Abstract
Aims
To investigate the use of diffusion weighted magnetic resonance imaging (DWI) and the apparent diffusion coefficient (ADC) values in the diagnosis of hemangioma.
Materials and methods
The study population consisted of 72 patients with liver masses larger than 1 cm (72 focal lesions). DWI examination with a b value of 600 s/mm2 was carried out for all patients. After DWI examination, an ADC map was created and ADC values were measured for 72 liver masses and normal liver tissue (control group). The average ADC values of normal liver tissue and focal liver lesions, the “cut-off” ADC values, and the diagnostic sensitivity and specificity of the ADC map in diagnosing hemangioma, benign and malignant lesions were researched.
Results
Of the 72 liver masses, 51 were benign and 21 were malignant. Benign lesions comprised 38 hemangiomas and 13 simple cysts. Malignant lesions comprised 9 hepatocellular carcinomas, and 12 metastases. The highest ADC values were measured for cysts (3.782±0.53×10−3 mm2/s) and hemangiomas (2.705±0.63×10−3 mm2/s). The average ADC value of hemangiomas was significantly higher than malignant lesions and the normal control group (p<0.001). The average ADC value of cysts were significantly higher when compared to hemangiomas and normal control group (p<0.001). To distinguish hemangiomas from malignant liver lesions, the “cut-off” ADC value of 1.800×10−3 mm2/s had a sensitivity of 97.4% and a specificity of 90.9%. To distinguish hemangioma from normal liver parenchyma the “cut-off” value of 1.858×10−3 mm2/s had a sensitivity of 97.4% and a specificity of 95.7%. To distinguish benign liver lesions from malignant liver lesions the “cut-off” value of 1.800×10−3 mm2/s had a sensitivity of 96.1% and a specificity of 90.0%.
Conclusion
DWI and quantitative measurement of ADC values can be used in differential diagnosis of benign and malignant liver lesions and also in the diagnosis and differentiation of hemangiomas. When dynamic examination cannot distinguish cases with vascular metastasis and lesions from hemangioma, DWI and ADC values can be useful in the primary diagnosis and differential diagnosis. The technique does not require contrast material, so it can safely be used in patients with renal failure.
Keywords: Liver, diffusion weighted magnetic resonance imaging, apparent diffusion coefficient, hemangioma
Introduction
Diffusion weighted magnetic resonance imaging (DWI) measures the random movement of water molecules in the tissues. The amount of diffusion determines the diffusion coefficient. When measuring the diffusion co-efficient, it can be affected by the heat of the tissue, microcirculation, perfusion, magnetic susceptibility or any type of movement, so, in clinical practice to measure diffusion, the apparent diffusion coefficient (ADC) is used1,2. DWI is a technique that can be obtained within the time of a single held breath, without requiring contrast material and was originally used in neuroradiology for early diagnosis of stroke3–5. In the literature, there have been published papers on the application of DWI to abdominal organs6–13.
These studies calculated the apparent diffusion coefficient of tissue and lesions from diffusion weighted images and have shown that the technique could be used for the differential diagnosis. Quantitative evaluations were made on images showing the amount of diffusion of water molecules called ADC maps. ADC combines the effects of capillary perfusion and water diffusion in extracellular and extravascular intervals14. As with solid lesions and abscess, while the cell density in the lesion increases, the diffusion becomes limited; so, cellular lesions on DWI with high b values (a factor indicating MR gradient strength and duration) (b=400–1000 s/mm2) gain a hyperintense signal property; showing low numeric values on the ADC map. As cell density decreases, such as in cysts, hemangiomas and necrotic lesions, diffusion is fast and high ADC values were found. Using high “b” values, DWI only reflects diffusion, however, low “b” value DWI is comprised of both diffusion and perfusion components15.
The aim of this study was to research the use of DWI and ADC values in the diagnosis of hemangioma; the most frequently observed benign tumor of the liver, and in its differentiation from other benign and malignant lesions.
Materials and methods
This study was planned prospectively. Ethics committee approval was obtained. The study population comprised 72 patients with liver masses larger than 1 cm (focal lesions). Patients with chronic liver disease caused by pathological situations other than viral hepatitis, alcohol and steatosis, biliary obstruction and metabolic causes were excluded. Patients with poor general condition, patients over the age of 70, patients without respiratory cooperation, and cases with prosthesis, implants or cardiac pacemakers were not included in the study. In addition, small-sized (less than 1 cm) lesions with difficult locations (proximity to subdiaphragmatic, subcapsular and falciform ligaments) were also excluded from the study because of difficulty in ADC measurements.
