Abstract
Background
The prevalence of Diabetes mellitus (DM) is on a rise in sub-Saharan Africa and will more than double by 2025. Cardiovascular disease (CVD) accounts for up to 2/3 of all deaths in the diabetic population. Of all the CVD deaths in DM, 3/4 occur in sub Saharan Africa (SSA). Non invasive identification of cardiac abnormalities, such as Left Ventricular Hypertrophy (LVH), diastolic and systolic dysfunction, is not part of diabetes complications surveillance programs in Uganda and there is limited data on this problem. This study sought to determine the prevalence, types and factors associated with echocardiographic abnormalities among newly diagnosed diabetic patients at Mulago National referral hospital in Uganda.
Methods
In this cross sectional study conducted between June 2014 and December 2014, we recruited 202 newly diagnosed adult diabetic patients. Information on patients' socio-demographics, bio-physical profile, biochemical testing and echocardiographic findings was obtained for all the participants using a pre-tested questionnaire. An abnormal echocardiogram in this study was defined as the presence of LVH, diastolic and/or systolic dysfunction and wall motion abnormality. Bivariate and multivariate logistic regression analyses were used to investigate the association of several parameters with echocardiographic abnormalities.
Results
Of the 202 patients recruited, males were 102(50.5%) and the mean age was 46±15 years. Majority of patients had type 2 DM, 156(77.2%) and type 1 DM, 41(20.3%) with mean HbA1C of 13.9±5.3%. Mean duration of diabetes was 2 months. The prevalence of an abnormal echocardiogram was 67.8 % (95% CI 60%-74%). Diastolic dysfunction, systolic dysfunction, LVH and wall motion abnormalities were present in 55.0%, 21.8%, 19.3% and 4.0% of all the participants respectively. In bivariate logistic regression analysis, the factors associated with an abnormal echocardiogram were age (OR 1.09 [95% CI 1.06–1.12], P <0.0001), type 2 DM (OR 5.8[95% CI 2.77–12.07], P<0.0001), hypertension (OR 2.64[95% CI 1.44–4.85], P=0.002), obesity (OR 3.51[955 CI 1.25–9.84], P=0.017 and increased waist circumference (OR 1.02[95% CI 1.00–1.04], P=0.024. On Multiple logistic regression analysis, age was the only factor associated with an abnormal echocardiogram (OR 1.09[95%CI 1.05–1.15], P<0.0001).
Conclusion
Echocardiographic abnormalities were common among newly diagnosed adults with DM. Traditional CVD risk factors were associated with an abnormal echocardiogram in this patient population. Due to a high prevalence of echocardiographic abnormalities among newly diagnosed diabetics, we recommend screening for cardiac disease especially in patients who present with traditional CVD risk factors. This will facilitate early diagnosis, management and hence better patient outcomes.
Keywords: Diabetes mellitus, echocardiography, cardiac abnormalities
Introduction
Diabetes is an important cause of mortality and morbidity worldwide1. The worldwide prevalence of diabe-tes mellitus (DM) has risen dramatically over the past 2 decades2. The global number of individuals with diabetes in 2000 was estimated to be 171 million (2.8% of the world's population), a figure projected to increase in 2030 to 366 million (6.5%), 298 million of whom will live in developing countries3. Type 2 diabetes and its associated long-term complications continue to accelerate among patients who reside in developing countries due to rapidly increasing life expectancy at birth4. The estimated prevalence of diabetes in Africa is 1% in rural areas, up to 5% to 7% in urban sub Saharan Africa, and between 8% and 13% in more developed areas such as South Africa and in populations of Indian origin5.
An aging population together with rapid urbanization will lead to an increase in the prevalence of diabetes by the year 20256 at which time the majority of the world's diabetespopulation will be living in the developing countries7. By the year 2025, the prevalence of diabetes in sub-Saharan Africa is expected to more than double the current figures (3).
Cardiovascular diseases, the commonest cause of death in patients with diabetes, accounts for up to two-thirds of all deaths in this group5. The risk of heart disease in diabetes is increased up to 15 years prior to the diagnosis of diabetes8. Management of established CVD in these SSA countries is unrewarding due to chronic shortage of resources. Early diagnosis of preclinical CVD in this patient population is therefore a priority as it will enable early intervention to prevent or delay complications. Subclinical cardiac abnormalities of structure and function, detectable using myocardial Tissue Doppler Imaging (TDI) and conventional echocardiographic imaging are common in adults with diabetes9. With increasing availability of this non-invasive cardiac ultrasound service including point-of-care hand-held echocardiographic machines, it is reasonable to argue that echo screening in all diabetes be part of routine care.
The absence of systematically collected data on pre-clinical CVD in these patients makes it impossible to implement policy change as suggested above as no studies have been carried out to assess the magnitude of the problem in this patient group.
Accordingly, the aim of this study was to determine the prevalence, types and factors associated with echocardiographic abnormalities among newly diagnosed diabetic patients at Mulago hospital.
