167, 168 (Fig. 4). Although the molecular mechanism remains to be defined, it has been suggested that apoptotic caspases might cleave cGAS, a regulator of cGAS or mitochondrial DNA itself 167, 168. Release of mitochondrial DNA could engage inflammasome sensors to potentiate activation of caspase 1 (Refs 146-149, 169, 170). Indeed, oxidized mitochondrial DNA released during apoptosis has been shown to bind directly to NLRP3 and activate the inflammasome in LPS-primed BMDMs 169, providing an example to illustrate that immunological silence during apoptosis is not always maintained 171.
Caspases and cell-autonomous immunity
The induction of cell-autonomous immunity is essential for host survival in response to infection. The functional roles of caspases and the cytoskeleton are in many ways inextricably linked to cell-autonomous host defense 172. Caspase 1 activation via the NLRC4 inflammasome controls replication of L. pneumophila by inducing maturation of the L. pneumophila-containing vacuole 150. Importantly, caspase 1 cleaves caspase 7 to promote fusion between phagosomes and pathogen-containing vacuoles 173, 174. A further study has shown that caspases 4, 5 and 11 are essential for the control of L. pneumophila replication in macrophages175. The mechanism by which caspase 11 contributes to the inhibition of L. pneumophila replication depends on the ability of caspase 11 to license actin polymerization through phosphorylation of the actin-binding protein cofilin, ultimately permitting fusion of the L. pneumophila vacuole with lysosomes 175. In addition, the CARD of caspase 11 has been shown to physically interact with the C-terminal WD40 propeller domain of actin-interacting protein 1 (AIP1), whereby this interaction potentiates cofilin-mediated actin depolymerization 176.
The relationship between caspases and cell-autonomous immunity is further demonstrated during S. Typhimurium infection. In infected macrophages, the NLRC4-caspase 1 axis is required to induce cellular stiffness, leading to impaired movement and uptake of additional bacteria as well as increased ROS production for bacterial killing 177. Earlier studies have validated a role for caspase 1 in cleaving β-actin and γ-actin, rendering them incapable to undergo polymerization173, 178-180. It is possible that caspase 1-dependent restriction of cytoskeletal functions prevents overwhelming infection at the single cell level, allowing the cell to direct resources to kill residing bacteria before phagocytosing more bacteria. This hypothesis would align with the idea that commitment to pyroptosis is made only when the cell no longer has the capacity to kill the residing bacteria 177.
In the absence of caspase 1, macrophages infected with S. Typhimurium and F. novicida or transfected with DNA undergo apoptosis and induce robust cleavage of caspases 3, 7, 8 and 9 or the apoptotic substrate PARP 112, 113, 181. It is likely that pyroptosis driven by caspase 1 overrides apoptotic cell death during infection by certain bacteria. However, if caspase 1-mediated cell death is somehow bypassed or compromised, by bacterial or viral virulence factors that strategically block or mediate evasion of the inflammasome, apoptosis eventually ensues to remove the replicative niche for these pathogens. The synergy between caspases in the context of cell-autonomous immunity further blurs the line between inflammatory and apoptotic caspases. Importantly, these findings provide elegant examples of how caspases control facets of immunity beyond cell death and inflammation.
Conclusions
The caspase family members are architects of the body. They maintain homeostasis by dismantling unwanted or damaged cells in a systematic manner. Although caspases are broadly classified into apoptotic and inflammatory caspases, new and exciting studies are continuously unveiling the multi-faceted nature of individual caspases in the immune system. Caspases function as pro-inflammatory and anti-inflammatory molecules, while having cell-intrinsic roles to orchestrate cell-autonomous immunity. Converging roles of caspases in the immune system are further exemplified by their ability to communicate with one another to maintain immunological silence or to promote inflammatory responses to eliminate invading pathogens. Furthermore, the capacity for inflammatory caspases to mediate sensing of LPS signifies the importance of these proteases in pattern recognition. These elegant and surprising findings challenge the existing functional classification of the caspase family members. The intertwining relationship between caspases will be further defined in the future. Understanding the expanding and interconnected roles of caspases will contribute to the development of therapies that target disease processes associated with dysregulated caspase activity.
Box 1 | Discovery of caspase 1 and inflammasomes.