Diagnosis of hemangioma and other benign liver lesions were based on previous characteristic computer tomography and/or dynamic liver magnetic resonance imaging results. No changes in size during 6 months of follow up was regarded as hemangiomas or benign liver lesions. A typical hemangiomas were not included in the study. Cases with malignant lesions or without characteristic imaging results were confirmed using biopsy and histopathological results.
A 1.5 Tesla superconducting MR scanner (Intera, Master Gyroscan, Philips Medical Systems, the Netherlands) was used for imaging, which was performed without any need for sedation, in a supine position with a 4 channel sense body coil over the liver. Before DWI measurement, coronal localized and T2A axial images were obtained. DWI images directed at the upper abdomen were taken in all patients and control group subjects. As a control group, ADC measurements were done from normal liver parenchyma of 72 cases. The measurements were taken from parenchyma areas at least 1 cm away from the capsule and not crossing major vascular structures as far as possible. By selecting TR 1523 ms, TE 60 ms, FOV 375 mm, matrix 512×512, NEX 4 in the single shot, spin echo, echo planar (SSSE-EP) DWI, images with b=0.600 s/mm2 values were obtained.
Diffusion weighted images with a “b” value of 600 s/mm2 were transferred to an independent work station (Extended MR Workspace, version 7.1.5.1, Philips Medical Systems) for processing and ADC maps were created. ADC values of lesions and normal liver parenchyma (control group) were measured by an experienced radiologist from these maps. The study was planned as a uni-center study and all the measurements were done by the same radiologist (O.T.). The radiologist (O.T.) who measured the ADC values was certainly blinded from the diagnosis of previous imaging of the patients. Circular Region of Interest (ROI) was used for the quantitative analysis of the ADC value of the normal hepatic parenchyma and lesion. The measured area of ROI was set at approximately 0.5 cm2. At least 3 measurements were made for every lesion and normal liver parenchyma and the average values were recorded. Using a water-filled phantom, the accuracy of the ADC measurements was tested. Average ADC values of normal liver parenchyma and focal liver lesions, ADC “cutoff ” values, and the diagnostic sensitivity and specificity of the ADC map for hemangioma, benign and malignant lesions were investigated.
For statistical analysis of data SPSS 18.0 program was used. Age and ADC values were summarized as average and standard deviation (if necessary, median and minimum-maximum). In situations with two or more groups (benign-malignant and control group, hemangioma-cyst and control group), and in general comparison of mean ages and ADC measurements, One Way Analysis of Variance was used. For significance of these two-way group comparisons, the Scheffe test was used. To distinguish mean ADC values of hemangioma, benign and malignant lesions and to determine a cut-off point, ROC analysis was completed. For all tests the statistical level of significance was set as 0.05.
Results
Of 72 focal liver masses, 51 were benign and 21 were malignant. The benign lesions were hemangiomas (n=38) and simple cysts (n=13). Malignant lesions were 9 hepatocellular carcinoma (n=9) and metastases (n=12). The highest ADC values were measured in cysts (3.782±0.53×10−3 mm2/s) and hemangiomas(2.705±0.63×10−3 mm2/s) (Table 1). The average ADC value was 2.912±0.73×10−3 mm2/s for benign lesions, 1.250±0.74×10−3 mm2/s for malignant lesions and 1.631±1.11×10−3 mm2/s for normal liver parenchyma (Table 1). The average ADC value for hemangioma was significantly higher than the averages for malignant lesions and the normal control group (p<0.001). The average ADC value for cysts was significantly higher than the value for hemangioma and the normal control group (p<0.001). For benign lesions, the average ADC value was significantly higher when compared to malignant lesions and normal control group (p<0.001). For malignant lesions, the average ADC value was significantly lower than for benign lesions and the normal control group (p<0.001).
Table 1.