Methods
Study design
This was a cross-sectional study among 202 newly diagnosed diabetic patients at Mulago National referral hospital in Uganda conducted from June to December 2014. The study was carried out in the diabetic outpatient clinic and the medical wards; 4B endocrine and 3BEM of Mulago National referral Hospital. Mulago serves as the National referral hospital for Uganda and Teaching Hospital for Makerere University with a bed capacity of 1500. A diagnosis of DM was made by one of the following: Fasting glucose >126mg/dL, 2 hour plasma glucose of >200mg/dl after oral Glucose tolerance test (OGTT), or glycated hemoglobin (HbA1C) >6.5%. We differentiated type 1 from type 2 DM using clinical assessment because we were unable to assess insulin levels which is the gold standard. Blood pressure was measured using a mercury sphygmomanometer and an average of the 2 readings was used in the analysis. Hypertension was defined as present if subjects were on anti-hypertensive medication, had history of hypertension and/or evidence of hypertension (blood pressure ≥140/90 mmHg).
All newly diagnosed diabetic patients aged 18 years and above attending the diabetic clinic or admitted to the medical wards of Mulago hospital during the study period who met the inclusion criteria and provided informed consent were recruited consecutively. Using prevalence of 72% for an abnormal echocardiogram among diabetics as determined by Piyush10, a sample size of 309 was estimated using the Kish Leslie (1965) formula11. However we were unable to achieve the required sample size due to limitations in logistics for the investigations.
Operational definitions
Type1 DM: This referred to patients who required insulin and were depending on insulin for glucose control since diagnosis.
Type 2 DM: Patients were classified as having type 2 diabetes mellitus if they required oral hypoglycemic agents or usage of combination of insulin and the oral hypoglycemic agents for glucose control.
Type 3 DM is diabetes secondary to an identifiable cause like pancreatitis or cancer of the pancreas.
Type 4 DM is gestational diabetes.
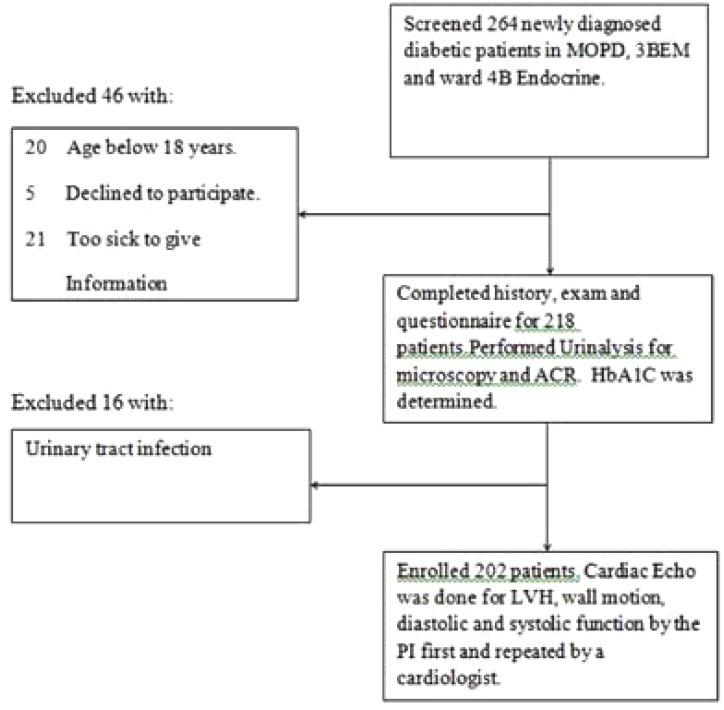
Fig. 1 illustrates the patient recruitment flow.
Figure 1.
Flow diagram showing newly diagnosed diabetic patients that participated in the study.
The following study parameters were collected using a pre tested questionnaire: patients' demographic data, history of hypertension, age, physical exercise, marital status, date of diagnosis of DM, drug history, occupation, education level, LNMP, Blood pressure, body Weight, height, Waist and hip circumferences. Glycated haemoglobin (HbA1C) was measured by automated high performance liquid chromatography. Other investigations included urinalysis and Microalbuminuria using ACR. Echocardiography parameters were acquired using a commercially available machine, Phillips HD11XE (Eindhoven, The Netherlands) with 2-D, M Mode and Doppler capabilities was used according to the American Society of Echocardiography12.
Ethics approval
The study was approved by the School of Medicine Research and Ethics Committee of the College of Health Sciences, Makerere University. Written informed consent for involvement in the study was obtained from the study participants. Enrolment was totally free and voluntary and participants were free to withdraw at any time without any consequences to them. The patients' records/information was anonymized and de-identified prior to analysis.
Data management and analysis
Data was captured on hard copy Case Report Forms, then transferred to the electronic EPIDATA version 3.1 and exported to STATA 13 for analysis. Continuous variables were summarized as mean (± standard deviation), median (± inter quartile range) and presented in tables. Categorical data was summarized using frequency and percentages and results are presented in frequency tables and bar charts. Logistic regression was used to determine odds for a given outcome and presented as Odds ratio (OR) and their 95% confidence interval (CI). Only factors with P- value <0.2 at bivariate analysis were considered for multivariate analysis. Multivariate logistic regression was used to determine factors associated with an abnormal echocardiogram and the different echo abnormalities (secondary outcomes): significance was at 5%.
