INTRODUCTION
Over the last few decades, cardiac magnetic resonance imaging (CMR) has evolved into a versatile imaging modality for the heart and is particularly useful for determining the underlying etiology of cardiomyopathies. In addition to accurate functional assessment, cardiac MR imaging plays a vital role in differentiating cardiomyopathies by tissue characterization as well as providing prognostic information depending upon the underlying etiology. The case being discussed is an example for which CMR would be an excellent first choice for imaging the patient as it offers a comprehensive assessment in a single study.1
MORPHOLOGY AND FUNCTION
Over the past 2 decades, cardiac MR has established itself as the gold standard for the quantification of LV mass, volume, and function.2,3 In the evaluation of dilated cardiomyopathies, the precise quantification of ventricular volumes and systolic function has great clinical relevance in regard to prognosis and clinical management such as ICD implantation.4 The excellent spatial and temporal resolution of cine imaging along with myocardial strain imaging allows for accurate and detailed analysis of global and regional myocardial function.5 Cardiac MR imaging also offers accurate and reproducible assessment of RV structure and function.1
TISSUE CHARACTERIZATION
The ability of CMR to characterize myocardial tissue is its greatest strength in determining the etiology of a dilated or infiltrative cardiomyopathy. Through the use of multiple techniques such as late gadolinium enhancement imaging (LGE), native and post-contrast T1 mapping, T2-weighted imaging, and T2* imaging, the differentiation between ischemic and non-ischemic etiology can be performed as well as further differentiation of the various etiologies of non-ischemic cardiomyopathies.4
Late Gadolinium Enhancement Imaging
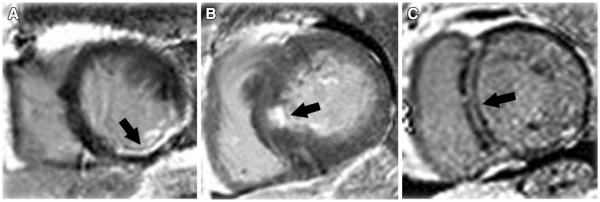
Late gadolinium enhancement imaging (LGE) has established itself as the gold standard for the depiction of myocardial replacement fibrosis and has been shown to be reasonably accurate in the differentiation between non-ischemic and ischemic cardiomyopathies.6,7 mechanism of LGE is a combination of an increased volume of distribution for the contrast agent and prolonged wash-out from fibrotic tissue secondary to decreased capillary density.8 LGE in ischemic cardiomyopathy typically follows a coronary distribution; however, lack of LGE does not completely rule out an ischemic etiology as there are patients with hibernating myocardium and severely reduced EF without prior infarction as evidenced by LGE.7 LGE in ischemic cardiomyopathy can vary from subendocardial to transmural but should always include the subendocardium as the wavefront of necrosis begins there and spreads toward the subepicardium (Figure 1).9
Figure 1.
Examples of late gadolinium enhancement (LGE) in different cardiomyopathies. Arrows indicate areas with LGE. (A) Ischemic cardiomyopathy with involvement of subendocardium; (B) hypertrophic cardiomyopathy with enhancement of the mid septum; (C) non-ischemic cardiomyopathy with a mid-myocardial stripe of LGE in the basal septum.
The various patterns of LGE also can help establish the underlying etiology of non-ischemic cardiomyopathies4 which include idiopathic, myocarditis, hypertrophic cardiomyopathy (HCM), as well as infiltrative processes such as sarcoidosis and amyloidosis. The different etiologies each have their own distinctive pattern of LGE. In non-ischemic dilated cardiomyopathies, LGE can be seen in the mid-myocardium in the basal interventricular septum in up to 30% of patients (Figure 1).9 LGE found in HCM varies, but occurs predominantly in the most hypertrophied regions in a patchy, multi-focal distribution as well as the RV insertion sites in the septum.9 In myocarditis, LGE is generally noted in the mid-myocardium and the subepicardium, especially common in the basal lateral wall, and correlates with areas of active inflammation by histopathology.9
In infiltrative cardiomyopathies, the LGE patterns are diverse. For example, sarcoidosis tends to demonstrate patchy mid-wall and epicardial enhancement but can also be present in the subendocardium or be transmural but not in a typical coronary distribution. Amyloidosis LGE patterns depend on the underlying type, TTR vs AL and may demonstrate global diffuse subendocardial enhancement or patchy enhancement in a noncoronary distribution.