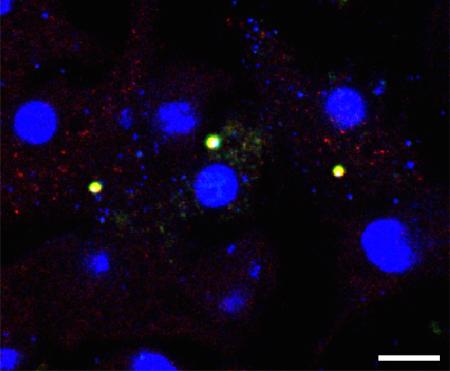
Caspase 1 was identified in 1989 and named interleukin-1β-converting enzyme (ICE) owing to its ability to convert a 31 kDa pro-IL-1β into an active 17.5 kDa fragment 182, 183. Characterization of purified caspase 1 in 1992 revealed that it is a heterodimeric cysteine protease composed of two subunits, p10 and p20 (Ref 184). Caspase 1 mediates proteolytic processing of IL-18 with similar efficiency 185. In 2002, Jürg Tschopp and colleagues described in an overexpression and cell free system the existence of a macromolecular protein complex comprised of caspase 1, caspase 5, an adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC; also known as PYCARD), and NOD-, LRR- and pyrin domain-containing protein 1 (NLRP1) 21. This elegant study provided evidence to show that caspase 1 is activated in a large multi-protein complex initiated by a member of the nucleotide-binding domain, leucine-rich repeat containing (NLR) family. The term inflammasome was then coined to describe its multi-protein composition. In 2004, Vishva Dixit and colleagues generated mutant mouse strains with a genomic deletion of ASC and NOD-, LRR- and CARD-containing 4 (NLRC4) and found that macrophages lacking these components failed to engage caspase 1 activation and do not process pro-IL-1β in response to infection by the intracellular bacteria Salmonella enterica serovar Typhimurium140, thereby providing the first line of genetic evidence to demonstrate the role of an NLR in the modulation of inflammasome activities under endogenous settings. In addition, three groups independently showed in 2006 that another member of the NLR family, NLRP3, assembles the inflammasome to activate caspase 1 in response to ATP, bacterial RNA molecules and uric acid crystals 141-143. The AIM2 and pyrin inflammasomes were subsequently identified in later studies 146-149, 186, 187. Inflammasomes can be visualized as a distinct speck by fluorescence or confocal microscopy95, 111, 144, 145, 188 (See image; arrows indicate ASC-caspase 1 inflammasomes in macrophages transfected with double stranded DNA; Scale bar, 10 μm).
Box 2 | Cross-species functionality of caspases 4, 5 and 11.
Murine caspase 11 was identified by Yuan and colleagues in 1996 (Ref 30). It shares 46% amino acid sequence identity with murine caspase 1 and 45% sequence identity with human caspase 1 (Ref 30). Human caspase 4 and caspase 5 share 78% nucleotide sequence identity79, indicating that these two genes evolved from a common ancestor by gene multiplication. The expression of caspases 5 and 11 can be upregulated in response to lipopolysaccharide (LPS), other Toll-like receptor (TLR) agonists, interferon-β (IFNβ) or IFNγ in a cell-type specific manner, whereas caspase 4 is constitutively and highly expressed in many cell types 5, 30, 37, 189. In addition, caspases 4, 5 and 11 are activated following recognition of cytoplasmic LPS from Gram-negative bacteria, leading to activation of the non-canonical NLRP3 inflammasome 32-34, 41. Caspases 4, 5 and 11 have cross-species activities and are functionally interchangeable in human and mouse cells. Stably expressed caspase 4 or caspase 5 induces pyroptosis in mouse macrophages lacking caspase 11, whereas caspase 11 when introduced into human 293T cells induces pyroptosis 49, 73.
Box 3 | Pyroptosis.
The term pyroptosis was first proposed by Cookson and Brennan in 2001 to describe the pro-inflammatory and caspase 1-dependent nature of Salmonella-induced cell death and to distinguish it from non-inflammatory apoptosis 52. “Pyro” describes fire or fever and “potosis” signifies a pro-inflammatory programmed cell death. Pyroptosis is characterized by rupture of the cell membrane and release of cellular contents, cell swelling, positivity for Annexin V and TUNEL staining, evidence of chromatin condensation and absence of DNA laddering 55, 188. The mitochondria of pyroptotic cells also lose membrane potential and release their contents 188. A number of investigators have also termed caspase 11-induced cell death as pyroptosis 35, 36, 53, 54. Caspase 11 activates pyroptosis in response to Escherichia coli and other non-canonical NLRP3 inflammasome triggers 29, 33. It is important, however, to note that caspase 11 induces cell death independently of caspase 1 and does not directly cleave IL-1β and IL-18. From this standpoint, it is likely that caspase 1-dependent pyroptosis and caspase 11-dependent pyroptosis feature their own set of molecular signatures, some of which might be common. Both caspase 1 and caspase 11 cleave a 53-kDa substrate called gasdermin D, generating a 31-kDa amino-terminal fragment which initiates pyroptosis and a 22-kDa carboxy-terminal fragment which has unknown functions 62-64.
