Abstract
Cholesterol is a structural component of cellular membranes, which is transported from liver to peripheral cells in the form of cholesterol esters (CE), residing in the hydrophobic core of low-density lipoprotein. Oxidized CE (OxCE) is often found in plasma and in atherosclerotic lesions of subjects with cardiovascular disease. Our earlier studies have demonstrated that OxCE activates inflammatory responses in macrophages via toll-like receptor-4 (TLR4). Here we demonstrate that cholesterol binds to myeloid differentiation-2 (MD-2), a TLR4 ancillary molecule, which is a binding receptor for bacterial lipopolysaccharide (LPS) and is indispensable for LPS-induced TLR4 dimerization and signaling. Cholesterol binding to MD-2 was competed by LPS and by OxCE-modified BSA. Furthermore, soluble MD-2 in human plasma and MD-2 in mouse atherosclerotic lesions carried cholesterol, the finding supporting the biological significance of MD-2 cholesterol binding. These results help understand the molecular basis of TLR4 activation by OxCE and mechanisms of chronic inflammation in atherosclerosis.
Keywords: cholesterol, MD-2, TLR4, LPS
1. Introduction
Endogenous lipids, proteins and nucleic acids often undergo, under pathological conditions, oxidation, fragmentation and other types of modification and form damage-associated molecular patterns (DAMPs)1 [1]. DAMPs activate innate immune receptors and induce inflammatory responses, which can be harmful if unconstrained and prolonged, such as in atherosclerosis, a chronic inflammation of large arteries leading to cardiovascular disease (CVD). We have demonstrated that oxidized cholesterol esters (OxCE), which are abundant in atherosclerotic lesions and in blood of patients with CVD, are such DAMPs, which induce inflammatory responses in macrophages via toll-like-receptor-4 (TLR4) [2–5]. OxCE induce TLR4 dimerization, activation of downstream kinases and transcription factors, resulting in secretion of inflammatory cytokines, cytoskeletal effects and macrophage lipid accumulation. Yet, molecular mechanisms of OxCE activation of TLR4 remain unclear.
Myeloid differentiation-2 (MD-2; also known as LY96) is a co-receptor of TLR4 and is required for bacterial lipopolysaccharide (LPS) binding and subsequent dimerization of two TLR4/MD-2/LPS molecular complexes. MD-2 has a β-cup fold structure composed of two antiparallel β sheets forming a hydrophobic pocket, with positively charged residues located near the opening rim of the cavity [6, 7]. The hydrophobic pocket accommodates fatty acid chains of LPS lipid A, and positively charged residues at its opening bind negatively charged phosphate groups of lipid A. In the molecule of cholesterol, a hydrocarbon chain linked to one side of the steroid form an elongated hydrophobic structure, which may dock in the hydrophobic pocket of MD-2, and a hydroxyl group linked to the other side of the steroid may stabilize cholesterol at the positively charged entrance to the pocket. The goal of this study was to provide experimental evidence to the hypothesis that MD-2 binds cholesterol. If confirmed, this hypothesis will help understand the molecular basis of TLR4 activation by OxCE and mechanisms of chronic inflammation in atherosclerosis.
2. Materials and methods
2.1. MD-2 expression and purification
MD-2 was expressed and purified from either human embryonic kidney (HEK) 293 cells or Spodoptera fruigiperda (Sf9) insect cells, with both preparations tested to bind cholesterol. HEK293 cells, cultured in DMEM supplemented with 10% FBS and 50 µg/ml gentamicin (Calbiochem), were transfected with either MD-2 subcloned into pSecTag2A (Invitrogen) or an empty vector, using GenJet In Vitro DNA transfection reagent (SignaGen Laboratories). After 3 days, cells and medium were harvested, lyzed, cleared by centrifugation, and loaded onto a pre-equilibrated Ni-NTA agarose (Qiagen) column. The column was extensively washed in a buffer containing 50 mM imidazole. MD-2 was eluted from the column with increasing concentrations of imidazole (100 to 500 mM). Collected fractions of the eluted protein were pooled and dialyzed against PBS. Protein concentration was determined using a BCA protein assay kit (Bio-Rad).
