Abstract
Autoinducer-2 (AI-2)-mediated quorum sensing (QS) is utilised for both intra- and inter-species communication by a wide variety of bacteria. An understanding of the mechanism of this communication has the potential to elucidate new targets for antibacterial therapeutics. Herein, we report the synthesis of DPD analogues with modified dynamic equilibria and the evaluation of their behaviour in Gram-negative bacteria. None of the compounds showed modulation of QS in S. Typhimurium, and although no antagonism of V. harveyi was observed, chloro-analogue C5-Cl-DPD showed modest agonism in this marine bacterium. This raises the possibility that access to a cyclic form of DPD may not be required for AI-2-mediated QS in V. harveyi.
Graphical abstract
1. Introduction
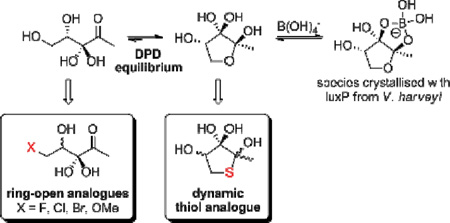
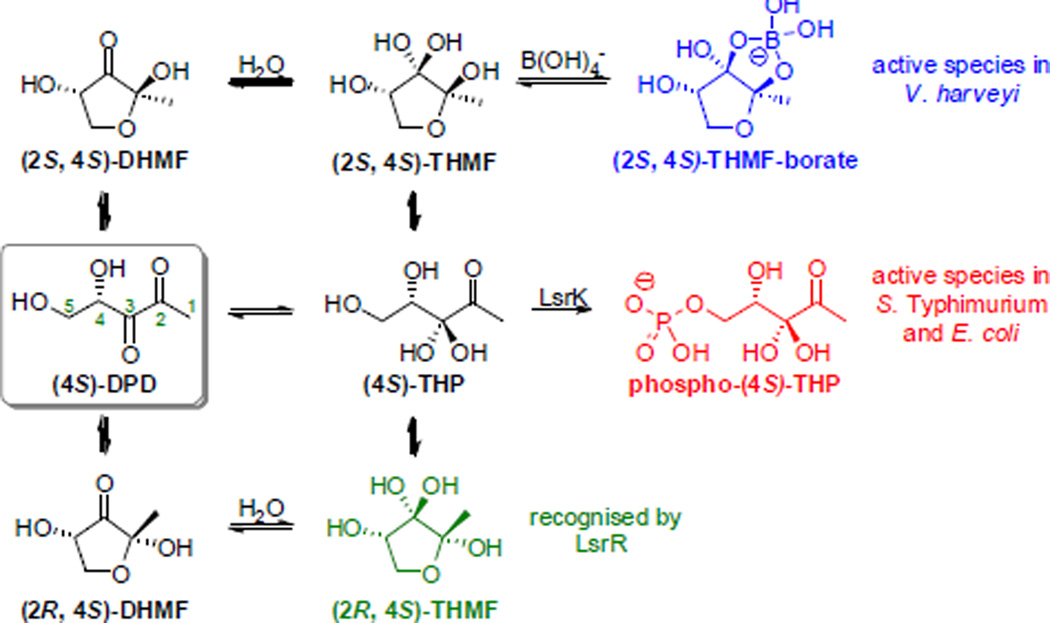
Quorum sensing (QS) is communication between bacteria through recognition of small molecule modulators. QS is responsible for the orchestration of bacterial behaviour in a density-dependent manner, including events that increase bacterial defences such as biofilm formation and virulence factor expression, and thus would be a powerful/important target for future therapeutics. Autoinducer-2 (AI-2) is a prolific modulator, recognised by over 70 species of both Gram-negative and - positive bacteria.1 However, AI-2-mediated QS has proved challenging to study given the propensity of the precursor, (4S)-DPD, to polymerisation at high concentration, and its existence as a complex equilibrium with recognition of different equilibrium species by different bacteria (Figure 1).2 Crystallisation of the AI-2 signal with LuxP, the AI-2 sensor protein in the marine organism Vibrio harveyi, suggests the requirement of a cyclic DPD species coordinating to boron, (2S, 4S)-THMF-borate.3 The corresponding sensor protein in the enteric bacterium Salmonella Typhimurium, LsrB, was also crystallised with a cyclic form of DPD, albeit recognising an alternative stereoisomer that lacked boron coordination, (2R, 4S)-THMF.4
Figure 1.
Structures of DPD analogues.
Although AI-2 is not internalised by V. harveyi, it appears this is not the case for all species. Binding to LsrB is the primary route through which AI-2 can enter S. Typhimurium, but an alternative, currently unknown, pathway is believed to exist.5 Thus the cyclic form may not be crucial for activity in the latter species. Downstream biological recognition of AI-2 in this species includes phosphorylation of the C5 alcohol by kinase LsrK, requiring a ring-opened form of DPD,6 before binding of the resulting phospho-(4S)-THP to transcriptional regulator LsrR.5
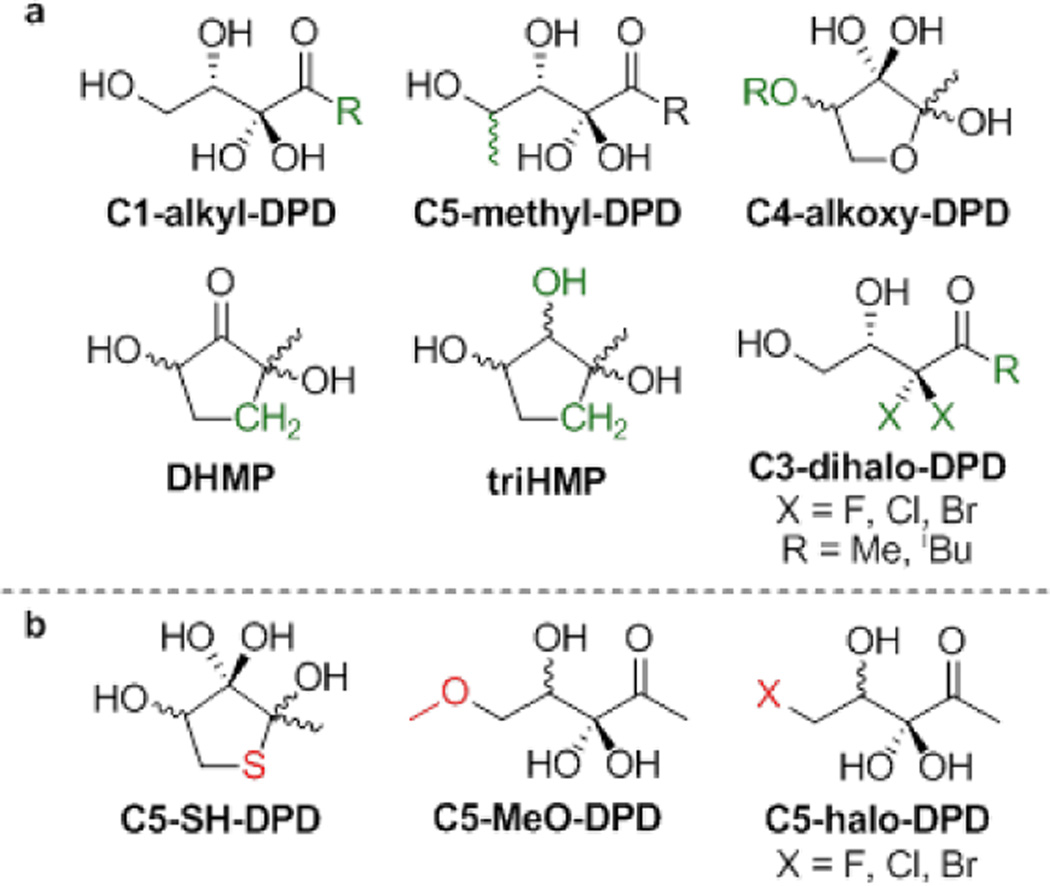
Given the effects of cyclisation on signalling activity have not been fully elucidated, we sought to explore the importance of linear and cyclic forms of DPD to investigate the requirements for the AI-2 structure in these systems. Analysis of the NMR spectra has shown that DPD predominantly exists as a mixture of ring-closed stereoisomers under physiological pH.2 We have previously investigated the use of carbocyclic analogues to mimic fixed ring-closed conformations of DPD (DHMP and triHMP, Figure 2).7 However, the direct analogue DHMP had no significant effect against either V. harveyi or S. Typhimurium, and triHMP showed only limited antagonism in V. harveyi. DFT calculations suggested limited hydration at the C3 ketone of DHMP, a requirement for formation of the borate diester. These compounds also lack the dynamic equilibrium that DPD utilises for multi-species recognition. We were therefore interested in designing new analogues that would exhibit both a predominantly ring-closed form and hydration of the C3 ketone. We identified C5-SH-DPD as a suitable target as DFT calculations suggested that the ring-closed form of this compound should exist mainly as the C3-hydrate (see Table S1, Supplementary material).