KEY POINTS.
■ Caspases have multifaceted roles in the immune system. They form structurally diverse and dynamic complexes to drive cell death and inflammation.
■ Inflammatory caspases caspases 1, 4, 5 and 11 mediate inflammasome-associated secretion of interleukin-1β (IL-1β) and IL-18 and initiate pyroptosis.
■ The inflammatory caspase caspase 12 have enigmatic roles in the immune system.
■ The apoptotic caspase caspase 8 contributes to initiation of inflammation by modulating NF-kB signalling, promotes direct or indirect cleavage of pro-IL-1β and pro-IL-18, but also play a role in the inhibition of inflammation by blocking inflammasome activation, IFN responses and necroptosis.
■ The functional roles of caspases and the cytoskeleton are linked to cell-autonomous immunity which contributes to the clearance of pathogens.
■ The converging and interconnected roles of caspases challenge the existing functional classification of the caspase family members.
Acknowledgements
Research studies from our laboratory are supported by the US National Institutes of Health (AR056296, CA163507 and AI101935 to T.-D.K.), the American Lebanese Syrian Associated Charities (to T.-D.K.), and the National Health and Medical Research Council of Australia (NHMRC/R.G. Menzies Early Career Fellowship to S.M.M.). We apologize to our colleagues whose work was not cited owing to space limitations.
Glossary
- Inflammasome
A multi-protein complex that activates caspase 1 to induce processing of pro-interleukin-1β (IL-1β) and pro-IL-18 and to drive pyroptosis.
- Pyroptosis
An inflammatory and lytic form of programmed cell death mediated by inflammatory caspases.
- Apoptosis
A non-inflammatory form of programmed cell death mediated by apoptotic caspases. Apoptotic cells exhibit membrane blebbing but have an intact cell membrane, DNA fragmentation and cell shrinkage.
- Death receptors
A subset of receptors within the tumour necrosis factor receptor superfamily that, upon binding to their ligands, convey cell death signals.
- FLICE-like inhibitory protein
(FLIP). The long isoform (FLIPL) heterodimerizes with pro-caspase 8 to inhibit apoptosis and potentially inhibits necroptosis. By contrast, the short isoform (FLIPS) promotes necroptosis.
- Apoptosome
A multi-protein complex comprised of apoptotic protease-activating factor 1 (APAF1) and caspase 9, which induces the intrinsic pathway of apoptosis.
- Microorganism-associated molecular patterns
(MAMPs). Conserved structures that make up microorganisms; for example, lipopolysaccharide or flagellin, which are recognized by pattern recognition receptors.
- Damage-associated molecular patterns
(DAMPs). Molecules that are released by injured cells; for example, ATP or host DNA, and are recognized by pattern recognition receptors.
- Alarmin
A damage-associated molecular pattern that is released by damaged or necrotic cells in response to infection or injury. Alarmins are recognized by pattern recognition receptors to further activate immune cells.
- Non-canonical NLRP3 inflammasome
An inflammasome containing NLRP3 that is activated by Gram-negative bacteria or lipopolysaccharide, requiring activation of caspases 4 or 5 in human cells and caspase 11 in mouse cells.
- Canonical inflammasomes
Inflammasomes that do not require caspases 4, 5 and 11 for their activation, including the canonical NLRP3, NLRC4 and AIM2 inflammasomes.
- Guanylate-binding proteins
(GBPs). A group of interferon-inducible GTPases produced by the host cell that often target pathogen-containing vacuoles, contributing to the release of pathogens from the vacuole and mediating pathogen killing.
- Necroptosis
A type of necrosis and a form of non-apoptotic cell death driven by kinases RIPK1 and RIPK3 under conditions in which caspase 8 is inhibited.
- Inhibitor of apoptosis proteins
(IAPs). A family of proteins that have multiple functions, including inhibition of apoptosis by binding to apoptotic caspases, E3 ubiquitin ligase activity, regulation of MAPK and NF-κB signalling pathways and regulation of signal transduction by pattern recognition receptors.
- Second mitochondrial-derived activator of caspases
(SMAC; also known as DIABLO). Endogenous antagonists of inhibitors of apoptosis proteins (IAPs) released from mitochondria in response to death stimuli. SMAC bind and degrade X-linked IAP (XIAP), cellular IAP1 (cIAP1) and cIAP2, resulting in elevated caspase activation.