Sf9 insect cells were maintained in Grace’s insect culture medium (Gibco) supplemented with 10% heat-inactivated FBS and 50 µg/ml gentamicin (Calbiochem) at 27°C. To produce recombinant MD-2 protein, Sf9 cells were co-transfected with the linearized wild type baculovirus DNA (Autographa californica nuclear polyhedrosis virus, AcNPV) and an MD-2 transfer vector DNA (pAcHLT-C) by the BD BaculoGold (BD Bioscience). The supernatant was harvested 4 days after transfection and used to infect Sf9 cells to produce high titers of recombinant viruses. Sf9 cells were infected with recombinant viruses and incubated at 27°C. Recombinant MD-2 protein was purified using a Ni-NTA agarose column as described above.
2.2. Cholesterol binding assay
The assay was performed similarly to the assay used to identify NPC1 cholesterol binding as previously described [8]. In brief, 2 µg purified MD-2 was incubated with 1 µM of 3H-cholesterol (Perkin Elmer) in a final volume of 100 µl of buffer A (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.004% NP-40) for 4 hours at room temperature. For competition assays, purified MD-2 was pre-incubated with either unlabeled cholesterol, LPS (Kdo2-lipidA from Avanti Polar Lipids), BSA or OxCE-BSA (see below) for 1 hour at room temperature, before adding 3H-cholesterol. The sample volume was adjusted to 1 ml with buffer A and eluted through a 0.5 ml Ni-NTA agarose column. The column was washed with 5 ml of buffer A containing 50 mM imidazole. MD-2 was eluted with 2 ml of buffer A containing 100 mM and 300 mM imidazole. Collected fractions were combined and 3H counts were measured in an LS6500 liquid scintillation counter (Beckman Coulter). Counts in the combined fractions eluted with 100 mM and 300 mM imidazole were normalized to total 3H counts. Kd was calculated using a total and nonspecific binding algorithm, and Ki using a one-site competitive binding algorithm, within the GraphPad Prism 5.0 software package.
2.3. OxCE-BSA
OxCE was prepared from arachidonic acid cholesteryl ester as described in our earlier work [4]. A hexane solution of OxCE was dried out in a glass tube under argon and reconstituted with endotoxin-free BSA (Gemini Bio-Products) dissolved in distilled water. After a one-hour incubation at 37°C, the reaction was reduced by adding NaBH3CN in PBS and incubation overnight at 37°C. The solution was dialyzed against PBS and sterile filtered using a 0.22 µm filter. The OxCE-BSA prep was free of endotoxin as measured with a LAL assay (Lonza). To measure a number of lysine residues modified by OxCE, we performed a trinitrobenzenesulfonic acid assay as reported [9].
2.4. Mouse atherosclerotic lesions
All animal experiments were approved by the UC San Diego Institutional Animal Care and Use Committee. Ldlr−/− mice were purchased from The Jackson Laboratories and starting from 8 weeks of age, were fed a high fat diet containing 1.25% cholesterol and 21% fat (Harlan Teklad, TD96121) for 12 weeks. Mice were terminated and aortas were isolated, cleaned from periaortic fat, homogenized and lyzed in a lysis buffer (50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 5 mM Na3VO4, 1 mM NaF, and Sigma’s protease inhibitor cocktail).
2.5. MD-2 ELISA
A 96-well plate was coated with a rabbit anti-human MD-2 antibody [10] (5 µg/ml) in PBS and incubated overnight at 4°C. Next day, the plate was washed three times with PBS containing 0.05% Tween-20 and blocked by adding PBS containing 1% BSA for 1 hour at room temperature. Protein lysates of mouse atherosclerotic lesions or human lipoprotein deficient plasma (LPDP) from which lipoproteins were removed by ultracentrifugation, were added to the plate and incubated for 2 hours at room temperature, followed by a biotin-conjugated rabbit anti-human MD-2 antibody [10] for 90 min, alkaline phosphatase-conjugated neutravidin (Pierce) for 1 hour, and LumiPhos 530 (Lumigen) for 90 min. Data are expressed as relative light units (RLU) counted per 100 ms.