Results
This study recruited 202 newly diagnosed diabetic patients between May 2014 and January 2015. Of these, 102(50.5%) were males. The mean age of the participants was 46 years (18–90). Majority of patients had type 2 DM, 156 (77.2%) or type 1 DM, 41 (20.3%). None of the patients had type 3 DM, and the remainder had gestational DM, 5 (2.5%). The mean HbA1C was 13.9%, (SD: 5.27%). Mean duration of diabetes was 2 months. Majority of patients 128 (63.6%) were unemployed. The education levels of participants were mainly primary which accounted for 78 (38.6%). The remainder of patients had had either secondary education 75 (37.1%), tertiary education 31 (15.4%), or no formal education at all 18 (8.9%). Majority of the study participants 118 (58.4%) were married. Study participants who had never been married were 30 (14.9%) while those who were once married and no longer married were 54(26.7%). Of all the participants, 125(61.95%) had hypertension according to the operational definition and only 38.1% were normotensive.
Echocardiographic abnormalities
Transthoracic echocardiography was performed on all 202 participants and the results are shown in Table 2. Prevalence of an abnormal echocardiogram was 67.8 % (95% CI: 60%–74%). Isolated Diastolic dysfunction was present in 55.0% of all the participants (95%CI: 47.8%–61.9%) while isolated systolic dysfunction was present in 21.8 %( 95% CI: 16.3%–28.1%) of all the participants. The prevalence of combined systolic and diastolic dysfunction was 13 (95% CI:8.9%–18.85). Left ventricular hypertrophy (LVH) and wall motion abnormality accounted for 19.3 %( 95%CI 14.1%–25.4%) and 4 %(95%CI 1.7%–7.6%) respectively.
Table 2.
Comparison of characteristics for hypertensive and normotensive patients who participated in the study (N=202)
| Characteristic | Total (N) | Total | Hypertensive | Normotensive N(%). | |
| (%). | N(%). | ||||
| Age | <40 years | 58 | 28.86 | 21(36.2) | 37(63.8) |
| >40 years | 143 | 71.14 | 105(73.4) | 38(26.6) | |
| Gender | Male | 102 | 50.75 | 54(52.9) | 48(47.06) |
| Female | 99 | 49.25 | 72(72.7) | 27(27.27) | |
| Employment | Employed | 76 | 38.0 | 41(53.9) | 35(46.05) |
| Unemployed | 124 | 62.00 | 85(68.6) | 39(31.45) | |
| Pregnancy | Yes | 6 | 5.41 | 3(50.0) | 3(50.00) |
| No | 105 | 94.59 | 74(70.5) | 31(29.52) | |
| Education | None | 17 | 8.46 | 10(58.8) | 7(41.18) |
| Primary | 78 | 38.81 | 50(64.1) | 28(35.90) | |
| Secondary | 75 | 37.31 | 45(60.0) | 30(40.00) | |
| Tertiary | 31 | 15.42 | 21(67.7) | 10(32.26) | |
| Marital status | Never married | 29 | 14.43 | 7(24.1) | 22(75.86) |
| Currently married |
119 | 59.20 | 83(69.8) | 36(30.25) | |
| No longer married |
53 | 26.4 | 36(67.9) | 17(32.1) | |
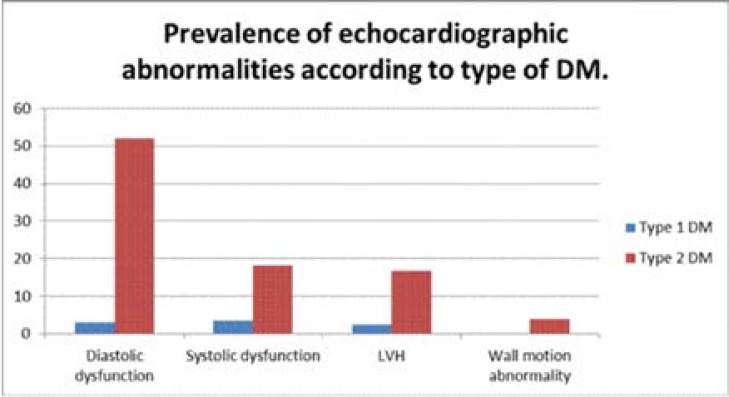
Echocardiographic Abnormalities and Type of DM
Generally, the prevalence of the different echocardiographic abnormalities was much higher among patients with type 2 compared to type 1 DM (Figure 2). Of these echocardiographic abnormalities 120 (87.6%) were seen among type 2 Diabetics while type 1 DM accounted for 15 (11.0%). Wall motion abnormalities were only present among type 2 DM patients; there were no patients with type 1 DM with wall motion abnormalities (Figure 2).
Figure 2.
Bar graph showing Prevalence of echocardiographic abnormalities according to type of DM.