- Cell-autonomous immunity
A defence mechanism used by the cell to control infection that is not traditionally considered as part of the immune system. For example, compartmentalization to prevent inappropriate entry of bacteria into the cytoplasm within a eukaryotic cell; and production of nitric oxide synthases to mediate killing of invading an microorganism.
Biography
Si Ming Man
Si Ming Man received his Ph.D. from the University of Cambridge, United Kingdom, for his work on inflammasomes and their roles in the host defence against Salmonella infection. He is a recipient of the National Health and Medical Research Council R.G. Menzies Early Career Fellowship and is currently a postdoctoral fellow in the laboratory of Thirumala-Devi Kanneganti at St. Jude Children's Research Hospital, Memphis, Tennessee, U.S.A.
Thirumala-Devi Kanneganti
Thirumala-Devi Kanneganti, Ph.D., is a Member (Professor) in the Department of Immunology at St. Jude Children's Research Hospital, Memphis, Tennessee, U.S.A. Her research focuses on innate immunity and inflammation. She has long standing interests in understanding the roles of NLRs, inflammasomes and their signalling molecules in the regulation of inflammatory, infectious and autoimmune diseases. She is a recipient of the American Association of Immunologists (AAI)-BD Biosciences Investigator Award from AAI for her contributions to the field of Immunology.
References
- 1.Alnemri ES, et al. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 2.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–22. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stowe I, Lee B, Kayagaki N. Caspase-11: arming the guards against bacterial infection. Immunol Rev. 2015;265:75–84. doi: 10.1111/imr.12292. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16:329–44. doi: 10.1038/nrm3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 8.Feoktistova M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–63. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenev T, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–48. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15:362–74. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 11.Blander JM. A long-awaited merger of the pathways mediating host defence and programmed cell death. Nat Rev Immunol. 2014;14:601–18. doi: 10.1038/nri3720. [DOI] [PubMed] [Google Scholar]
- 12.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–13. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 13.Li P, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–57. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 15.Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–56. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Benedict MA, Ding L, Nunez G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J. 1999;18:3586–95. doi: 10.1093/emboj/18.13.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckhart L, et al. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol. 2000;115:1148–51. doi: 10.1046/j.1523-1747.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- 18.Lippens S, et al. Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ. 2000;7:1218–24. doi: 10.1038/sj.cdd.4400785. [DOI] [PubMed] [Google Scholar]
- 19.Creagh EM. Caspase crosstalk: integration of apoptotic and innate immune signalling pathways. Trends Immunol. 2014;35:631–640. doi: 10.1016/j.it.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011;12:757–63. doi: 10.1038/nrm3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [This paper described the existence an inflammasome complex which is assembled in the cytoplasm to mediate cleavage of IL-1β. This paper also characterised the basic architecture of human NLRP1 inflammasome.] [DOI] [PubMed] [Google Scholar]
- 22.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–32. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–98. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Veerdonk FL, Joosten LA, Netea MG. The interplay between inflammasome activation and antifungal host defense. Immunol Rev. 2015;265:172–80. doi: 10.1111/imr.12280. [DOI] [PubMed] [Google Scholar]
- 25.Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol. 2012;13:343–51. doi: 10.1038/ni.2224. [DOI] [PubMed] [Google Scholar]
- 26.Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol. 2012;13:352–7. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–45. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 28.Kang SJ, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149:613–22. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21. doi: 10.1038/nature10558. [showed that caspase 11 activation in response to infection by Gram-negative bacteria leads to non-canonical NLRP3 inflammasome activity.] [DOI] [PubMed] [Google Scholar]
- 30.Wang S, et al. Identification and characterization of Ich-3, a member of the interleukin-1beta converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J Biol Chem. 1996;271:20580–7. doi: 10.1074/jbc.271.34.20580. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–9. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 32.Rathinam VA, et al. TRIF Licenses Caspase-11-Dependent NLRP3 Inflammasome Activation by Gram-Negative Bacteria. Cell. 2012;150:606–19. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broz P, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–91. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurung P, et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem. 2012;287:34474–83. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–9. doi: 10.1126/science.1240248. [identified an unknown cytoplasmic sensor which mediates recognition of cytosolic LPS independently of TLR4. This sensor was later identified to be caspase 11 (see Reference 73).] [DOI] [PubMed] [Google Scholar]