2.6. MD-2-associated cholesterol in LPDP and atherosclerotic lesions
Five hundred µl of human LPDP was pre-cleared by incubation with 100 µl of protein A/G beads (GE Healthcare) for 2 hours at 4°C. After centrifugation, LPDP was incubated with 2 µg of either control rabbit IgG or rabbit anti-human MD-2 antibody [10] overnight at 4°C. Next day, protein A/G beads were added and incubated for 2 hours at 4°C. The samples were washed 5 times with PBS containing 0.1% Triton X-100 (PBS-T) and resuspended in 0.5 ml PBS. Cholesterol was extracted with 200 µl of chloroform:isopropanol:NP40 (7:11:0.1). After sonication and centrifugation, the organic phase was collected and dried out under argon. Unesterified cholesterol was measured using a cholesterol detection kit from BioVision. To verify cholesterol measurements, some samples, prior to cholesterol extraction and measurement, were cholesterol-depleted by incubation with 10 mM methyl-beta-cyclodextrin (MβCD) for 30 min at 37°C, washed 4 times with PBS-T and resuspended in 500 µl PBS. The same procedure was used to measure MD-2 associated cholesterol in mouse atherosclerotic lesions.
2.7. Statistical analyses
Graphs represent means ± standard error from 3-4 independent experiments. Results were analyzed using Student's t-test (for differences between 2 groups) or two-way ANOVA with the Bonferroni post hoc test (for curves), and the differences with p<0.05 were considered statistically significant.
3. Results and Discussion
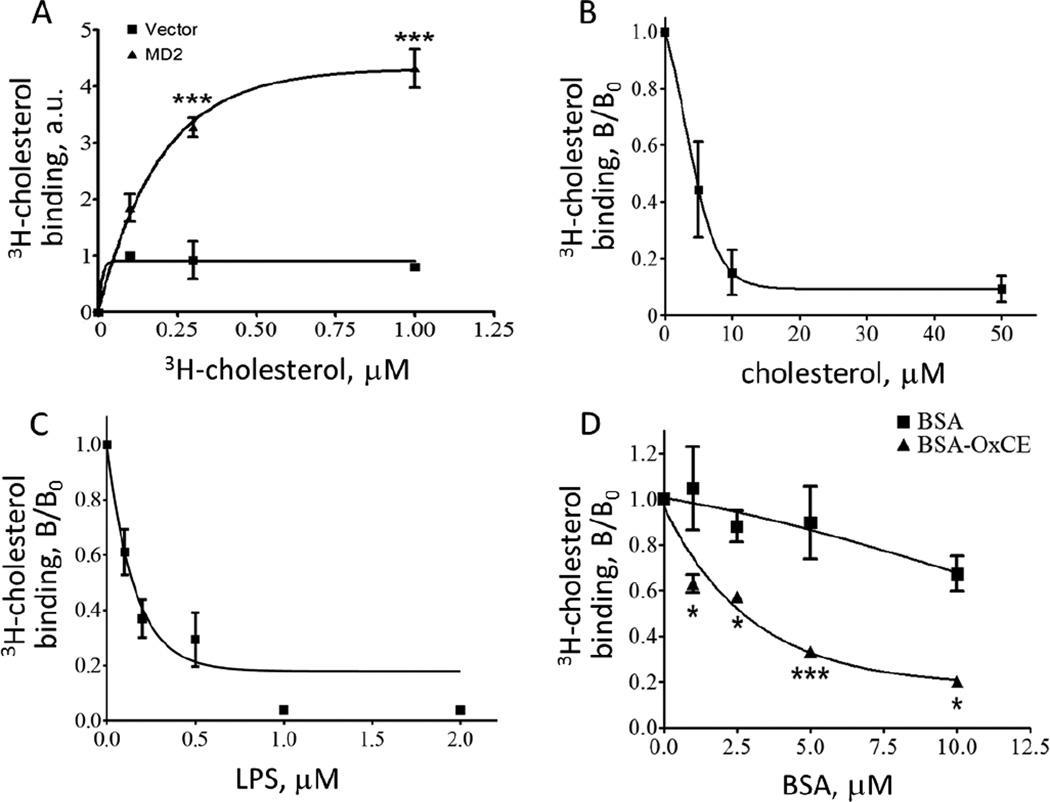
His-tagged MD-2 was expressed in HEK293 cells and purified using a Ni-NTA column. Material from HEK293 cells transfected with an empty vector served as a negative control. Radiolabeled cholesterol bound MD-2 in a concentration-dependent and saturable manner (Fig. 1A). An apparent Kd was calculated as 155 nM (R2=0.93). To confirm the specificity of binding, the 3H-cholesterol binding was competed with non-labeled cholesterol, with an apparent Ki of 399 nM (R2=0.86) (Fig. 1B). Kdo2-lipidA, the active moiety of LPS, which binds MD-2 [11], competed MD-2 3H-cholesterol binding with an apparent Ki of 19 nM (R2=0.84). This value is lower that an earlier reported Kd of 65 nM for MD-2-LPS binding [10], which can be explained by different LPS preparations, Re595 LPS in [10] and E.coli Kdo2-lipid A in our study, and different analytical methods.