Figure 2.
Complex equilibrium of AI-2 including the known biologically recognised species.
a. Existing DPD analogues. b. Targets of this research.
In a parallel, complementary approach we proposed the installation of a C5-halide in order to mimic the (4S)-THP open-chain form of DPD. Fluoride is considered a classical bioisostere for oxygen, being of similar size and able to (weakly) participate as a hydrogen-bond acceptor.8 The larger, less electronegative halides have been shown to form halogen-bonding interactions,9 potentially mimicking the hydrogen-bond donor character of the hydroxyl group. Indeed, this strategy has been utilised in replacement of the hydrated ketone in C3-dihalo-DPD analogues, with both the dichloro and dibromo analogues displaying comparable agonism to DPD itself.10 Although non-fluoro alkyl halides have the potential to undergo nucleophilic displacement and are widely considered to be genotoxic fragments, drugs containing alkyl chloride motifs are generally stable in vivo and are relatively common.11 There are also examples of alkyl bromides being used in medicinal chemistry with apparent stability (cannabinoid receptor agonists O-806 and O-123612), although electrophilic reactivity is anticipated. However, alkyl iodides are scarcely reported in this context due to their instability; in our context, C5-I-DPD would almost certainly act as an alkylating agent or simply hydrolyse to form DPD. We therefore proposed the synthesis of the C5-fluoro, -chloro and -bromo analogues to allow for investigation of both hydrogen-bond donors and acceptors in place of the C5-hydroxy group. We also investigated the biological activity of previously synthesised open-chain analogue C5-MeO-DPD,2 for its ability to act as a hydrogen-bond acceptor.
2. Results and Discussion
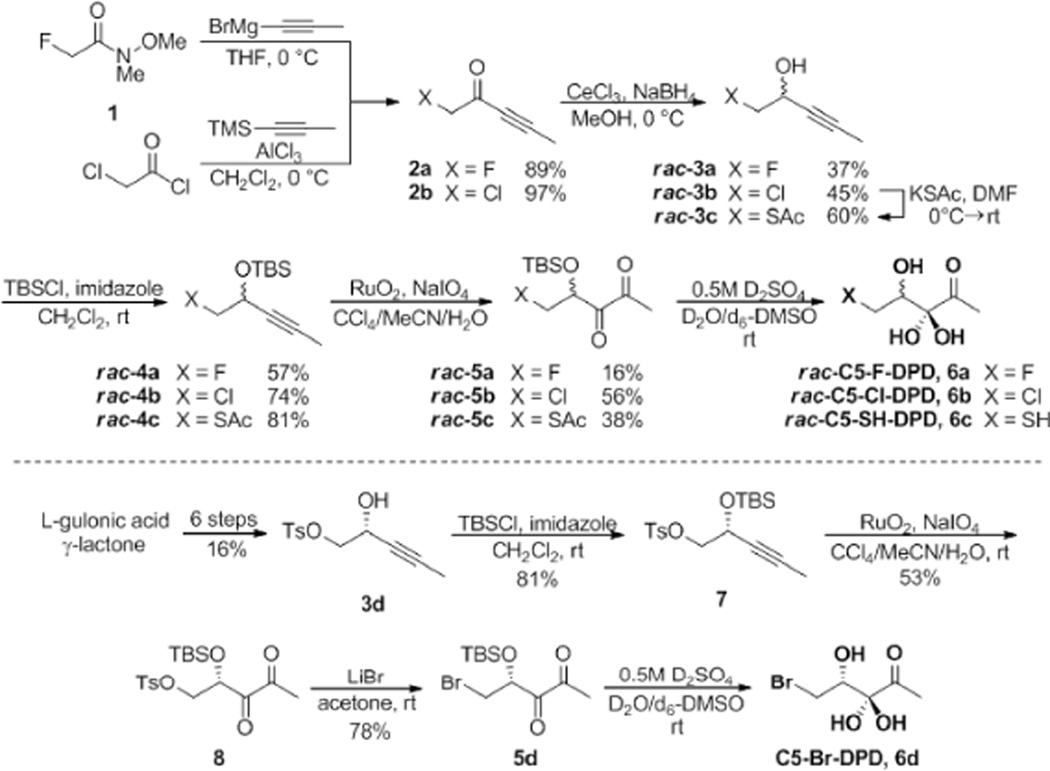
Three of our targets, rac-C5-SH-DPD, rac-C5-Cl-DPD and rac-C5-F-DPD could be readily synthesised in a similar manner to rac-C5-MeO-DPD (Scheme 1).2 Alkynyl Grignard addition to Weinreb amide 113 and alkynylation of chloroacetyl chloride afforded ketones rac-2a and rac-2b in high yield, which could be readily reduced by Luche reduction. Displacement of the chloride in rac-3b allowed the introduction of a thioacetate group; acetate protection was chosen as this was stable to the subsequent reaction conditions but would ultimately allow for facile deprotection either under mild conditions or in situ as was the case for diacetylated-DPD14; in the latter case, the absence of base should render the resulting thiol resistant to oxidation. Subsequent TBS-protection and alkyne oxidation15 proceeded smoothly to give the three DPD precursors 5a–c in low to moderate yields. As with all α-diketone DPD analogues, the final acid-catalysed deprotection was carried out without purification or concentration of the resulting solution; it has previously been shown that the tert-butyldimethylsilanol by-product is non-toxic and inactive up to 100 µM against both V. harveyi and S. Typhimurium.7
Scheme 1.