- 36.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–3. doi: 10.1126/science.1240988. [identified an unknown cytoplasmic sensor which mediates recognition of cytosolic LPS independently of TLR4. This sensor was later identified to be caspase 11 (see Reference 73).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casson CN, et al. Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1421699112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid-Burgk JL, et al. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015 doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 39.Baker PJ, et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol. 2015 doi: 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- 40.Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K efflux. Eur J Immunol. 2015 doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 41.Sander LE, et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474:385–9. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fassy F, et al. Enzymatic activity of two caspases related to interleukin-1beta-converting enzyme. Eur J Biochem. 1998;253:76–83. doi: 10.1046/j.1432-1327.1998.2530076.x. [DOI] [PubMed] [Google Scholar]
- 43.Knodler LA, et al. Noncanonical Inflammasome Activation of Caspase-4/Caspase-11 Mediates Epithelial Defenses against Enteric Bacterial Pathogens. Cell Host Microbe. 2014;16:249–56. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casson CN, et al. Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog. 2013;9:e1003400. doi: 10.1371/journal.ppat.1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukens JR, et al. RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature. 2013;498:224–7. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 47.Lamkanfi M, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol. 2010;185:4385–92. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarkar A, et al. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med. 2006;174:1003–10. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kajiwara Y, et al. A critical role for human caspase-4 in endotoxin sensitivity. J Immunol. 2014;193:335–43. doi: 10.4049/jimmunol.1303424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Moltke J, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–11. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–31. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 52.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–4. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 53.Aachoui Y, et al. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–8. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilla DM, et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci U S A. 2014;111:6046–51. doi: 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130–42. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–42. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sellin ME, et al. Epithelium-Intrinsic NAIP/NLRC4 Inflammasome Drives Infected Enterocyte Expulsion to Restrict Salmonella Replication in the Intestinal Mucosa. Cell Host Microbe. 2014;16:237–48. doi: 10.1016/j.chom.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Storek KM, Monack DM. Bacterial recognition pathways that lead to inflammasome activation. Immunol Rev. 2015;265:112–29. doi: 10.1111/imr.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen KW, et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 2014;8:570–82. doi: 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 60.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–9. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doitsh G, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–14. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signaling. Nature. 2015 doi: 10.1038/nature15541. [demonstrated that the N-terminal fragment of cleaved gasdermin D drives pyroptosis.] [DOI] [PubMed] [Google Scholar]
- 63.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015 doi: 10.1038/nature15514. [demonstrated that the N-terminal fragment of cleaved gasdermin D drives pyroptosis.] [DOI] [PubMed] [Google Scholar]
- 64.Agard NJ, Maltby D, Wells JA. Inflammatory stimuli regulate caspase substrate profiles. Mol Cell Proteomics. 2010;9:880–93. doi: 10.1074/mcp.M900528-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–20. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–56. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dupaul-Chicoine J, et al. The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunity. 2015 doi: 10.1016/j.immuni.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 68.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A. 2010;107:21635–40. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Demon D, et al. Caspase-11 is expressed in the colonic mucosa and protects against dextran sodium sulfate-induced colitis. Mucosal Immunol. 2014;7:1480–91. doi: 10.1038/mi.2014.36. [independently identified a role for caspase 11 in mediating protection against colitis-associated colorectal cancer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams TM, et al. Caspase-11 Attenuates Gastrointestinal Inflammation and Experimental Colitis Pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2014;308:G139–50. doi: 10.1152/ajpgi.00234.2014. [independently identified a role for caspase 11 in mediating protection against colitis-associated colorectal cancer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oficjalska K, et al. Protective Role for Caspase-11 during Acute Experimental Murine Colitis. J Immunol. 2014;194:1252–60. doi: 10.4049/jimmunol.1400501. [independently identified a role for caspase 11 in mediating protection against colitis-associated colorectal cancer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–60. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 73.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–92. doi: 10.1038/nature13683. [This study demonstrated that mouse caspase 11 and human caspase 4 and 5 are sensors of cytosolic LPS. The pathogen-sensing capability of caspases clearly broadens their functional repertoire.] [DOI] [PubMed] [Google Scholar]
- 74.Case CL, et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A. 2013;110:1851–6. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meunier E, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–70. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 76.Kalai M, et al. Regulation of the expression and processing of caspase-12. J Cell Biol. 2003;162:457–67. doi: 10.1083/jcb.200303157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–94. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakagawa T, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 79.Fischer H, Koenig U, Eckhart L, Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun. 2002;293:722–6. doi: 10.1016/S0006-291X(02)00289-9. [DOI] [PubMed] [Google Scholar]