Figure 1. Cholesterol binds to MD-2.
A, Recombinant, His-tagged MD-2 was purified from HEK293 cells, and 2 µg MD-2 was incubated with 3H-cholesterol. MD-2 was then isolated on a Ni-NTA agarose column eluted with imidazole, and the amounts of MD-2-associated 3H counts were measured. The material isolated from HEK293 cells transfected with an empty vector carrying His-tag was used as a negative control. Mean±SEM; n=3; ***, p < 0.005. B-D, Binding of 3H-cholesterol (1 µM) to MD-2 (2 µg) was competed with increasing concentrations of unlabeled cholesterol (B), LPS (Kdo2-lipidA, panel C), or OxCE-BSA and BSA (D). The competitors were preincubated with MD-2 for 1 hour at room temperature before adding 3H-cholesterol. Then, the experiment was performed as in panel A. Mean±SEM; n=3-4; *, p < 0.05; ***, p < 0.005.
Oxidation of CE yields many hydroperoxide and bicyclic endoperoxide products in the fatty acyl chain [2, 4], which are stable when reside in the hydrophobic core of lipoproteins, but rapidly react with proteins under protic conditions (e.g. in aqueous environments) to form lipid-protein adducts [12]. The chemical reactivity of OxCE prevented us from measuring its binding to MD-2. For that reason, we used OxCE-modified BSA. OxCE-BSA competed 3H-cholesterol binding to MD-2 (Fig. 1D), with the affinity comparable to that of unesterified cholesterol (Fig. 1B). However, here we did not calculate the Ki because we do not know how many OxCE moieties on OxCE-BSA can bind MD-2 and how protein presentation of immobilized OxCE moieties influences the binding. We measured that 13.3±1.2 lysine residues of BSA were modified in our OxCE-BSA preparations. However, BSA is a 67 kDa and MD-2 is a 19 kDa protein and we do not know how many of the OxCE moieties on BSA are accessible to MD-2 and/or if there are more complex interactions.
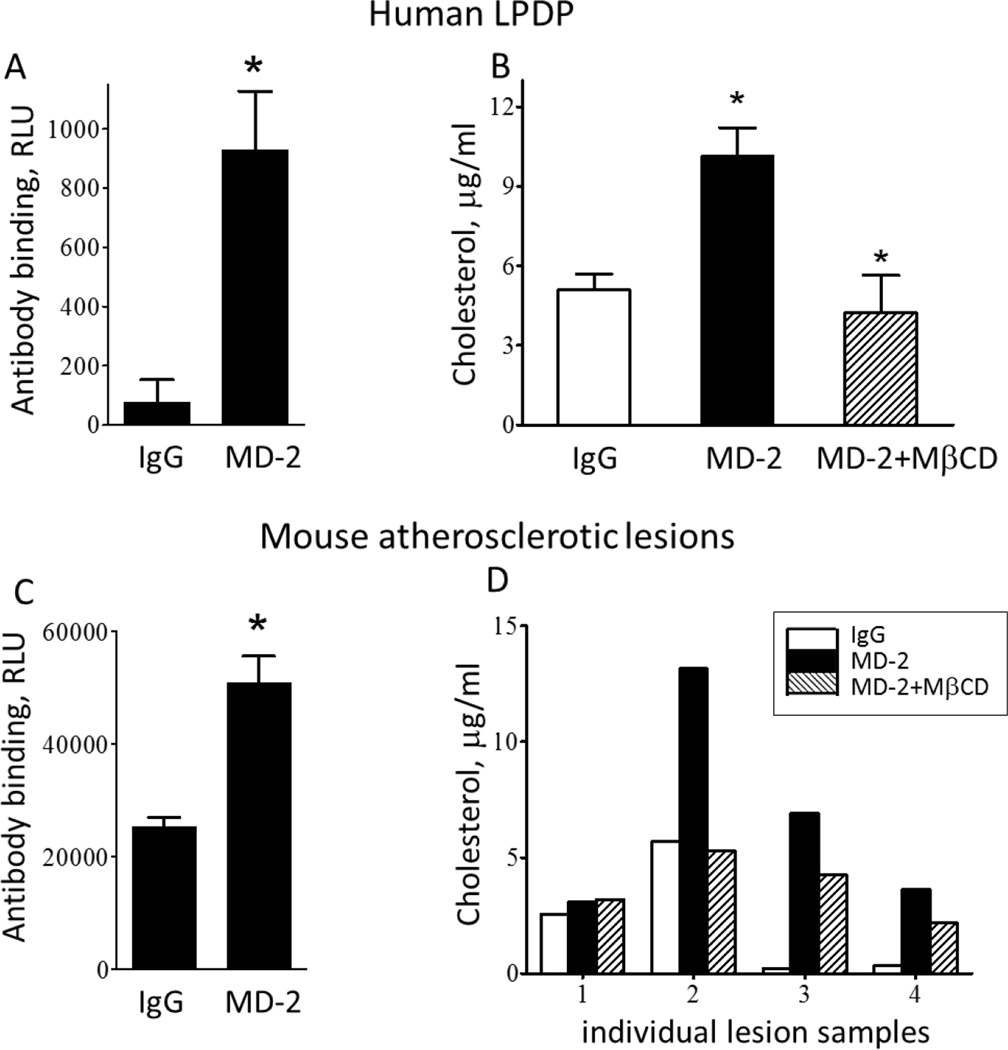
MD-2 exists in cell-surface-expressed and soluble forms, and soluble MD-2 is found in blood plasma. Soluble MD-2 is a co-factor essential for the activation of TLR4-expressing cells [13]. We removed all lipoproteins from human plasma by gradient density ultracentrifugation and confirmed the presence of MD-2 in LPDP using a sandwich ELISA (Fig. 2A). Next, we pulled down MD-2 using an MD-2 specific antibody or an isotype control, non-specific IgG and measured MD-2-associated cholesterol. In a separate set of samples, the MD-2 immunoprecipitate was preincubated with MβCD, a detergent which effectively solubilizes cholesterol. The results shown in Fig. 2B demonstrate that soluble MD-2 