Synthesis of open- and closed-ring DPD analogues.
The final target C5-Br-DPD was synthesised in enantiopure form via an alternative route. Tosylate 3d could be prepared in 6 steps from L-gulonic acid γ-lactone,15–17 subsequent TBS-protection of the alcohol and oxidation of the alkyne provided diketone 7. The bromide was then installed by displacement of the tosylate in good yield, before acidic deprotection to give C5-Br-DPD.
Deprotection of the resulting C5-DPD analogues was monitored by NMR. As was previously observed, rac-C5-MeO-DPD gave a clean spectrum with one compound, the linear hydrated form. The same was observed for both rac-C5-F-DPD and rac-C5-Cl-DPD; detailed analysis of the spectra of these derivatives showed no evidence of instability. However, C5-Br-DPD was found to slowly hydrolyse to form DPD under the deprotection conditions, and therefore this compound was not further analysed. TBS deprotection of thioacetate analogue rac-5c, initially formed intermediate rac-C5-SAc-DPD, and neutralisation subsequently initiated deprotection of the thiol (to give rac-C5-SH-DPD). As predicted, the spectra of this compound supported the presence of a complex equilibrium, analogous to that seen upon deprotection of DPD.
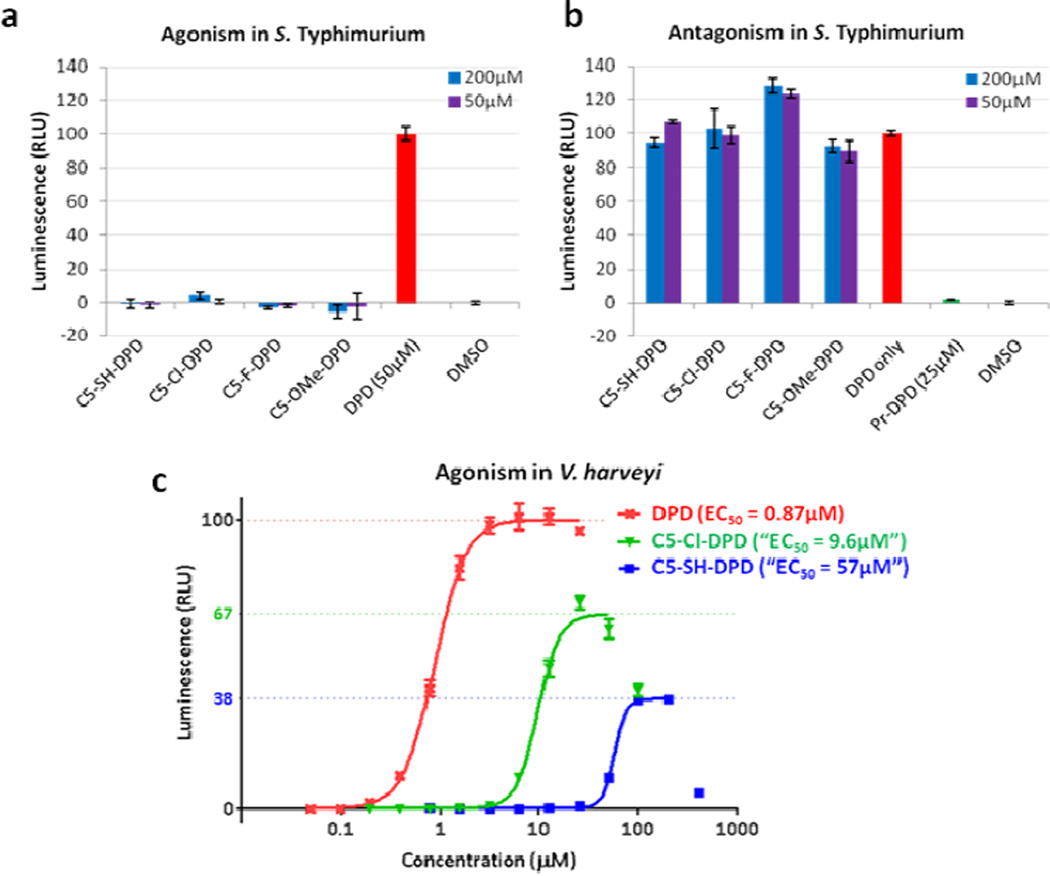
All four stable C5-DPD analogues were then tested for both agonism and antagonism of AI-2-mediated QS in Gram negative bacteria S. Typhimurium and V. harveyi using previously established reporter assays.18,19 Although one might expect that the linear analogues cannot bind LsrB, which is believed to require cyclisation for recognition, another pathway for DPD uptake has been postulated, albeit, much slower than LsrB-modulated transport for AI-2 and analogues.5
S. Typhimurium strain Met844 (ΔluxS), a knockout of the gene encoding DPD synthase luxS, was chosen for these assays. This strain also contains a lacZ-lsr fusion, coupling activation of the AI-2-dependent lsr promoter to the biosynthesis of β-galactosidase, enabling quantification of AI-2-based QS. The strain can be used in the absence of DPD to identify AI-2 QS agonists, and DPD can be added for the detection of antagonists or synergistic agonists, which have previously been reported.20,21 All four compounds were tested at two concentrations (50 and 200 µM) in the presence or absence of DPD (50 µM), using DPD itself as an agonism control and C1-Pr-DPD20 as an antagonism control (Figure 3a–b). No significant agonism or antagonism was observed for any of the tested compounds, which aligned with our expectations for the open-chain analogues. The lack of activity shown by rac-C5-SH-DPD is harder to interpret due to the complexity of the AI-2 pathway in this bacterium. There are multiple steps involved in the processing of DPD; binding to LsrB, phosphorylation by LsrK and subsequent binding to transcriptional regulator LsrR. Further work is required to identify what effect if any, rac-C5-SH-DPD has on the individual stages of AI-2 processing.
Figure 3.
Agonism and antagonism of C5-DPD analogues in V. harveyi and S. Typhimurium.
a. and b. Agonism and antagonism, respectively, of C5-DPD analogues in S. Typhimurium strain Met844 in the absence and presence of DPD (50 µM); Pr-DPD used as a positive antagonism control. Bioluminescence in and β-galactosidase activity in S. Typhimurium were normalised to cell density. c. Agonism of C5-DPD analogues using V. harveyi strain MM32. All data was performed in triplicate, errors represent SEM.