- 80.Saleh M, et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75–9. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- 81.Xue Y, et al. Spread of an inactive form of caspase-12 in humans is due to recent positive selection. Am J Hum Genet. 2006;78:659–70. doi: 10.1086/503116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kachapati K, O'Brien TR, Bergeron J, Zhang M, Dean M. Population distribution of the functional caspase-12 allele. Hum Mutat. 2006;27:975. doi: 10.1002/humu.9448. [DOI] [PubMed] [Google Scholar]
- 83.Yavari M, et al. Presence of the functional CASPASE-12 allele in Indian subpopulations. Int J Immunogenet. 2012;39:389–93. doi: 10.1111/j.1744-313X.2012.01107.x. [DOI] [PubMed] [Google Scholar]
- 84.Ferwerda B, et al. Caspase-12 and the inflammatory response to Yersinia pestis. PLoS One. 2009;4:e6870. doi: 10.1371/journal.pone.0006870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saleh M, et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064–8. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- 86.Marshall L, et al. CASPASE-12 and rheumatoid arthritis in African-Americans. Immunogenetics. 2014;66:281–5. doi: 10.1007/s00251-014-0762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosentul DC, et al. The impact of caspase-12 on susceptibility to candidemia. Eur J Clin Microbiol Infect Dis. 2012;31:277–80. doi: 10.1007/s10096-011-1307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen J, et al. Lack of association of the caspase-12 long allele with community-acquired pneumonia in people of African descent. PLoS One. 2014;9:e89194. doi: 10.1371/journal.pone.0089194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McCall MB, et al. Persistence of full-length caspase-12 and its relation to malaria in West and Central African populations. Eur Cytokine Netw. 2010;21:77–83. doi: 10.1684/ecn.2010.0187. [DOI] [PubMed] [Google Scholar]
- 90.Hervella M, et al. The loss of functional caspase-12 in Europe is a pre-neolithic event. PLoS One. 2012;7:e37022. doi: 10.1371/journal.pone.0037022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roy S, et al. Confinement of caspase-12 proteolytic activity to autoprocessing. Proc Natl Acad Sci U S A. 2008;105:4133–8. doi: 10.1073/pnas.0706658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.LeBlanc PM, et al. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe. 2008;3:146–57. doi: 10.1016/j.chom.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 93.Chun HJ, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–9. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 94.Lemmers B, et al. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem. 2007;282:7416–23. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- 95.Man SM, et al. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1beta production. J Immunol. 2013;191:5239–46. doi: 10.4049/jimmunol.1301581. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gurung P, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–46. doi: 10.4049/jimmunol.1302839. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allam R, et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982–90. doi: 10.15252/embr.201438463. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su H, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–8. doi: 10.1126/science.1104765. [This study identified that a human mutation in caspase 8 leads to impaired activation of NFκB signalling, demonstrating a role for caspase 8 in inflammation.] [DOI] [PubMed] [Google Scholar]
- 99.Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838–44. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 100.Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol. 2004;24:2627–36. doi: 10.1128/MCB.24.7.2627-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maelfait J, et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–73. doi: 10.1084/jem.20071632. [This study demonstrated a requirement for TRIF in the processing of IL-1β by caspase 8, but not by caspase 1 in vitro.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vince JE, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–27. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 103.Luthi AU, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 104.Gringhuis SI, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [This study characterised a caspase 8-containing unit induced by certain fungal and mycobacterial infection, leading to direct processing of IL-1β independently of caspase 1.] [DOI] [PubMed] [Google Scholar]
- 105.Moriwaki K, Bertin J, Gough PJ, Chan FK. A RIPK3-Caspase 8 Complex Mediates Atypical Pro-IL-1beta Processing. J Immunol. 2015;194:1938–44. doi: 10.4049/jimmunol.1402167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1beta via caspase-8 in dendritic cells. J Immunol. 2013;191:4789–803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.England H, Summersgill HR, Edye ME, Rothwell NJ, Brough D. Release of interleukin-1alpha or interleukin-1beta depends on mechanism of cell death. J Biol Chem. 2014;289:15942–50. doi: 10.1074/jbc.M114.557561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shenderov K, et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. 2014;192:2029–33. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bossaller L, et al. Cutting Edge: FAS (CD95) Mediates Noncanonical IL-1beta and IL-18 Maturation via Caspase-8 in an RIP3-Independent Manner. J Immunol. 2012;189:5508–12. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uchiyama R, Yonehara S, Tsutsui H. Fas-mediated inflammatory response in Listeria monocytogenes infection. J Immunol. 2013;190:4245–54. doi: 10.4049/jimmunol.1203059. [DOI] [PubMed] [Google Scholar]