in human plasma exists in a cholesterol-bound form. Similarly, we detected MD-2 in lysates of mouse atherosclerotic lesions (Fig. 2C). In this case, the assay measured a total pool of soluble, cell surface-associated and intracellular MD-2. This and different extent of atherosclerosis are likely the reasons for a large variation in the amounts of cholesterol found associated with MD-2 in atherosclerotic lesion lysates. However, 3 out of 4 experiments unequivocally detected MD-2-bound cholesterol in atherosclerotic lesions (Fig. 2D).
Figure 2. Cholesterol is associated with MD-2 in human plasma and in mouse atherosclerotic lesions.
A and C, MD-2 was measured in human lipoprotein-deficient plasma (LPDP, panel A) and in lysates of mouse atherosclerotic lesions (C) using a sandwich ELISA. B and D, Unesterified cholesterol was measured using a standard enzymatic/chromogenic assay in immunoprecipitates of LPDP (B) and atherosclerotic lesions (D), which were pulled down with a non-specific IgG, a specific MD-2 antibody, or the MD-2 antibody followed by a MβCD treatment. Mean±SEM; n=3-4. *, p < 0.05.
The results of this study suggest that MD-2 carries cholesterol, constitutively docked in MD-2's hydrophobic pocket. It is unlikely that this unesterified cholesterol binding to MD-2 activates TLR4. However, under pathologic conditions, bacterial LPS or endogenous DAMPs, such as OxCE or OxCE-protein adducts, replace cholesterol in MD-2's binding pocket and provide additional interfaces that can interact with TLR4 and induce its dimerization and inflammatory signaling.
MD-2 shares a significant structural homology with Niemann-Pick disease type C2 (NPC2), a lysosomal cholesterol-binding protein [14], which suggests that MD-2 may also bind cholesterol, but our study is the first to provide experimental evidence for MD-2 cholesterol binding, including in biologic materials. Structural studies have suggested that MD-2 also binds hydroxylated phospholipids, leading to TLR4 activation [15]. In that work, phosphatidylethanolamine (PE) in extracellular vesicles oxidized by 15-lipoxygenase (15LO) was the most prominent oxidized phospholipid, which induced TLR4/MD-2-mediated inflammatory responses [15]. Remarkably, in our work, 15LO-oxygenated CE were the OxCE species that stimulated TLR4/MD-2 responses in macrophages [2, 4]. In both cases, advanced oxidation products of CE or PE failed to activate TLR4. These findings will be useful for detailed structural studies to identify exact oxidized lipid moieties responsible for MD-2-dependent TLR4 activation.