The compounds were subsequently tested against V. harveyi strain MM32 (ΔluxN; ΔluxS) to look for agonism and strain BB170 (ΔluxN) to identify antagonism; the inherent bioluminescence of this bacterium allows facile quantification of AI-2 mediated QS. Both strains lack the gene encoding AHL receptor luxN, excluding the detection of QS modulation through this pathway, and strain MM32 has also had the AI-2 synthase, luxS, removed. No antagonism was observed for any of the species, but rac-C5-Cl-DPD and rac-C5-SH-DPD exhibited moderate and weak agonism, respectively (Figure 3c). Direct comparison with DPD showed a reduced response of ca. 10-fold in the case of rac-C5-Cl-DPD and ca. 100-fold in the case of rac-C5-SH-DPD. It should be noted that (4R)-DPD has negligible activity compared with (4S)-DPD,22 and thus this assay likely underestimates the true potency of the active enantiomer of these racemic analogues. Furthermore, although partial agonism was indicated by the lower comparative maximum bioluminescence, some or all of this decrease resulted from toxicity of the compounds at higher concentrations, as evidenced both by the decrease in bioluminescence and cell density at higher concentrations of all compounds, including DPD. To rule out the possibility of non-AI-2-mediated QS being responsible for the resulting agonism, rac-C5-Cl-DPD was tested against V. harveyi strain BB886 (ΔluxPQ), wherein no agonism was observed at concentrations up to 200 µM, likely owing to the absence of the AI-2 receptor protein.
As discussed previously, activity of DPD is believed to result from (2S,4S)-THMF-borate, requiring cyclisation and borate complexation, as observed in a crystal structure of DPD bound to the receptor protein, LuxP. However, the observed agonism of rac-C5-Cl-DPD, which is locked in the linear form in V. harveyi directly counters this binding requirement.
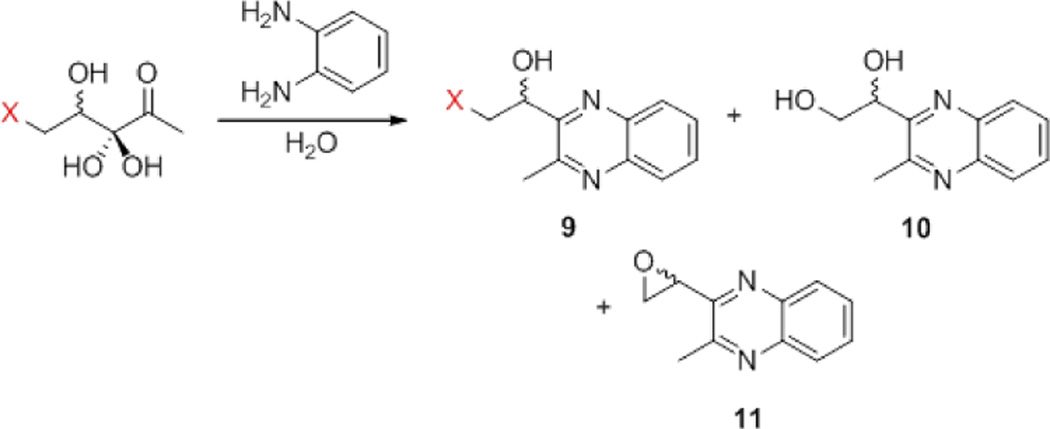
Given the instability of rac-C5-Br-DPD, it was proposed that the chloro analogue may also form small amounts of DPD that could conceivably account for the agonism observed in V. harveyi. Although no DPD was observed by 1H- or 13C-NMR, the characteristic signals of the complex DPD equilibrium are much smaller in magnitude than those of the chloride, which exists as a single, hydrated species; the 20% rac-DPD required to account for the observed agonism may not be detectable via this method. Direct LCMS analysis is not possible on the DPD analogues due to their inherent instability, but derivatisation with 1,2-phenylenediamine allowed for analysis of the resulting quinoxaline products.23,24 Reaction of rac-C5-Cl-DPD did in fact show evidence of DPD derivative 10, suggesting that a small quantity of DPD may have been present, potentially explaining the agonism observed in the V. harveyi assay (Scheme 2). Analysis of the supernatant from an assay of the compound against V. harveyi does not indicate increased amounts of DPD after an 8-hour incubation at 30 °C, ruling out the possibility of slow DPD formation at neutral pH. It is possible that DPD is produced upon reaction of rac-C5-Cl-DPD with 1,2-phenylenediamine, via formation of the corresponding epoxide derivative 11, which was also detected. However, this does not rule out the possibility that both rac-DPD and rac-epoxy-DPD formation occurred during the acidic deprotection step or the subsequent neutralization.
Scheme 2.
Reaction of C5-halo-DPD analogues and DPD with 1,2-phenylenediamine.
Strong evidence for the absence of a DPD impurity present in the sample of rac-C5-Cl-DPD can be inferred from the S. Typhimurium assays, wherein no agonism was observed at concentrations up to 200 µM compared to the DPD positive control at 50 µM. This combination of assay results suggests that AI-2-mediated QS may not be dependent upon access to a cyclic form of DPD, despite analytical challenges preventing a definitive characterisation.
3. Conclusion
A series of DPD analogues were synthesised and evaluated for their ability to modulate QS in two gram-negative bacteria. Neither dynamic DPD analogue rac-C5-SH-DPD nor the ring opened analogues showed any activity in S. Typhimurium, but linear chloride, rac-C5-Cl-DPD, was found to be a moderate agonist of AI-2 mediated QS in V. harveyi in spite of the presumed requirement for a cyclic DPD signal. As such, the activity of this molecule may hinge on the presence of an unidentified alternative mechanism or binding mode to the LuxP receptor protein, and ultimately may suggest a broader lexicon for V. harveyi communication than currently understood.
4. Experimental section
Reactions were performed under an inert atmosphere at rt using flame-dried glassware with dry solvents. Reagents were used as commercially supplied, unless otherwise specified. TLC was performed on glass-backed plates pre-coated with silica (EMD 60 F254, 0.25–1 mm) and developed using standard visualising agents: UV fluorescence (254 nm) and KMnO4, cerium ammonium nitrate or ninhydrin with appropriate heating. 1H and 13C-NMR were performed on Bruker spectrometers, with the reference from the residual solvent peak for 1H-NMR (7.26 ppm for CDCl3, 3.31 ppm for CD3OD), and the solvent peak for 13C-NMR (77.1 ppm for CDCl3, 49.0 ppm for CD3OD), coupling constants (J values) are given in Hz. HRMS (ESI) were performed on an Agilent 1100 Series LC/MSD-TOF.