Factors associated with an abnormal echocardiogram
In bivariate analysis, the factors associated with an abnormal echocardiogram included: age (P<0.0001), hypertension (P=0.002), obesity (P=0.017) and increase in waist circumference (P=0.024).
After adjusting for patients' sex, hypertension, BMI, waist hip ratio and waist circumference the only significant factor associated with an abnormal echocardiogram was age (OR1.09 [1.05–1.15] P<0.0001). Hypertension, obesity, waist hip ratio and waist circumference were no longer significantly associated with an abnormal echocardiogram in this model.
Discussion
Prevalence of echocardiographic abnormalities among newly diagnosed diabetic patients. Abnormal echocardiogram (Generally)
The prevalence of an abnormal echocardiogram as defined in this study was 67.8% among newly diagnosed diabetic patients of whom 77.2% had type 2 DM. Of these echocardiographic abnormalities 120 (87.6%) were seen among type 2 Diabetics while type 1 DM accounted for 15 (11.0%). Diastolic dysfunction was present in 55% of all participants while LVH and systolic dysfunction were present in 19.3% and 21.8% respectively. Only 4% of all participants had regional wall motion abnormality.
Our findings are in tandem with evidence from earlier studies, which revealed that: the main forms of structural heart disease associated with DM are coronary heart disease and diabetic cardiomyopathy, which is characterized by LV hypertrophy, LV diastolic and systolic dysfunction13.
These findings are comparable to what was found in a cohort study among 229 type 2 diabetic patients in Australia performed in 200810. An abnormal echocardiogram was present in 166 (72%) of all patients. In this study diastolic dysfunction alone was present in 109(48 %) of all patients10, similar to other studies.
Unlike type 1 DM, type 2DM may have a latent period before diagnosis which can go up to 10 years and patients may only be diagnosed with a complication. This may explain why majority of the newly diagnosed type 2 diabetics presented with an abnormal echocardiogram in our study.
Type 1 DM patients in our study had a very low prevalence of echocardiographic abnormalities; abnormal echocardiogram was present in only 15 (11.0 %) of all patients.
Our findings are different from what Bryan et al found; their study assessed 141 type 1 diabetic patients and found the prevalence to be 2.5 times higher than what we found. The major abnormalities on the echocardiogram was diastolic dysfunction, LVH and systolic dysfunction14. The difference in the studies is that the latter looked at patients with an average duration of diabetes of 21 years and only type 1 DM, yet in our study we enrolled only newly diagnosed diabetics with mean duration of DM of only 2 months.
Diastolic dysfunction
In Africa, Diabetes-related cardiovascular disease complications were considered to be rare but are on the rise5. We found the prevalence of LVDD to be 55% in our study. This is in keeping with earlier studies in Africa which showed that Diabetic cardiomyopathy, which is largely represented by diastolic dysfunction, may affect up to 50% of all patients5.
Over the last three decades several studies have demonstrated the presence of left ventricular (LV) diastolic dysfunction in patients with normal LV ejection fraction (LVEF)10,14–17. Majority of these studies have found a high prevalence of Left ventricular diastolic dysfunction ranging from 40% to 75%10,14–16,18.
The prevalence of LVDD in patients with T2DM has been shown to vary from 25% to 60% in different studies19–21. It is noted that majority of patients in our study had type 2 DM and were newly diagnosed as cited earlier. This could account for the similarity in the results. In Senegal LVDD has been found in 62% of type 2 DM patients22.
Prior to the development of symptomatic heart failure, subclinical LV dysfunction (systolic and diastolic) may exist for some time and are associated with a poor prognosis13,15.
We used mitral E/A ratio to define diastolic dysfunction unlike in the studies quoted where they used both E/A and TDI for diastolic dysfunction a method that increases the sensitivity in diagnosis. We used mitral E/A because it is the standard of care in our setting and TDI has not been widely used. This means that the prevalence of diastolic dysfunction we reported of 55% among newly diagnosed diabetics may be an under estimate of a bigger problem.
Omission of TDI may eliminate up to 25% of patients with pseudonormalization which may go unmasked10. This may call for longitudinal studies to evaluate this problem further using TDI.
The clinical implication of this diastolic dysfunction which was found in majority of our patients is the inevitable progression to clinical heart failure. In fact Progression of diastolic dysfunction to heart failure is 4 years in type 2 DM9,10 and 6 years in type 1 DM14. There is great need to follow up these patients before overt clinical outcomes occur.
Left ventricular hypertrophy (LVH)
From a recent cohort in Australia among type 1 DM patients10, the prevalence of LVH alone was 9%, which is twice the prevalence in the general population; however, in our study, we found a higher prevalence up to 19.3%. This could be explained by the high prevalence of hypertension (62%), which was strongly associated with LVH in our study.