In addition to establishing an underlying etiology of a dilated cardiomyopathy, LGE also provides important prognostic information regarding mortality and sudden cardiac death. Multiple studies have shown that the presence of LGE is associated with an increase in mortality, cardiovascular events, sudden cardiac death, and ventricular arrhythmias independent of other prognostic markers such as LVEF.10–12
Native (Non-contrast) T1 Mapping
Native T1 changes can detect pathologic processes related to excess water in edema, protein deposition, and lipid or iron infiltration.13 Alterations in the native myocardial T1 signal can represent isolated cardiac disease processes or systemic diseases with cardiac involvement.13 Quantification of myocardial T1 values can be useful in determining the etiology of a dilated or infiltrative cardiomyopathy. For example, cardiac amyloidosis has a significantly higher T1 value than in normal controls and in patients with LVH due to aortic stenosis14,15 due to the infiltration of the amyloid protein in the myocardium. Abnormally high T1 values can be seen in cardiomyopathies due to acute myocarditis (related to increased edema)16 or hypertrophic cardiomyopathy (increase in myocardial fibrosis).17
T2-Weighted Imaging/T2 Mapping
T2 relaxation times are sensitive to myocardial edema and are elevated in acute MI, acute myocarditis, and sarcoidosis.18 T2 mapping, like T1 mapping, allows for quantification of the T2 signal in the myocardium. In patients with acute myocarditis or Takotsubo cardiomyopathy, which involve acute inflammation of the myocardium, affected portions of the myocardium demonstrated an increased T2 value.19
T2* Mapping
T2* mapping is the most clinically established quantitative cardiac MR tissue characterization mapping techniques and it has revolutionized the detection and monitoring of iron-overload cardiomyopathy.18 A reduction of the T2* to <20 ms is diagnostic of iron-overload cardiomyopathy.20 This technique can be used to track the beneficial effects of iron chelation therapy in iron-overload diseases.
Limitations
With all the advantages of CMR, there are also some limitations to the technique particularly relevant to patients with dilated or infiltrative cardiomyopathies. Nephrogenic systemic fibrosis (NSF) is an extremely rare but serious disease associated with gadolinium use in patients with severe kidney dysfunction on dialysis. The risk of NSF restricts the use of gadolinium in patients with type 4 or 5 chronic kidney disease, thus limiting LGE imaging and differentiating the etiologies of dilated or infiltrative cardiomyopathies. Another limitation is that the presence of an implantable cardioverter defibrillator is a contraindication to CMR, which limits its use in patients with more advanced stages of heart failure. However, this is changing with the advent of MR-compatible pacemakers and recognition that some non-pacer dependent patients can be safely imaged under certain stringent conditions.
CONCLUSION AND CASE SUMMARY
When it comes to evaluation of a new-onset dilated or infiltrative cardiomyopathy, CMR is the imaging modality of choice. Aside from its accurate and reproducible evaluation of cardiac structure and function, CMR readily differentiates between ischemic and non-ischemic cardiomyopathies but also is able to further characterize the type of non-ischemic cardiomyopathy which in turn drives appropriate therapy. Additionally, CMR is able to provide prognostic information which can help guide further heart failure management. In the case presented, CMR would be useful to accurately measure LV volumes and EF and help identify the underlying etiology based on tissue characterization. LGE patterns and T1 and T2 mapping may help point to a particular diagnosis as well as prognosis. CMR has essentially become a noninvasive myocardial biopsy.
Acknowledgments
Dr. Kramer is supported by R01 HL075792 and U01HL117006-01A1 and receives research equipment support from Siemens Healthcare.
References
- 1.Shehata ML, Turkbey EB, Vogel-Claussen J, Bluemke DA. Role of cardiac magnetic resonance imaging in assessment of nonischemic cardiomyopathies. Top Magn Reson Imaging. 2008;19:43–57. doi: 10.1097/RMR.0b013e31816fcb22. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP., Jr Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- 3.van der Geest RJ, Reiber JH. Quantification in cardiac MRI. J Magn Reson Imaging. 1999;10:602–8. doi: 10.1002/(sici)1522-2586(199911)10:5<602::aid-jmri3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Muzzarelli S, Ordovas K, Higgins CB. Cardiovascular MRI for the assessment of heart failure: Focus on clinical management and prognosis. J Magn Reson Imaging. 2011;33:275–86. doi: 10.1002/jmri.22433. [DOI] [PubMed] [Google Scholar]
- 5.Isbell DC, Kramer CM. Cardiovascular magnetic resonance: structure, function, perfusion, and viability. J Nucl Cardiol. 2005;12:324–36. doi: 10.1016/j.nuclcard.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 7.Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743–8. doi: 10.1016/j.jacc.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 10.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 11.Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: A systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7:250–8. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks J, Kramer CM, Salerno M. Markedly increased volume of distribution of gadolinium in cardiac amyloidosis demonstrated by T1 mapping. J Magn Reson Imaging. 2013;38:1591–5. doi: 10.1002/jmri.24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamitsos TD, Piechnik SK, Banypersad SM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6:488–97. doi: 10.1016/j.jcmg.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira VM, Piechnik SK, Dall'Armellina E, et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: Comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging. 2013;6:1048–58. doi: 10.1016/j.jcmg.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Puntmann VO, Voigt T, Chen Z, et al. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC. 2013;6:475–84. doi: 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6:806–22. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thavendiranathan P, Walls M, Giri S, et al. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging. 2012;5:102–10. doi: 10.1161/CIRCIMAGING.111.967836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]