4.1. General Method for Luche Reduction
CeCl3.7H2O (1.1 eq) was added to a solution of crude ketone (1.0 eq) in MeOH (ca. 70 mM) at 0 °C. After 5 min, NaBH4 (1.2 eq) was added portionwise over 5 min, before the solution was stirred at 0 °C (1 h). The reaction was quenched with sat. aq. NH4Cl, the solution extracted with CH2Cl2 (× 3), the combined organic layers dried (MgSO4), filtered and the solvent removed in vacuo. Purification by column chromatography eluting with EtOAc/hexane (gradient, 0:1 → 1:2) gave the title compound.
4.2. General Method for TBS Protection
TBSCl (2.5 eq) was added to a solution of alcohol (1.0 eq) and imidazole (5.0 eq) in CH2Cl2 (ca. 200 mM) and the solution stirred at rt (17 h). Sat. aq. NH4Cl and CH2Cl2 were added, the aqueous layer was extracted with CH2Cl2 (× 2), the combined organic layers were dried (MgSO4), filtered and the solvent removed in vacuo. Purification by column chromatography eluting with EtOAc/hexane (gradient, 0:1 → 1:19) gave the title compound.
4.3. General Method for Alkyne Oxidation
A solution of NaIO4 (2.3 eq) in H2O (ca. 700 mM) was added to a solution of alkyne (1.0 eq) in CCl4/MeCN (1:1, ca. 225 mM) and stirred vigorously at rt under air for 3 min before the addition of RuO2.xH2O (0.02 eq) and the solution stirred vigorously (15 min) before being filtered through a silica pad, washing with CH2Cl2. Purification by column chromatography eluting with EtOAc/hexane (gradient, 0:1 → 1:9) gave the title compound.
4.4. General Method for TBS-Deprotection
D2SO4 (0.82 µL) was added to a solution of silyl ether (10 µmol) in 4:1 D2O/d6-DMSO (500 µL) and the solution incubated at rt (24 h). Upon NMR confirmation of complete deprotection, the sample was carefully neutralised to pH 7 from ca. pH 1 by the addition of NaOD.
4.5. 1-Fluoropent-3-yn-2-one (2a)
1-propynylmagnesium bromide (52 mL, 0.5M in THF, 26.0 mmol) was added to a solution of Weinreb amide 1 (2.10 g, 17.3 mmol) in THF (165 mL) at 0 °C and the solution stirred at 0 °C (4.5 h). The reaction was quenched with sat. aq. NH4Cl (120 mL) and H2O (30 mL) was added. The organic layer was separated and the aqueous layer extracted with Et2O (2 × 100 mL), the combined organic layers were dried (MgSO4), filtered and the solvent removed in vacuo to give the ketone 2a as a yellow oil, which was used without further purification (1.54 g, 89%), δH (500 MHz, CDCl3) 4.82 (2H, d, J 47.2, CH2), 2.02 (3H, d, J 1.2, CH3); δC (126 MHz, CDCl3) 181.5 (d, J 22.0), 95.9 (d, J 2.2), 84.83 (d, J 188.1), 76.58, 4.19; δF (377 MHz, CDCl3) −225.0. Found: 101.0402. C5H6FO requires 101.0397.
4.6. 1-Chloropent-3-yn-2-one, (2b)
A solution of chloroacetyl chloride (478 mL, 60 mmol) and 1-(trimethylsilyl)propyne (8.88 mL, 60 mmol) in CH2Cl2 (30 mL) was cannulated into a suspension of AlCl3 (8.80 g, 66 mmol) in CH2Cl2 (120 mL) at 0 °C over ca. 30 min. The solution was stirred at 0 °C (1 h) and rt (1 h) before being poured into ice/conc. HCl (200 mL). The aqueous layer was extracted with CH2Cl2 (2 × 100 mL), the combined organic layers were washed with brine (2 × 150 mL), dried (MgSO4), filtered and the solvent removed in vacuo to give ketone 2b as a brown liquid, which was used without further purification (6.8 g, 97%), δH (400 MHz, CDCl3) 4.17 (2H, s, CH2), 2.05 (3H, s, CH3); δC (101 MHz, CDCl3) 178.7, 95.1, 77.7, 59.7, 4.3.
4.7. 1-Fluoropent-3-yn-2-ol (rac-3a)
Synthesised using the general method for Luche reduction, giving alcohol rac-3a as a colourless oil (321 mg, 37%), Rf 0.22 (1:19 EtOAc/hexane); δH (500 MHz, CDCl3) 4.62-4.52 (1H, br m, CHOH), 4.42 (1H, ddd, J 46.9, 9.3, 3.3, CHHF), 4.33 (1H, ddd, J 47.8, 9.3, 7.2, CHHF), 2.89 (1H, br s, OH), 1.82 (3H, d, J 2.4, ≡-CH3); δC (101 MHz, CDCl3) 85.8 (d, J 175.5), 83.4 (d, J 1.8), 75.1 (d, J 12.6), 61.8 (d, J 23.9), 3.55.
4.8. 1-Chloropent-3-yn-2-ol (rac-3b)
Synthesised using the general method for Luche reduction, giving alcohol rac-3b as a yellow oil (2.50 g, 66% over 2 steps), Rf 0.33 (1:4 EtOAc/hexane); δH (500 MHz, CDCl3) 4.57-4.53 (1H, m, CHOH), 3.69 (1H, dd, J 11.1, 3.9, CHHCl), 3.61 (1H, dd, J 11.1, 6.8, CHHCl), 2.27 (1H, br s, OH), 1.87 (3H, d, J 2.0, CH3); δC (101 MHz, CDCl3) 83.2, 76.6, 62.8, 49.6, 3.7.
4.9. S-(2-Hydroxypent-3-yn-1-yl) ethanethioate (rac-3c)
A solution of KSAc (21 mg, 0.186 mmol) in DMF (1 mL) was added dropwise to a solution of chloride rac-3b (20 mg, 0.169 mmol) in DMF (1 mL) at 0 °C, and the solution allowed to warm to rt and stirred (72 h). EtOAc (20 mL) and H2O (20 mL) were added and the aqueous layer extracted with EtOAc (2 × 20 mL), washed combined organic layers with brine (2 × 30 mL), dried (MgSO4), filtered and the solvent removed in vacuo. Purification by column chromatography eluting with EtOAc/hexane (gradient, 0:1 → 1:2) gave alcohol rac-3c as a pale yellow oil (16 mg, 60%), Rf 0.40 (1:2 EtOAc/hexane); δH (400 MHz, CDCl3) 4.49-4.43 (1H, m, CHOH), 3.24 (1H, dd, J 13.8, 4.9, CHHS), 3.14 (1H, dd, J 13.8, 7.0 Hz, CHHS), 2.37 (3H, s, COCH3), 1.84 (3H, d, 2.1, ≡-CH3); δC (101 MHz, CDCl3) 196.0, 82.4, 78.5, 61.9, 36.9, 30.7, 3.7. Found: MH+, 159.0471. C7H11O2S requires 159.0474.