ADC values of lesions that were measured using b value 600 s/ mm2
| ADC values (mm2/sec) |
Number of patients (n) | |
| Normal liver parenchyma | 1.631±1.11×103 | 72 |
| Hemangioma | 2.705±0.63×103 | 38 |
| Cyst | 3.782±0.53×103 | 13 |
| Benign lesion | 2.912±0.73 ×10−3 | 51 |
| Malignant lesion | 1.250±0.74 ×10−3 | 21 |
ADC: apparent diffusion coefficient
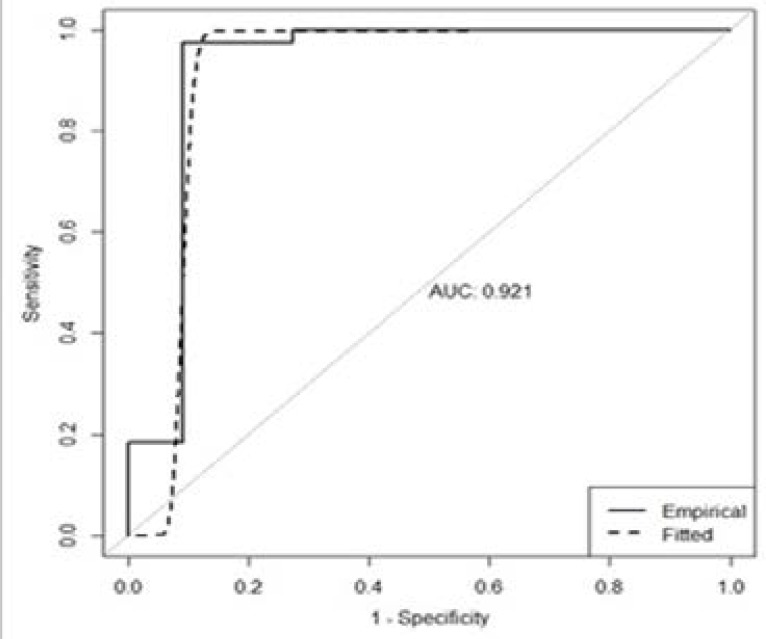
To distinguish hemangioma from normal liver parenchyma the “cut-off ” ADC value of 1.858×10−3 mm2/s had a sensitivity of 97.4% and a specificity of 95.7%. Distinguishing hemangioma from malignant liver lesions, the “cut-off ” ADC value of 1.800×10−3 mm2/s had a sensitivity of 97.4% and a specificity of 90.9% (Table 2, Fig. 1).
Table 2.
Cut-off values for ADC with relevant sensitivity and specificity values
| Cut-off value for ADC mm2/sec) |
Sensitivity (%) |
Specificity (%) |
|
|
Differentiation between hemangioma and malignant lesion |
1.800 ×10−3 | 97.4 | 90.9 |
|
Differentiation between hemangioma and normal liver parenchyma |
1.858 ×10−3 | 97.4 | 95.7 |
ADC: apparent diffusion coefficient
Figure 1.
ROC curve used to distinguish hemangioma from malignant lesions.
To distinguish benign liver lesions from normal liver parenchyma the “cut-off ” ADC value of 1.844×10−3 mm2/s had a sensitivity of 96.1% and a specificity of 95.0% while the “cut-off ” ADC value of 1.504×10−3 mm2/s to distinguish malignant liver lesions from normal liver parenchyma had a sensitivity of 88.2% and a specificity of 81.8%. The ADC “cut-off ” value to distinguish benign liver lesions from malignant liver lesions was 1.800×10−3 mm2/s which had a sensitivity of 96.1% and a specificity of 90.0% (Table 3).
Table 3.
Cut-off values for ADC with relevant sensitivity and specificity values
| Cut-off value for ADC mm2/sec) |
Sensitivity (%) |
Specificity (%) |
|
|
Differentiation between Benign lesion and normal liver parenchyma |
1.844 ×10−3 | 96.1 | 95.0 |
|
Differentiation between malignant lesion and normal liver parenchyma |
1.507 ×10−3 | 88.2 | 81.8 |
|
Differentiation between benign and malignant lesion |
1.800 ×10−3 | 96.1 | 90.9 |
ADC: apparent diffusion coefficient
Discussion
Cavernous hemangioma is the most frequently observed benign liver tumor. Typical hemangioma appears hypointense on T1AG and hyperintense on T2AG. Hemangiomas have higher ADC values than solid lesions in the liver and lower ADC values than cysts (Fig. 2). In addition, to distinguish cavernous hemangiomas from other pathologies, contrast dynamic examination is necessary. Mass characterization with magnetic resonance imaging (MRI) can evaluate the general lesion morphology, signal intensity and contrast pattern.