Acknowledgments
This study was supported by grants HL124174, HL055798 and HL088093 (Y.I.M.) from the National Institutes of Health, and SDG14710028 (S.-H.C.) from the American Heart Association.
Footnotes
Abbreviations: CE, cholesterol esters; CVD, cardiovascular disease; DAMPs, damage-associated molecular patterns; MβCD, methyl-beta-cyclodextrin; MD-2, myeloid differentiation-2; OxCE, oxidized cholesterol esters; TLR4, toll-like receptor-4.
References
- 1.Miller YI, Choi S-H, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized LDL. J. Biol. Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, Pattison J, Deer E, Sayaphupha T, Dennis EA, Witztum JL, Tsimikas S, Miller YI. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high-cholesterol diet: macrophage binding and activation. J. Biol. Chem. 2010;285:32343–32351. doi: 10.1074/jbc.M110.137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi S-H, Yin H, Ravandi A, Armando A, Dumlao D, Kim J, Almazan F, Taylor AM, McNamara CA, Tsimikas S, Dennis EA, Witztum JL, Miller YI. Polyoxygenated cholesterol ester hydroperoxide activates TLR4 and SYK dependent signaling in macrophages. PLoS One. 2013;8:e83145. doi: 10.1371/journal.pone.0083145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravandi A, Leibundgut G, Hung MY, Patel M, Hutchins PM, Murphy RC, Prasad A, Mahmud E, Miller YI, Dennis EA, Witztum JL, Tsimikas S. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J. Am. Coll. Cardiol. 2014;63:1961–1971. doi: 10.1016/j.jacc.2014.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 8.Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 9.Habeeb AF. Chemical evaluation of conformational differences in native and chemically modified proteins. Biochim. Biophys. Acta. 1966;115:440–454. doi: 10.1016/0304-4165(66)90442-9. [DOI] [PubMed] [Google Scholar]
- 10.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J. Biol. Chem. 2001;276:38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 11.Raetz CRH, Garrett TA, Reynolds CM, Shaw WA, Moore JD, Smith DC, Jr, Ribeiro AA, Murphy RC, Ulevitch RJ, Fearns C, Reichart D, Glass CK, Benner C, Subramaniam S, Harkewicz R, Bowers-Gentry RC, Buczynski MW, Cooper JA, Deems RA, Dennis EA. Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J. Lipid Res. 2006;47:1097–1111. doi: 10.1194/jlr.M600027-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Salomon RG. Isolevuglandins, oxidatively truncated phospholipids, and atherosclerosis. Ann.N Y.Acad.Sci. 2005;1043:327–342. doi: 10.1196/annals.1333.040. [DOI] [PubMed] [Google Scholar]
- 13.Tissieres P, Dunn-Siegrist I, Schappi M, Elson G, Comte R, Nobre V, Pugin J. Soluble MD-2 is an acute phase protein and an opsonin for Gram-negative bacteria. Blood. 2007 doi: 10.1182/blood-2007-06-097782. blood-2007. [DOI] [PubMed] [Google Scholar]
- 14.Gruber A, Mancek M, Wagner H, Kirschning CJ, Jerala R. Structural Model of MD-2 and Functional Role of Its Basic Amino Acid Clusters Involved in Cellular Lipopolysaccharide Recognition. J. Biol. Chem. 2004;279:28475–28482. doi: 10.1074/jbc.M400993200. [DOI] [PubMed] [Google Scholar]
- 15.Mancek-Keber M, Frank-Bertoncelj M, Hafner-Bratkovic I, Smole A, Zorko M, Pirher N, Hayer S, Kralj-Iglic V, Rozman B, Ilc N, Horvat S, Jerala R. Toll-like receptor 4 senses oxidative stress mediated by the oxidation of phospholipids in extracellular vesicles. Science signaling. 2015;8:ra60. doi: 10.1126/scisignal.2005860. [DOI] [PubMed] [Google Scholar]