Valensi, in an African population, found LVH in 33.6% of the study population17. The population he assessed had one or more CVD risk factors and used both rest echocardiography and myocardial scintigraphy which could have led to a higher prevalence compared to what we found. Piyush in a cohort among type 2 DM patients found LVH in 51% of all patients10. The difference between his and our findings may be due to a long duration of DM of 10 years in his study while our patient population was newly diagnosed. Furthermore, our patients were younger; mean age 46 years compared to Piyush's cohort which had 61 years as their mean age. Screening for left ventricular hypertrophy in patients with type 2 diabetes mellitus in the community revealed a prevalence of up to 56%. This prevalence was much higher than what we found, possibly because our patient population was newly diagnosed and the duration of DM was only 2 months23. In the former, patients had a minimum duration of diabetes of 5 years. This may call for longitudinal studies to assess the burden of LVH and its association with duration of diabetes in our setting.
Systolic dysfunction
We found a high prevalence of systolic dysfunction at 21.8% among newly diagnosed diabetic patients in this study. Majority of these patients 37(84.1%) had type 2 DM. Piyush in Australia found a lower prevalence of systolic dysfunction among type 2 DM patients which was present in 5%10. This is greatly different from what we found yet patients in Australia were much older; mean age 65 compared to 46 and duration of DM of 10 years compared to 2 months in our study. This calls for more studies to clear the difference.
There is evidence of high prevalence of reduced LVEF in diabetic patients without known heart disease. In one study the prevalence of reduced LVEF was 16.7%24, comparable to our findings. Valensi in an African population found a lower prevalence of systolic dysfunction of 3.2% compared to what we found. The reason for the difference could be the fact that all patients assessed had no clinical features of heart failure in Valensi's cohort17. In our study we did no clinical assessment.
We found that 27 (13.4%) of all patients had combined systolic and diastolic dysfunction in our study. This finding is similar to what was found in a cohort in Australia which found combined systolic and diastolic dysfunction at 11%10. Both studies assessed type 2 diabetic patients.
Wall motion abnormalities
Regional wall abnormalities were present among 8 (4.0 %) of all the study participants in our study. In Africa, the findings have been similar to what earlier studies in the developed world have shown15,17,22. Valensi generated evidence from an African diabetic population: Rest echocardiography revealed that hypokinesia was found in 6.1% of the study population17. This finding is comparable to what we revealed in this study. The slight difference may be due to the fact that in the former study, both rest echocardiography and myocardial scintigraphy were used; hence a higher sensitivity.
Factors associated with echocardiographic abnormalities among newly diagnosed diabetic patients at Mulago Hospital
In this study, we found that traditional CVD risk factors such as advancing age, hypertension, obesity, increasing waist circumference and type 2 DM were associated with an abnormal echocardiogram in newly diagnosed adult diabetic patients in Uganda (in the unadjusted model).
These findings are similar to what was found in other previous studies on echocardiographic abnormalities among diabetic patients10,14. The increasing prevalence of factors like hypertension and obesity has led to an increase in the risk and prevalence of cardiac abnormalities in DM25. This is in line with what we are seeing in this study.
We found a high burden of hypertension at 62% among the study population. It was strongly associated with an abnormal echocardiogram in our study. This is in agreement with earlier studies which described that hypertension is more prevalent in diabetic patients in sub-Saharan Africa26. The other reason which can explain this association is that: Individuals with hypertension are at increased risk of DM compared with normotensive persons. Furthermore, up to 75% of cases of cardiac disease in DM can be attributed to hypertension27.
Piyush, in a longitudinal study in Australia among type 2 DM patients found the independent predictors of an abnormal heart echocardiogram were obesity, age and the number of antihypertensive agents used. Age was the strongest predictor of an abnormal echocardiogram, with the risk increasing by 9% for each year over 50 years of age10. This is similar to what we found that every 1 year increase in age has a 9% increased odds of having an abnormal echocardiogram.
After multivariate adjustment, however, only age was independently associated with echocardiographic abnormalities among newly diagnosed diabetic patients. Life expectancy in sub-Saharan Africa; including Uganda has risen in the past 50 years. Many more people living with DM are therefore exposed to the CVD risk factors for long periods for the cardiac complications to develop5.This may be the reason why age is an important factor in this study. In addition, advancing age is a known risk factor for diastolic dysfunction in the general population; this could have contributed to the high prevalence among the elderly.
Gough described that glycemic control does not affect cardiac abnormalities in the short term among diabetics 26. We reached a similar conclusion in this study: glycemic control was not associated with cardiac abnormalities despite very profound hyperglycemic states, mean HbA1C was 13.4%.
Similarly microalbuminuria measured by urine ACR was not associated with an abnormal echocardiogram, despite the fact that significant microalbuminuria was present in 54% of all patients. This is in contrast with the Strong Heart Study in diabetes, which found urinary albumin excretion was independently associated with cardiac dysfunction28. In the later study ACR was measured at least twice but in our study only one measurement was taken.
Limitation of the study
We recognize that the lack of an non diabetic control population is a limitation of our study; however, this study was not designed to determine the effect of diabetes on cardiac structure and function, but rather to explore the magnitude of subclinical cardiac abnormalities among newly diagnosed diabetic patients in the SSA context, including the risk associated with traditional CVD risk factors. Secondly, we used spot urine ACR as a single measure yet persistent microalbuminuria would be diagnosed by at least 2 measurements depicting 20mg/g according to our laboratory. This was due to logistical limitations.