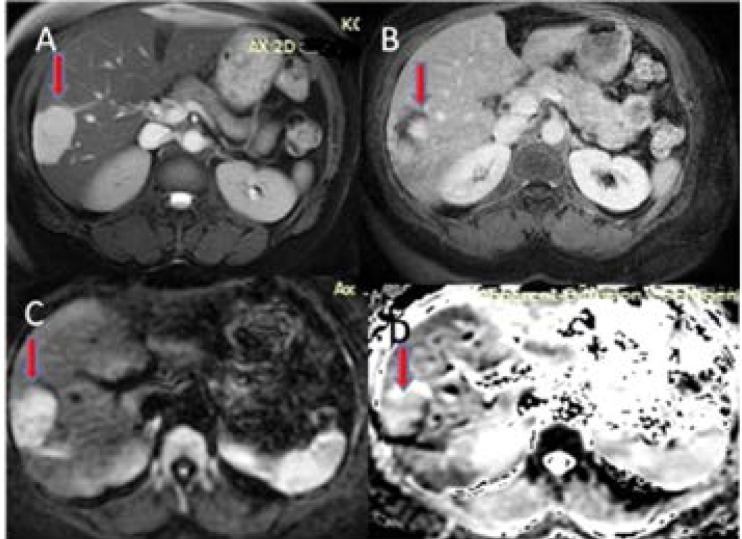
Figure 2.
Liver right lobe posterior hemangioma
(A)T2 weighted MR image showing hyper intense lesion
(B) Fat suppressed T1 weighted image after IV contrast showing compatible hemangioma
(C) b=600 s/mm2 diffusion weighted image showing hyper intense lesion
(D) ADC map showing the lesion's hyper intense signal.
However even if all the results were evaluated together, there might still be an overlap between benign and malignant lesions. While dynamic contrast examination has become a component of routine abdominal scans, the contrast material used increases costs and carries the risk of side effects. Additionally, in some cases, distinguishing vascular metastasis from hemangioma is not possible even with dynamic examination16. DWI is a technique which can be used within the duration of a held breath with no necessity for contrast material, and it helps to distinguish the “benign-malignant” nature of many focal lesions. This advantage, especially in renal failure patients due to the risk of “nephrogenic systemic sclerosis”, is important as it reduces the need for IV gadolinium chelate compounds.
Diffusion is the name given to the randomized microscopic movement of water molecules. At the microscopic level, diffusion is known to be a sensitive parameter for tissue characterization. Today, the measurement of diffusion in vivo is possible using DWI and ADC measurements17. DWI queries and quantifies the movement of water molecules in tissues and thus the image is based on differences in movement in tissues which produces contrast. Tissue with high density of cell membranes, such as tumors, limits the diffusion of water protons. In contrast, water molecules move more freely in cystic or necrotic tissues and the water protons ADC is defined freely. As a result DWI provides information about cellularity and cellular membrane integrity, and even microcapillary perfusion. The presence of water diffusion means signal loss of DWI and corresponds with high ADC values. In contrast tumor cells have limited diffusion, producing DWI with high signal intensity and correspondingly low ADC values appear. The development of echoplanar imaging (EPI), a type of fast MRI method, has shown that, diffusion weighted MRI can be used to evaluate abdominal organs18,19. The diffusion properties of tissues are related to the interstitial free fluid and amount of permeability. In general, more limitation on diffusion is observed in cancer tissues. On DWI, the foci of liver tumors have limited diffusion and a reduction in ADC values20,21. In cancer tissues, the normal structure of tissues is disrupted. This obstructs the macromolecular movement of water limiting diffusion in cancer cases and thus reduces measured ADC.
Our study supports previous studies in showing that ADC measurements are significantly different in benign and malignant liver masses21,22,7,23,24.
In our study, cysts and hemangiomas had the highest ADC values while malignant lesions had lower ADC values. The average ADC value for cysts was 3.782±0.53×10−3 mm2/s while hemangiomas had an average ADC value of 2.705±0.63×10−3 mm2/s. The average ADC value of cysts were statistically significantly higher than hemangioma ADC values (p<0.001). All simple cysts had higher ADC values than the hemangioma average ADC values21,22. In our study, the average ADC value of benign lesions was 2.912±0.73×10−3 mm2/s, whereas the average ADC value of malignant lesions was 1.250±0.74×10−3 mm2/s.
For normal liver parenchyma, the average ADC value was 1.631±1.11×10−3 mm2/s. Our study supports previous studies in finding that ADC values for hemangioma were statistically significantly higher than malignant lesions and normal liver parenchyma (p<0.001)20,21. The “cut-off” ADC value to distinguish hemangioma from normal liver parenchyma was 1.858×10−3 mm2/s with a sensitivity of 97.4% and a specificity of 95.7%. The “cut-off” ADC value to distinguish hemangioma from malignant liver lesions was 1.800×10−3 mm2/s with a sensitivity of 97.4% and a specificity of 90.9%.