4.10. tert-Butyl((1-fluoropent-3-yn-2-yl)oxy)dimethylsilane (rac-4a)
Synthesised using the general method for TBS protection, giving silyl ether rac-4a as a pale yellow oil (768 mg, 77%), Rf 0.60 (1:19 EtOAc/hexane); δH (500 MHz, CDCl3) 4.61-4.54 (1H, m, CHOSi), 4.33 (1H, ddd, J 46.8, 9.1, 3.8, CHHF), 4.28 (1H, ddd, J 47.9, 9.1, 7.5, CHHF), 1.82 (3H, d, J 2.1, ≡-CH3), 0.91 (9H, s, C(CH3)3), 0.13 (3H, s, SiCH3), 0.12 (3H, s, SiCH3); δC (126 MHz, CDCl3) 86.1 (d, J 178.3), 82.4 (d, J 2.4), 76.2 (d, J 13.1), 62.7 (d, J 24.9), 28.9, 18.4, 3.60, −4.6, −4.9; δF (377 MHz, CDCl3) −219.6.
4.11. tert-Butyl((1-chloropent-3-yn-2-yl)oxy)dimethylsilane (rac-4b)
Synthesised using the general method for TBS protection, giving silyl ether rac-4b as a pale yellow oil (73 mg, 74%), Rf 0.67 (1:19 EtOAc/hexane); δH (500 MHz, CDCl3) 4.48-4.44 (1H, m, CHOH), 3.55 (1H, ddd, J 10.8, 5.0, 0.6, CHHCl), 3.51 (1H, ddd, J 10.8, 7.3, 0.7, CHHCl), 1.83 (3H, dd, J 2.0, 0.7, ≡-CH3), 0.91 (9H, d, J = 0.8 Hz, C(CH3)3), 0.14 (3H, s, SiCH3), 0.13 (3H, s, SiCH3); δC (126 MHz, CDCl3) 82.1, 77.9, 64.1, 49.1, 25.9, 18.4, 3.6, −4.5, −4.9.
4.12. S-(2-((tert-Butyldimethylsilyl)oxy)pent-3-yn-1-yl) ethanethioate (rac-4c)
Synthesised using the general method for TBS protection, giving silyl ether rac-4c as a pale yellow oil (84 mg, 81%), Rf 0.43 (1:9 EtOAc/hexane); δH (400 MHz, CDCl3) 4.39-4.34 (1H, m, CHOSi) 3.15 (1H, dd, 13.4, 5.9 Hz, CHHS), 3.09 (1H, dd, J 13.4, 7.0, CHHS), 2.32 (3H, s, COCH3), 1.81 (3H, d, J 2.1, ≡-CH3), 0.89 (9H, s, C(CH3)3), 0.11 (3H, s, SiCH3), 0.09 (3H, s, SiCH3); δC (101 MHz, CDCl3) 195.4, 81.2, 79.3, 62.4, 37.5, 30.6, 25.9, 18.4, 3.6, −4.6, −4.9. Found: MH+, 273.1345. C13H25O2SSi requires 273.1339.
4.13. 4-((tert-Butyldimethylsilyl)oxy)-5-fluoropentane-2,3-dione (rac-5a)
Synthesised using the general method for alkyne oxidation, giving diketone rac-5a as a yellow oil (162 mg, 40%), Rf 0.20 (1:19 EtOAc/hexane); δH (400 MHz, CDCl3) 5.02 (1H, ddd, J 22.9, 5.1, 3.5, CHOSi), 4.67 (1H, ddd, J 47.2, 9.8, 5.1, CHHF), 4.56 (1H, ddd, J 46.9, 9.8, 3.5, CHHF), 2.36 (3H, s, COCH3), 0.89 (9H, s, C(CH3)3), 0.10 (3H, s, SiCH3), 0.09 (3H, s, SiCH3); δC (151 MHz, CDCl3) 199.3, 196.7 (d, J 6.9), 84.3 (d, J 174.4), 73.5 (d, J 20.9), 25.7, 24.9, 18.3, −4.9, −5.0; δF (377 MHz, CDCl3) −228.9. Also recovered alkyne rac-4a as a colourless oil (126 mg, 36%).
4.14. 4-((tert-Butyldimethylsilyl)oxy)-5-chloropentane-2,3-dione (rac-5b)
Synthesised using the general method for alkyne oxidation, giving diketone rac-5b as a yellow oil (364 mg, 56%), δH (400 MHz, CDCl3) 5.06 (1H, t, J 5.0, CHOSi), 3.84-3.79 (1H, m, CHHCl), 3.79-3.74 (1H, m, CHHCl), 2.41 (3H, s, COCH3), 0.92 (9H, s, C(CH3)3), 0.13 (3H, s, SiCH3), 0.12 (3H, s, SiCH3); δC (126 MHz, CDCl3) 199.1, 196.1, 73.2, 45.0, 25.7, 24.9, 18.3, −4.8, −4.9.
4.15. S-(2-((tert-Butyldimethylsilyl)oxy)-3,4-dioxopentyl) ethanethioate (rac-5c)
Synthesised using the general method for alkyne oxidation, giving diketone rac-5c as a yellow oil (33 mg, 38%), Rf 0.50 (1:9 EtOAc/hexane); δH (400 MHz, CDCl3) 5.10 (1H, dd, J 6.8, 3.9, CHOSi), 3.38 (1H, dd, J 13.9, 3.9, CHHS), 3.08 (1H, dd, J 13.9, 6.8, CHHS), 2.35 (3H, s, SCOCH3), 2.29 (3H, s, OCOCH3), 0.87 (9H, s, C(CH3)3), 0.09 (3H, s, SiCH3), 0.06 (3H, s, SiCH3); δC (126 MHz, CDCl3) 198.6, 195.9, 195.4, 71.1, 71.0, 32.7, 30.5, 25.8, 24.6, 18.3, −4.9. Also recovered alkyne rac-4c as a colourless oil (18 mg, 21%).
4.16. 5-Fluoro-4-hydroxypentane-2,3-dione (rac-C5-F-DPD, rac-6a)
Synthesised using the general method for in situ TBS-deprotection, giving a colourless solution of rac-C5-F-DPD, rac-6a, δH (400 MHz, 4:1 D2O/d6-DMSO) 4.64 (1H, ddd, J 46.2, 10.2, 3.3, CHOH), 4.48 (1H, ddd, J 47.9, 10.1, 6.6, CHHF), 4.09 (1H, ddd, J 19.8, 6.6, 3.3, CHHF), 2.30 (3H, s, COCH3), 0.85 (4.5H, s, C(CH3)3), 0.03 (3H, s, SiCH3); δC (151 MHz, 4:1 D2O/d6-DMSO) 210.8, 97.4 (d, J 7.5), 84.9 (d, J 164.8), 73.7 (d, J 18.6), 26.6 (TBS-OH), 25.8 (TBS-OH), 18.8, −3.0 (TBS-OH); δF (377 MHz, 4:1 D2O/d6-DMSO) −232.1.