We measured diastolic dysfunction using mitral E/A ratios because it is the standard of care in our setting. Although rigorous TDI in addition to E/A ratios from conventional Doppler studies was necessary to unmask pseudonormalization, it was not done due to lack of capacity. This may result in missing up to 25% of cases of diastolic dysfunction with pseudonormalization. Therefore the prevalence of diastolic dysfunction we are reporting may be an under estimate of a bigger problem. Patients with clinical heart failure were not excluded and this may confound our findings because patients with heart failure may develop diabetes and conversely diabetics are at risk of heart failure. This is one of the limitations in this study.
We differentiated type1 and type 2 DM using clinical assessment because we could not assess insulin levels which is the gold standard: this is a limitation for this study.
We were unable to enroll the desired number of participants according to the sample size estimated. This was due to limitations in logistics for the required investigations.
Conclusion
Overall prevalence of an abnormal echocardiogram among newly diagnosed diabetic patients was high at 67.8% with majority having diastolic dysfunction as the main abnormality. Left ventricular hypertrophy, systolic dysfunction and regional wall abnormalities were common findings in this study. Traditional CVD risk factors including: Advancing age, hypertension, type 2 DM, obesity and increasing waist circumference were associated with an abnormal echocardiogram in this population. Microalbuminuria, a known CVD risk factor measured using urine ACR was not associated with any of the echocardiographic abnormalities despite a high prevalence at 54%. Similarly HbA1C, which is a measure of glycemic control, was not associated with echocardiographic abnormalities although this patient population had profound hyperglycemic states: mean HbA1C was 13.4%.
Table 1.
Characteristics of newly diagnosed diabetic patients at Mulago National referral hospital who participated in the study.(N=202)
| Characteristic | Total (N) | Total | Abnormal | Abnormal | |
| (%). | echo(N). | echo(%). | |||
| Gender | Male | 102 | 50.5 | 63 | 46.0 |
| Female | 100 | 49.5 | 74 | 54.0 | |
| Employment | Employed | 74 | 36.4 | 50 | 36.5 |
| Unemployed | 128 | 63.6 | 87 | 63.5 | |
| Pregnancy | Yes | 5 | 5.0 | 2 | 2.7 |
| No | 95 | 95.0 | 72 | 93.3 | |
| Education | None | 18 | 8.9 | 17 | 12.4 |
| Primary | 78 | 38.6 | 50 | 36.5 | |
| Secondary | 75 | 37.1 | 49 | 35.8 | |
| Tertiary | 31 | 15.4 | 21 | 15.3 | |
| Marital status | Never married | 30 | 14.9 | 14 | 10.2 |
| Currently married |
118 | 58.4 | 84 | 61.3 | |
| No longer married |
54 | 26.7 | 39 | 28.5 | |
| DM type | Type 1 | 41 | 20.3 | 15 | 10.9 |
| Type 2 | 156 | 77.2 | 120 | 87.6 | |
| Secondary | 0 | 0.0 | 0.0 | 0.0 | |
| Gestational | 5 | 2.5 | 2 | 1.5 | |
| BP status | Normotensive | 77 | 38.1 | 42 | 30.7 |
| Hypertensive | 125 | 61.9 | 95 | 69.3 | |
| Microalbumin | Absent | 79 | 44.9 | 52 | 44.1 |
| In urine | Present | 97 | 55.1 | 66 | 55.9 |
| BMI | Underweight | 40 | 19.8 | 22 | 16.1 |
| Normal weight | 76 | 37.6 | 49 | 35.8 | |
| Over weight | 49 | 24.3 | 36 | 26.3 | |
| Obesity | 37 | 18.3 | 30 | 21.9 | |
| Waist | Normal | 121 | 59.9 | 84 | 66.7 |
| circumference | Abnormal | 81 | 40.1 | 42 | 33.3 |
| Waist hip | Normal | 141 | 69.8 | 92 | 67.2 |
| ratio | Abnormal | 61 | 30.2 | 45 | 32.9 |
| HbA1C | Normal | 15 | 8.4 | 11 | 9.2 |
| Abnormal | 164 | 91.6 | 109 | 90.8 | |
| Drugs | ACEI/ARBS | 19 | 33.9 | 19 | 41.3 |
| CCB | 15 | 26.8 | 13 | 28.3 | |
| Diuretics | 3 | 5.4 | 3 | 6.5 | |
| Beta blockers | 5 | 8.9 | 4 | 8.7 | |
| Not on drugs | 30 | 55.6 | 21 | 47.7 |
Table 3.