- 111.Man SM, et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci U S A. 2014;111:7403–8. doi: 10.1073/pnas.1402911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pierini R, et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19:1709–21. doi: 10.1038/cdd.2012.51. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sagulenko V, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20:1149–60. doi: 10.1038/cdd.2013.37. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Antonopoulos C, et al. Caspase-8 as an Effector and Regulator of NLRP3 Inflammasome Signaling. J Biol Chem. 2015 doi: 10.1074/jbc.M115.652321. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weng D, et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A. 2014;111:7391–6. doi: 10.1073/pnas.1403477111. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Philip NH, et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc Natl Acad Sci U S A. 2014;111:7385–90. doi: 10.1073/pnas.1403252111. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karki R, et al. Concerted Activation of the AIM2 and NLRP3 Inflammasomes Orchestrates Host Protection against Aspergillus Infection. Cell Host Microbe. 2015;17:357–68. doi: 10.1016/j.chom.2015.01.006. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ganesan S, et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1beta production in response to beta-glucans and the fungal pathogen, Candida albicans. J Immunol. 2014;193:2519–30. doi: 10.4049/jimmunol.1400276. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lukens JR, et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014;516:246–9. doi: 10.1038/nature13788. [showed that caspase 1 and 8 operate in a concerted manner to drive production of IL-1β in the development of bone inflammation and destruction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 121.Rajput A, et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–51. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 122.Kovalenko A, et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–77. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vercammen D, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–85. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 125.Varfolomeev EE, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 126.Sakamaki K, et al. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 2002;9:1196–206. doi: 10.1038/sj.cdd.4401090. [DOI] [PubMed] [Google Scholar]
- 127.Yeh WC, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 128.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 129.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–7. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dillon CP, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1:401–7. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ando M, et al. Cancer-associated missense mutations of caspase-8 activate nuclear factor-kappaB signaling. Cancer Sci. 2013;104:1002–8. doi: 10.1111/cas.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Labbe K, McIntire CR, Doiron K, Leblanc PM, Saleh M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity. 2011;35:897–907. doi: 10.1016/j.immuni.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 134.Yabal M, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 135.Menu P, et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bronner DN, et al. Endoplasmic Reticulum Stress Activates the Inflammasome via NLRP3- and Caspase-2-Driven Mitochondrial Damage. Immunity. 2015;43:451–62. doi: 10.1016/j.immuni.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lebeaupin C, et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6:e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gurung P, Kanneganti TD. Novel Roles for Caspase-8 in IL-1beta and Inflammasome Regulation. Am J Pathol. 2015;185:17–25. doi: 10.1016/j.ajpath.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Monie TP, Bryant CE. Caspase-8 functions as a key mediator of inflammation and pro-IL-1beta processing via both canonical and non-canonical pathways. Immunol Rev. 2015;265:181–93. doi: 10.1111/imr.12284. [DOI] [PubMed] [Google Scholar]
- 140.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–8. doi: 10.1038/nature02664. [described a role for NLRC4 and ASC in the engagement of a caspase 1 inflammasome complex under endogenous settings in response to Salmonella infection.] [DOI] [PubMed] [Google Scholar]
- 141.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [identified that NLRP3 assembles an inflammasome complex to activate caspase 1 in response to ATP, bacterial RNA molecules and uric acid crystals.] [DOI] [PubMed] [Google Scholar]
- 142.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–6. doi: 10.1038/nature04517. [identified that NLRP3 assembles an inflammasome complex to activate caspase 1 in response to ATP, bacterial RNA molecules and uric acid crystals.] [DOI] [PubMed] [Google Scholar]
- 143.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [identified that NLRP3 assembles an inflammasome complex to activate caspase 1 in response to ATP, bacterial RNA molecules and uric acid crystals.] [DOI] [PubMed] [Google Scholar]
- 144.Broz P, et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–55. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–83. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–8. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–13. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roberts TL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–60. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 149.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–72. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 150.Amer A, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–23. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 151.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–82. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 152.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–75. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 153.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–5. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao Y, Shao F. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion. Immunol Rev. 2015 doi: 10.1111/imr.12293. [DOI] [PubMed] [Google Scholar]
- 155.Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci U S A. 2013;110:14408–13. doi: 10.1073/pnas.1306376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rayamajhi M, Zak DE, Chavarria-Smith J, Vance RE, Miao EA. Cutting Edge: Mouse NAIP1 Detects the Type III Secretion System Needle Protein. J Immunol. 2013;191:3986–9. doi: 10.4049/jimmunol.1301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kang S, et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun. 2015;6:7515. doi: 10.1038/ncomms8515. [represent a collection of important studies which deciphered the functional relevance of caspase 8 in governing the activities of various caspase 1 inflammasomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ben Moshe T, et al. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology. 2007;45:1014–24. doi: 10.1002/hep.21495. [DOI] [PubMed] [Google Scholar]