The limitations to our study should be considered. Firstly, our study population was relatively small when the study groups were divided into subgroups. Secondly, another limitation was related with the technique. Diffusion weighted examination is made by sequences sensitive to respiratory, cardiac or peristaltic physiological movements which affect image quality and make the evaluation more difficult. Another limitation was the spatial resolution of the sequence which was too low for lesions less than 1 cm in size and these lesions were not included in the study. New studies using faster parallel imaging methods have developed image quality and reduced the artifacts due to EPI22. Recently, diffusion MR studies using 3 Tesla MR devices have improved image quality and new papers are available25.
On the other hand, diffusion weighted MRI sequence can be obtained within the duration of a single held breath (about 24 seconds), the technique itself requires no contrast material. The technique can aid differential diagnosis of benign and malignant liver masses in cases where conventional sequences cannot be used. We think that, this MRI sequence is necessary to complete routine liver MR imaging protocols. We suspect the ADC values would be lower for complex cysts like abscesses and infestations. Because of the dense nature of the cysts, this is not surprising. In T2A images, hemangiomas and simple cysts were both seen as hyperintense lesions. On diffusion weighted images (with high b values), hemangiomas were noticed as hyperintense; however, simple cysts were seen as hypointense lesions. After ADC mapping, cysts had higher ADC values, whereas hemangiomas had lower ADC values than cysts. Without using contrast material, this differentiation could be done easily with the aid of ADC measurement.
Conclusion
DWI and quantitative measurement of ADC values can be used in differential diagnosis of benign and malignant liver lesions. Conventional MR imaging with added DWI increases the accuracy of conventional MR methods in characterizing benign and malignant lesions. Hemangiomas have higher ADC values than malignant lesions and normal parenchyma, but they have lower values ADC values than cysts. DWI and quantitative measurement of ADC values can be used in the diagnosis and differentiation of hemangiomas. In case where dynamic examination cannot distinguish vascular metastasis and lesions from hemangioma, DWI and ADC values can be useful both in primary and differential diagnosis. The technique does not require contrast material, so it can safely be used in patients with renal failure.
References
- 1.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with single-shot echo-planar imaging in the upper abdomen: preliminary clinical experience in 61 patients. Abdom Imaging. 1999;24:456–461. doi: 10.1007/s002619900539. [DOI] [PubMed] [Google Scholar]
- 2.Moteki T, Horikoshi H, Oya N, Aoki J, Endo K. Evaluation of hepatic lesions and hepatic parenchyma using diffusion-weighted reordered turboFLASH magnetic resonance images. J Magn Reson Imaging. 2002;15:564–572. doi: 10.1002/jmri.10101. [DOI] [PubMed] [Google Scholar]
- 3.Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology. 1992;42:1717–1723. doi: 10.1212/wnl.42.9.1717. [DOI] [PubMed] [Google Scholar]
- 4.Back T, Hoehn-Berlage M, Kohno K, Hossmann KA. Diffusion nuclear magnetic resonance imaging in experimental stroke: correlation with cerebral metabolites. Stroke. 1994;25:494–500. doi: 10.1161/01.str.25.2.494. [DOI] [PubMed] [Google Scholar]
- 5.Lutsep HL, Albers GW, DeCrespigny A, Kamat GN, Marks MP, Moseley ME. Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol. 1997;41:574–580. doi: 10.1002/ana.410410505. [DOI] [PubMed] [Google Scholar]
- 6.Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393–398. doi: 10.2214/ajr.173.2.10430143. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998;170:397–402. doi: 10.2214/ajr.170.2.9456953. [DOI] [PubMed] [Google Scholar]