4.17. 5-Chloro-4-hydroxypentane-2,3-dione (rac-C5-Cl-DPD, rac-6b)
Synthesised using the general method for in situ TBS-deprotection, giving a colourless solution of rac-C5-Cl-DPD, rac-6b, δH (600 MHz, 4:1 D2O/d6-DMSO) 4.11 (1H, dd, J 9.4, 2.5, CHOH), 3.92 (1H, dd, J 11.8, 2.5, CHHCl), 3.61 (1H, dd, J 11.8, 9.4, CHHCl), 2.39 (3H, s, COCH3), 0.93 (9H, s, C(CH3)3), 0.12 (6H, s, 2 × SiCH3); δC (151 MHz, 4:1 D2O/d6-DMSO) 210.7, 97.8, 75.6, 46.0, 26.6 (TBS-OH), 25.7, 18.8 (TBS-OH), -3.0 (TBS-OH).
4.18. 4-Hydroxy-5-mercaptopentane-2,3-dione (rac-C5-SAc-DPD, rac-6c precursor)
Synthesised using the general method for in situ TBS-deprotection, giving a colourless solution of rac-C5-SAc-DPD, rac-6c precursor, δH (400 MHz, 4:1 D2O/d6-DMSO) 3.88 (1H, dd, J 10.0, 2.6, CHOH), 3.30 (1H, dd, J 14.1, 2.6, CHHS), 2.86 (1H, dd, J 14.1, 10.0, CHHS), 2.36 (3H, s, COCH3), 2.31 (3H, s, COCH3), 0.87 (9H, s, C(CH3)3), 0.05 (6H, s, 2 × SiCH3); δH (151 MHz, 4:1 D2O/d6-DMSO) 211.2, 201.6, 98.1, 73.7, 31.6, 31.5, 26.6 (TBS-OH), 25.7, 18.8 (TBS-OH), −3.0 (TBS-OH).
4.19. (R)-2-((tert-Butyldimethylsilyl)oxy)pent-3-yn-1-yl 4-methylbenzenesulfonate (7)
Synthesised using the general method for TBS protection, giving silyl ether 7 as a colourless oil (209 mg, 81%), Rf 0.34 (1:9 EtOAc/hexane); [α]D25 −46.1 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 7.79 (2H, d, J 7.8, Ar-H), 7.33 (2H, d, J 7.8, Ar-H), 4.55-4.49 (1H, m, CHOSi), 4.01 (1H, dd, J 9.6, 3.6, CHHOS), 3.91 (1H, dd, J 9.8, 7.9, CHHOS), 2.44 (3H, s, CH3Ar), 1.77 (3H, s, ≡-CH3), 0.85 (9H, s, C(CH3)2), 0.09 (3H, s, SiCH3), 0.06 (3H, s, SiCH3); δC (101 MHz, CDCl3) 144.9, 135.1, 129.9, 128.1, 82.7, 76.3, 73.0, 61.6, 25.8, 21.8, 18.3, 3.6, −4.7, −4.9. Found: MH+, 369.1551. C18H29O4SSi requires 359.1556.
4.20. (S)-2-((tert-Butyldimethylsilyl)oxy)-3,4-dioxopentyl 4-methylbenzenesulfonate (8)
Synthesised using the general method for alkyne oxidation, giving diketone 5d as a yellow oil (232 mg, 53%), Rf 0.54 (1:2 EtOAc/hexane); δH (500 MHz, CDCl3) 7.76 (2H, d, J 8.3, Ar-H), 7.35 (2H, d, J 7.9, Ar-H), 5.00-4.98 (1H, m, CHOSi), 4.27 (1H, ddd, J 10.4, 5.2, 0.8 Hz, CHHOS), 4.22 (1H, ddd, J 10.5, 4.1, 0.8, CHHOS), 2.45 (3H, s, CH3Ar), 2.33 (3H, s, COCH3), 0.83 (9H, s, C(CH3)3), 0.05 (3H, s, SiCH3), 0.04 (3H, s, SiCH3); δC (126 MHz, CDCl3) 198.8, 195.4, 145.3, 132.6, 130.1, 128.2, 71.7, 70.2, 25.7, 24.8, 21.8, 18.3, −4.9. Found: MH+, 401.1454. C18H29O6SSi requires 401.1449. Also recovered alkyne 8 as a colourless oil (71 mg, 18%).
4.21. (R)-5-Bromo-4-((tert-butyldimethylsilyl)oxy)pentane-2,3-dione (5d)
LiBr (6.5 mg, 0.050 mmol) was added to a solution of tosylate 8 (20 mg, 0.050 mmol) in acetone (1.5 mL) and stirred at rt (24 h), whereupon the solution was filtered and washed through with acetone before the solvent was removed in vacuo. Purification by column chromatography eluting with EtOAc/hexane (gradient, 0:1 → 1:24) gave bromide 5d as a yellow oil (12 mg, 78%), Rf 0.24 (1:19 EtOAc/hexane); [α]D25 +22.3 (c 0.8, CHCl3); δH (500 MHz, CDCl3) 5.09 (1H, t, J 5.0, CHOSi), 3.63 (1H, dd, J 10.5, 4.7, CHHBr), 3.58 (1H, dd, J 10.5, 5.3, CHHBr), 2.39 (3H, s, COCH3), 0.90 (9H, s, C(CH3)3), 0.11 (3H, s, SiCH3), 0.09 (1H, s, SiCH3); δC (101 MHz, CDCl3) 199.0, 195.9, 72.6, 32.6, 25.7, 24.9, 18.3, −4.8, −4.8.
4.22. 5-Bromo-4-hydroxypentane-2,3-dione (C5-Br-DPD, 6d)
Synthesised using the general method for in situ TBS-deprotection, giving a colourless solution of C5-Br-DPD, 6d, δH (600 MHz, 4:1 D2O/d6-DMSO) 4.10 (1H. d, J 9.9, CHOH), 3.74 (1H, d, J 11.0, CHHBr), 3.42-3.37 (1H, m, CHHBr), 2.33 (3H, s, CH3); δC (151 MHz, 4:1 D2O/d6-DMSO) 210.7, 97.9, 75.6, 34.5, 26.6, 25.6, 18.8, −3.0.