- 159.Cuda CM, et al. Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. J Immunol. 2014;192:5548–60. doi: 10.4049/jimmunol.1400122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee P, et al. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–23. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–26. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–67. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 163.O'Donnell MA, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–42. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gunther C, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–9. doi: 10.1038/nature10400. [demonstrated using in vivo models that caspase 8 inhibits inflammation by means of suppressing necroptosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Weinlich R, et al. Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Rep. 2013;5:340–8. doi: 10.1016/j.celrep.2013.08.045. [demonstrated using in vivo models that caspase 8 inhibits inflammation by means of suppressing necroptosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Welz PS, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–4. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 167.Rongvaux A, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–77. doi: 10.1016/j.cell.2014.11.037. [found that apoptotic caspases of the intrinsic apoptosis pathway negatively regulate cGAS and STING signalling to suppress type I IFN production.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.White MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–62. doi: 10.1016/j.cell.2014.11.036. [found that apoptotic caspases of the intrinsic apoptosis pathway negatively regulate cGAS and STING signalling to suppress type I IFN production.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shimada K, et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity. 2012;36:401–14. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kailasan Vanaja S, et al. Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2014;111:7765–70. doi: 10.1073/pnas.1400075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Davidovich P, Kearney CJ, Martin SJ. Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol Chem. 2014;395:1163–71. doi: 10.1515/hsz-2014-0164. [DOI] [PubMed] [Google Scholar]
- 172.Mostowy S, Shenoy AR. The cytoskeleton in cell-autonomous immunity: structural determinants of host defence. Nat Rev Immunol. 2015;15:559–73. doi: 10.1038/nri3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lamkanfi M, et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–63. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akhter A, et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009;5:e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Akhter A, et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity. 2012;37:35–47. doi: 10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li J, et al. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat Cell Biol. 2007;9:276–86. doi: 10.1038/ncb1541. [DOI] [PubMed] [Google Scholar]
- 177.Man SM, et al. Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc Natl Acad Sci U S A. 2014;111:17588–93. doi: 10.1073/pnas.1419925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kayalar C, Ord T, Testa MP, Zhong LT, Bredesen DE. Cleavage of actin by interleukin 1 beta-converting enzyme to reverse DNase I inhibition. Proc Natl Acad Sci U S A. 1996;93:2234–8. doi: 10.1073/pnas.93.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mashima T, Naito M, Tsuruo T. Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene. 1999;18:2423–30. doi: 10.1038/sj.onc.1202558. [DOI] [PubMed] [Google Scholar]
- 180.Mashima T, Naito M, Fujita N, Noguchi K, Tsuruo T. Identification of actin as a substrate of ICE and an ICE-like protease and involvement of an ICE-like protease but not ICE in VP-16-induced U937 apoptosis. Biochem Biophys Res Commun. 1995;217:1185–92. doi: 10.1006/bbrc.1995.2894. [DOI] [PubMed] [Google Scholar]
- 181.Puri AW, Broz P, Shen A, Monack DM, Bogyo M. Caspase-1 activity is required to bypass macrophage apoptosis upon Salmonella infection. Nat Chem Biol. 2012;8:745–7. doi: 10.1038/nchembio.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kostura MJ, et al. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989;86:5227–31. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Black RA, Kronheim SR, Sleath PR. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 1989;247:386–90. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- 184.Thornberry NA, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–74. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 185.Ghayur T, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–23. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 186.Gavrilin MA, et al. Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia. J Immunol. 2012;188:3469–77. doi: 10.4049/jimmunol.1102272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xu H, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–41. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 188.Fernandes-Alnemri T, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lin XY, Choi MS, Porter AG. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-gamma. J Biol Chem. 2000;275:39920–6. doi: 10.1074/jbc.M007255200. [DOI] [PubMed] [Google Scholar]
- 190.Hellmich KA, et al. Anthrax lethal factor cleaves mouse nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS One. 2012;7:e49741. doi: 10.1371/journal.pone.0049741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chavarria-Smith J, Vance RE. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog. 2013;9:e1003452. doi: 10.1371/journal.ppat.1003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–93. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Man SM, et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 2015;16:467–75. doi: 10.1038/ni.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Meunier E, et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol. 2015;16:476–84. doi: 10.1038/ni.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]