- 8.Namimoto T, Yamashita Y, Sumi S, Tang Y, Takahashi M. Focal liver masses: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 1997;204:739–744. doi: 10.1148/radiology.204.3.9280252. [DOI] [PubMed] [Google Scholar]
- 9.Stahlberg F, Brockstedt S, Thomsen C, Wirestam R. Single-shot diffusion-weighted echo-planar imaging of normal and cirrhotic livers using a phased-array multicoil. Acta Radiol. 1999;40:339. [PubMed] [Google Scholar]
- 10.Amano Y, Kumazaki T, Ishihara M. Singleshot diffusion-weighted echo-planar imaging of normal and cirrhotic livers using a phased-array multicoil. Acta Radiol. 1998;39:440–442. doi: 10.1080/02841859809172460. [DOI] [PubMed] [Google Scholar]
- 11.Moteki T, Ishizaka H, Horikoshi H, Matsumoto M. Differentiation between hemangiomas and hepatocellular carcinomas with the apparent diffusion coefficient calculated from turbo FLASH MR images. J Magn Reson Imaging. 1995;5:187–191. doi: 10.1002/jmri.1880050214. [DOI] [PubMed] [Google Scholar]
- 12.Ries M, Jones RA, Basseau F, Moonen CT, Grenier N. Diffusion tensor MRI of the human kidney. J Magn Reson Imaging. 2001;14:42–49. doi: 10.1002/jmri.1149. [DOI] [PubMed] [Google Scholar]
- 13.Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging. 1999;9:832–837. doi: 10.1002/(sici)1522-2586(199906)9:6<832::aid-jmri10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Koike N, Cho A, Nasu K, Seto K, Nagaya S, Ohshima Y, Ohkohchi N. Role of diffusion-weighted magnetic resonance imaging in the differential diagnosis of focal hepatic lesions. World J Gastroenterol. 2009;15:5805–5812. doi: 10.3748/wjg.15.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 16.Semelka RC, Shoenut JP, Kroeker MA, Greenberg HM, Simm FC, Minuk GY, Kroeker RM, Micflikier AB. Focal liver disease: comparison of dynamic contrast-enhanced CT and T2-weighted fatsuppressed, FLASH, and dynamic gadolinium-enhanced MR imaging at 1.5 T. Radiology. 1992;184:687–694. doi: 10.1148/radiology.184.3.1324509. [DOI] [PubMed] [Google Scholar]
- 17.Le Bihan D, Turner R, Douek P, Patronas N. Diffusion MR imaging: clinical applications. AJR Am J Roentgenol. 1992;159:591–599. doi: 10.2214/ajr.159.3.1503032. [DOI] [PubMed] [Google Scholar]
- 18.Muller MF, Prasad P, Siewert B, Nissenbaum MA, Raptopoulos V, Edelman RR. Abdominal diffusion mapping with use of a whole-body echo-planar system. Radiology. 1994;190:475–483. doi: 10.1148/radiology.190.2.8284402. [DOI] [PubMed] [Google Scholar]
- 19.Reimer P, Saini S, Hahn PF, Brady TJ, Cohen MS. Clinical application of abdominal echoplanar imaging (EPI): optimization using a retrofitted EPI system. J Comput Assist Tomogr. 1994;18:673–679. doi: 10.1097/00004728-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Naganawa S, Sato C, Nakamura T, Kumada H, Ishigaki T, Miura S, Maruyama K, Takizawa O. Diffusion-weighted images of the liver: comparison of tumor detection before and after contrast enhancement with superparamagnetic iron oxide. J Magn Reson Imaging. 2005;21:836–840. doi: 10.1002/jmri.20346. [DOI] [PubMed] [Google Scholar]
- 21.Demir OI, Sağol O, Dicle O. Contribution of diffusion-weighted MRI to the differential diagnosis of hepatic masses. Diagn Interv Radiol. 2007;13:81–86. [PubMed] [Google Scholar]
- 22.Yoshikawa T, Kawamitsu H, Mitchell DG, Ohno Y, Ku Y, Seo Y. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006;187:1521–1530. doi: 10.2214/AJR.05.0778. [DOI] [PubMed] [Google Scholar]
- 23.Sandrasegaran K, Akisik FM, Lin C, Tahir B, Rajan J, Aisen AM. The value of diffusion-weighted imaging in characterizing focal liver masses. Acad Radiol. 2009;16:1208–1214. doi: 10.1016/j.acra.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Sun XJ, Quan XY, Huang FH, Xu YK. Quantitative evaluation of diffusion-weighted magnetic resonance imaging of focal hepatic lesions. World J Gastroenterol. 2005;11:6535–6537. doi: 10.3748/wjg.v11.i41.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunsche S, Moseley ME, Stoeter P, Hedehus M. Diffusion-tensor MR imaging at 1.5 and 3.0 T: initial observations. Radiology. 2001;221:550–556. doi: 10.1148/radiol.2212001823. [DOI] [PubMed] [Google Scholar]