4.23. Modulation of bioluminescence in V. harveyi
Agonistic and antagonistic activity was evaluated in V. harveyi using the procedure of Taga.25 Briefly, V. harveyi strain BB170 (ATCC BAA-1117), MM32 (ATCC BAA-1121) or BB886 (ATCC BAA-1118) was grown in AB medium (14 h, 30 °C). The cells were diluted in fresh AB medium (1/2,500) and added (100 µL/well) to the serially diluted test compounds (previously adjusted to pH 7 using NaOD) in AB medium (100 µL/well) at a final concentration of 0.5% DMSO. The plate was incubated (30 °C, ca. 8 h), whereupon the bioluminescence (OD490) and cell density (OD600) were measured. EC50 values were calculated using GraphPad Prism 5 from a plot of the bioluminescence (normalised to cell density) versus log(concentration) as the concentration corresponding to 50% maximum bioluminescence.
4.24. Modulation of bioluminescence in S. Typhimurium
Agonistic and antagonistic activity was evaluated in S. Typhumurium using the procedure of Wolf.26 Briefly, S. Typhumurium strain Met8445 was grown in LB medium (16 h, 37 °C). The cells were diluted in fresh LB medium (1/25) and added (50 µL/well) to the serially diluted test compounds (previously adjusted to pH 7 using NaOD) with or without DPD in LB medium (150 µL/well) at a final concentration of 0.5% DMSO in deep-well polypropylene plates. The plates were incubated (37 °C, ca. 4 h), whereupon an aliquot (50 µL) was diluted ¼ and the cell density (OD600) measured. A second aliquot (50 µL) was added to Z buffer (500 µL, containing 0.35% BME), whereupon CHCl3 (20 µL) was added and the solution thoroughly mixed. ONPG (20 uL, 4 mg/mL in Z buffer) was added to an aliquot of the aqueous top layer (100 µL)and the resulting luminescence (OD420) was recorded every 1 min for 20 min. β-Galactosidase activity was calculated according to the following equation: “activity = Vmax/OD600”, where “Vmax = maximum slope of kinetic display of OD420/min”.
Supplementary Material
Acknowledgments
We gratefully acknowledge Prof. Bonnie Bassler (Princeton University) for providing us with S. Typhimurium strain Met844. This work was funded by the National Institute of Allergy and Infectious Diseases grant AI077644. This is manuscript #29163 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Additional tables and figures containing DFT calculations, numerical antagonism data and 1H- and 13C-NMR spectra.
References and notes
- 1.Waters CM, Bassler BL. Annu. Rev. Cell. Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 2.Globisch D, Lowery CA, McCague KC, Janda KD. Angew. Chem. Int. Ed. 2012;51:4204–4208. doi: 10.1002/anie.201109149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 4.Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Mol. Cell. 2004;15:677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Taga ME, Miller ST, Bassler BL. Mol. Microbiol. 2003;50:1411–1427. doi: 10.1046/j.1365-2958.2003.03781.x. [DOI] [PubMed] [Google Scholar]
- 6.Xavier KB, Miller ST, Lu W, Kim JH, Rabinowitz J, Pelczer In, Semmelhack MF, Bassler BL. ACS Chem. Biol. 2007;2:128–136. doi: 10.1021/cb600444h. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchikama K, Lowery CA, Janda KD. J. Org. Chem. 2011;76:6981–6989. doi: 10.1021/jo200882k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smart BE. J.Fluorine Chem. 2001;109:3–11. [Google Scholar]
- 9.Sirimulla S, Bailey JB, Vegesna R, Narayan M. J. Chem. Inf. Model. 2013;53:2781–2791. doi: 10.1021/ci400257k. [DOI] [PubMed] [Google Scholar]
- 10.Guo M, Zheng Y, Terell JL, Ad M, Opoku-Temeng C, Bentley WE, Sintim HO. Chem. Commun. 2015;51:2617–2620. doi: 10.1039/c4cc09361e. [DOI] [PubMed] [Google Scholar]
- 11.Chung W-J, Vanderwal CD. Acc. Chem. Res. 2014;47:718–728. doi: 10.1021/ar400246w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin G, Wray EJ, Rorrer WK, Crocker PJ, Ryan WJ, Saha B, Razdan RK, Martin BR, Abood ME. Br. J. Pharmacol. 1999;126:1575–1584. doi: 10.1038/sj.bjp.0702469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witte MD, Horst D, Wiertz EJHJ, van der Marel GA, Overkleeft HS. J. Org. Chem. 2008;74:605–616. doi: 10.1021/jo801906s. [DOI] [PubMed] [Google Scholar]
- 14.Frezza M, Soulère L, Balestrino D, Gohar M, Deshayes C, Queneau Y, Forestier C, Doutheau A. Bioorg. Med. Chem. Lett. 2007;17:1428–1431. doi: 10.1016/j.bmcl.2006.11.076. [DOI] [PubMed] [Google Scholar]
- 15.Semmelhack MF, Campagna SR, Federle MJ, Bassler BL. Org. Lett. 2005;7:569–572. doi: 10.1021/ol047695j. [DOI] [PubMed] [Google Scholar]
- 16.Meijler MM, Hom LG, Kaufmann GF, McKenzie KM, Sun C, Moss JA, Matsushita M, Janda KD. Angew. Chem. Int. Ed. 2004;43:2106–2108. doi: 10.1002/anie.200353150. [DOI] [PubMed] [Google Scholar]
- 17.Witulski B, Zimmermann A, Gowans ND. Chem. Commun. 2002:2984–2985. doi: 10.1039/b209573d. [DOI] [PubMed] [Google Scholar]
- 18.Griffith KL, Wolf RE., Jr Biochem. Biophys. Res. Commun. 2002;290:397–402. doi: 10.1006/bbrc.2001.6152. [DOI] [PubMed] [Google Scholar]
- 19.Taga ME, Xavier KB. Curr. Protoc. Microbiol. John Wiley & Sons, Inc.; 2005. [Google Scholar]
- 20.Lowery CA, Park J, Kaufmann GF, Janda KD. J. Am. Chem. Soc. 2008;130:9200–9201. doi: 10.1021/ja802353j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rui F, Marques JC, Miller ST, Maycock CD, Xavier KB, Ventura MR. Bioorganic & Medicinal Chemistry. 2012;20:249–256. doi: 10.1016/j.bmc.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Lowery CA, McKenzie KM, Qi L, Meijler MM, Janda KD. Bioorg. Med. Chem. Lett. 2005;15:2395–2398. doi: 10.1016/j.bmcl.2005.02.069. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Hu X, Dizin E, Pei D. J. Am. Chem. Soc. 2003;125:13379–13381. doi: 10.1021/ja0369663. [DOI] [PubMed] [Google Scholar]
- 24.Nedvidek W, Ledl F, Fischer P. Z. Lebensm. Unters. Forsch. 1992;194:222–228. [Google Scholar]
- 25.Taga ME, Xavier KB. Curr. Protoc. Microbiol. John Wiley & Sons, Inc.; 2005. [Google Scholar]
- 26.Griffith KL, Wolf RE., Jr Biochem. Biophys. Res. Commun. 2002;290:397–402. doi: 10.1006/bbrc.2001.6152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.