Prevalence of echocardiographic abnormalities among newly diagnosed diabetic patients at Mulago hospital (N=202)
| Characteristic | Normal | Abnormal | CI (95%) |
| Echocardiogram | 65(32.2) | 137(67.8) | (60.0–74.0) |
| Isolated Diastolic function | 91(45.0) | 111(55.0) | (47.8–61.9) |
| Isolated Systolic function | 158(78.2) | 44(21.8) | (16.3–28.1) |
| Combined systolic and diastolic function | 175(86.6) | 27(13.4) | (8.9–18.8) |
| Left ventricular hypertrophy | 163(80.7) | 39(19.3) | (14.1–25.4) |
| Wall motion | 194(96.0) | 8(4.0) | (1.7–7.6) |
* Note: CI= Confidence Interval
Table 4.
Logistic regression for factors associated with an abnormal echocardiogram among newly diagnosed diabetic patients at Mulago hospital
| Factor | Echocardiogram | Crude OR(CI). |
P-value | Adjusted OR |
P-value | ||
| Normal No(%) |
Abnormal No(%) |
||||||
| Sex | Male | 39(60.0) | 63(46.0) | 1 | |||
| Female | 26(40.0) | 74(54.0) | 1.76(0.97–3.21) | 0.064 | 1.46(0.62–3.45) | 0.3810 | |
| Age | 1.09(1.06–1.12) | <0.0001 | 1.09(1.05–1.15) | <0.0001 | |||
| HbA1C | Normal | 4(6.78) | 11(9.2) | 1 | |||
| Abnormal | 55(93.2) | 109(90.8) | 0.72(0.22–2.37) | 0.589 | |||
| BP status | Normotensive | 35(53.9) | 42(30.7) | 1 | |||
| Hypertensive | 30(46.2) | 95(69.3) | 2.64(1.44–4.85) | 0.002 | 0.89(0.37–2.19) | 0.8101 | |
| Microalbuminuria | Normal | 27(46.6) | 52(44.1) | 1 | |||
| Abnormal | 31(53.5) | 66(55.9) | 1.11(0.59–2.08) | 0.756 | |||
| BMI | Underweight | 18(27.7) | 22(16.1) | 1 | |||
| Normal weight | 27(41.5) | 49(35.8) | 1.49(0.68–3.24) | 0.321 | 1.09(0.41–2.88) | ||
| Overweight | 13(20.0) | 36(26.3) | 2.27(0.93–5.51) | 0.071 | 1.46(0.44–4.89) | ||
| Obesity | 7(10.8) | 30(21.9) | 3.51(1.25–9.84) | 0.017 | 2.30(0.57–9.30) | 0.6241 | |
| Waist/Hip ratio | 14.5(0.4–474.9) | 0.134 | 0.80(0.32–2.05) | 0.6484 | |||
| Waist circumference | 1.02(1.00–1.04) | 0.024 | |||||
* Note: OR= Odds ratio, CI= Confidence Interval
Some figures may not sum to total due to missing data.
Adjusted for sex, hypertension, BMI and waist hip ratio.
Acknowledgement
Research reported in this manuscript was supported by the Fogarty International Center of the National Institutes of Health under Award Number R24TW008861. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are grateful to the following persons for their invaluable support: Prof. Nelson Sewankambo, Prof. Moses R. Kamya, the staff of ward 4B endocrine, diabetic clinic, the echocardiography and clinical laboratory of Mulago hospital.
Abbreviations
- DM
Diabetes Mellitus
- CVD
Cardiovascular Disease
- LVH
Left Ventricular Hypertrophy
- ECHO
Echocardiography (Transthoracic)
- ECG
12-lead Rest Electrocardiography
- MR
Mitral Regurgitation
- AR
Aortic Regurgitation
- LVEF
Left Ventricular Ejection Fraction
- IVS
Interventricular septum
- LVPW
Left ventricular posterior wall
- TDI
Tissue Doppler Imaging
References
- 1.ADA, author. Standards of medical care in diabetes. Diabetes Care. 2013;36(supplement 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodarz D, Carlene M, Lawes, Stephen V, Hoorn, Christopher J, et al. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet. 2006;368:1651–1659. doi: 10.1016/S0140-6736(06)69700-6. [DOI] [PubMed] [Google Scholar]
- 3.Amos A, McCarty D. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(suppl 5):S1–S85. [PubMed] [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.André PK, Albert GB, Amoah, Mbanya Jean-Claude. Cardiovascular Complications of Diabetes Mellitus in Sub-Saharan Africa. Circulation. 2005;112:3592–3601. doi: 10.1161/CIRCULATIONAHA.105.544312. [DOI] [PubMed] [Google Scholar]
- 6.Motala A, Omar MA, Pirie FJ. Diabetes in Africa: epidemiology of type1 and type 2 diabetes in Africa. J Cardiovasc Risk. 2003;10:77–83. doi: 10.1097/01.hjr.0000060843.48106.31. [DOI] [PubMed] [Google Scholar]
- 7.Cities in a Globalizing World: Global Report on Human Settlements 2001. London, Sterling, Va: Earthscan Publications; 2001. [Google Scholar]
- 8.Geiss L, Herman W H, Smith PJ, National Diabetes Data Group . Diabetes in America. 2 edn. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 1995. Mortality in non-insulin-dependent diabetes; pp. 233–258. [Google Scholar]
- 9.Zvonko M, Itamar Raz, Scott D, Beattie, Barbara N, Campaigne Samiha, Sarwat M, Elwira Gromniak, et al. Natural History of Cardiovascular Disease in Patients With Diabetes Role of hyperglycemia. Diabetes care. 2008 Feb;31(suppl 2):S155–S160. doi: 10.2337/dc08-s240. [DOI] [PubMed] [Google Scholar]
- 10.Piyush M, Srivastava Paul Calafiore, Richard J, et al. Prevalence and predictors of cardiac hypertrophy and dysfunction in patients with Type 2 diabetes. Clinical Science. 2008;114:313–320. doi: 10.1042/CS20070261. [DOI] [PubMed] [Google Scholar]
- 11.Kish aL. survey sampling. John Wiley and Sons NY; 1965. [Google Scholar]
- 12.Lang RM, Bierig M, Devereux R B, Flachskampf F A, Foster E, Pellikka P A, et al. Recommendations for chamber quantification: A report fromthe American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Somaratne JB, Whalley GA, Bagg W, Doughty RN. Early detection and significance of structural cardiovascular abnormalities in patients with Type 2 diabetes mellitus. Expert Rev Cardiovasc Ther. 2008;6:109–125. doi: 10.1586/14779072.6.1.109. [DOI] [PubMed] [Google Scholar]
- 14.Bryan W, Sheila K, et al. Prevalence, predictors and evolution of echocardiographically defined cardiac abnormalities in adults with type 1 diabetes: an observational cohort study. Journal of Diabetes and Its Complications. 2014;28:22–28. doi: 10.1016/j.jdiacomp.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Sotonye T, Dodiyi-Manuel, Akpa Maclean R, Odia Osaretin J. Left ventricular dysfunction in normotensive type II diabetic patients in Port Harcourt, Nigeria. Vascular Health and Risk Management. 2013;9:529–533. doi: 10.2147/VHRM.S44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacomo Faden, et al. The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: Data from the SHORTWAVE study. Diabetes Research and Clinical Practice. 2013;07:004. doi: 10.1016/j.diabres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen M T, et al. Transthoracic echocardiographic abnormalities in asymptomatic diabetic patients: Association with microalbuminuria and silent coronary artery disease. Ann Cardiol Angeiol (Paris) 2013;62(1):3–7. doi: 10.1016/j.diabet.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Poulsen Mikael Kjaer, et al. Left Ventricular Diastolic Function in Type 2 Diabetes Mellitus: Prevalence and Association With Myocardial and Vascular Disease. Circ Cardiovasc Imaging. 2010;3:24–31. doi: 10.1161/CIRCIMAGING.109.855510. [DOI] [PubMed] [Google Scholar]
- 19.Boyer J, Thanigaraj S, Schechtman KB, Perez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–875. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with wellcontrolled type 2 diabetes mellitus. Am J Cardiol. 2001;87:320–323. doi: 10.1016/s0002-9149(00)01366-7. [DOI] [PubMed] [Google Scholar]
- 21.Ojji D, Parsonage W, Dooris M, et al. Left ventricular diastolic function in normotensive type-2 diabetic subjects. J Natl Med Assoc. 2008 Sep;100(9):1066–1072. doi: 10.1016/s0027-9684(15)31446-2. [DOI] [PubMed] [Google Scholar]
- 22.Yaméogo NV, et al. Study of echocardiographic parameters of type 2 black African diabetics at high cardiovascular risk. A cross-sectional study of 79 cases in Senegal. Ann Cardiol Angeiol (Paris) 2013;62(1):3–7. doi: 10.1016/j.ancard.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Somaratne Jithendra B, et al. Screening for left ventricular hypertrophy in patients with type 2 diabetes mellitus in the community. Cardiovascular Diabetology. 2011;10:29. doi: 10.1186/1475-2840-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panithaya C, Sorajja Paul, Rajagopalan Navin, Miller Todd D, et al. Prevalence and prognosis of left ventricular systolic dysfunction in asymptomatic diabetic patients without known coronary artery disease referred for stress single-photon emission computed tomography and assessment of left ventricular function. American Heart Journal. 2007;154(3) doi: 10.1016/j.ahj.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 25.Cooper R, Rotimi C, Ataman S, McGee D, et al. The prevalence of hypertension in sevenpopulations of west African origin. Am J Public Health. 1997;87:160–168. doi: 10.2105/ajph.87.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kengne A, Mbanya JC. Cardiovascular risk profile of newly diagnosed type 2 diabetic patient at the central Hospital of Yaounde [final dissertation, end-of-specialisation in internal medicine] Yaounde: The University of Yaounde; 2004. p. 1. [Google Scholar]
- 27.Sowers J. Recommendations for special populations: diabetes mellitus and the metabolic syndrome. Am J Hypertens. 2003;16:41S–50S. doi: 10.1016/j.amjhyper.2003.07.009. (11 pt 2) [DOI] [PubMed] [Google Scholar]
- 28.Bella J, Palmieri V, Roman MJ, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the StrongHeart Study. Circulation. 2002;105:1928–1933. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]