Abstract
Background
Hookworm eggs identification and quantification is usually carried out by Kato-Katz method. However various structures present in the smear may be confused with eggs of such parasites.
Objective
To document the presence of structures in Kato-Katz slides that could initially be misinterpreted as hookworm eggs.
Method
497 faecal samples were analysed by Kato-Katz technique, diphasic concentration technique, agar-plate coprocultive and larvae obtained were analysed by PCR and characterized by sequencing.
Result
Hookworm-like eggs were found in 159 (32%) of the samples by Kato-Katz, finally identified as Caenorhabditis elegans by PCR technique.
Conclusion
The diagnosis of human hookworm eggs, only by the use of Kato-Katz technique can lead to false positives because of similarities with eggs of other free-living worms, from wet soils like those of Rwanda that could contaminate stool samples.
Keywords: Hookworm eggs, Kato-Katz method, misclassification, Rwanda
Introduction
Soil Transmitted Helminths (STH) infect more than 1 billion people worldwide and is an enormous problem of public health in tropical countries1. A national survey in Rwanda gave data of 66% prevalence of STH, being hookworm infection of about 31%2.
One of the most commonly stool examination techniques applied for detection of STH ova in stools is the Kato-Katz technique3–5 because of its easy use in the field, its low cost and its non-invasive nature. For those reasons, the Kato-Katz technique is the method of choice in parasitological surveys6,7. However, for the detection of hookworms this technique has a relatively poor sensitivity5, this may occur, because of the rapid clearing of hookworm eggs with time5,8. A long period from ejection of faeces until the preparation of slides, together with the time from Kato-Katz preparation until the beginning of the microscope examination, makes this technique less sensitive4. In addition, another problem is the similar shape of hookworm eggs with other structures, diverse in nature, which may lead to erroneous diagnoses.
The purpose of this report was to document the presence of eggs in Kato-Katz slides that could initially be misinterpreted as hookworm eggs.
Methods
Samples: In this cross-sectional study a total of 497 faecal samples from a population of children (aged between 6 and 18 years old; mean: 11.0; SD: 2.32) from the rural area of Nemba Sector, that it is placed in the Gakenke District (located at an altitude between 1.700 and 2.700 meters), on the Northern Province of Rwanda, were collected during the dry season between August and September of 2011. For the samples collection, children were instructed to place the sample directly on the plastic collection tube. Samples were analysed by a) diphasic concentration technique, to know the entire parasite spectrum6; b) Kato-Katz technique for the identification and quantification of eggs of helminths9, and c) by agar-plate coproculture for the detection and identification of STH species with free-living larvae10. Considering that eggs of different hookworm species are difficult to distinguish and can be confused with those of hookworm-like species, to avoid this, proper identification can be made after hatching the eggs and cultivating larvae11.
Ethics statement: Written informed consent was obtained from each parent or tutor before sampling. The research protocol was reviewed and approved by the local authorities and by the bioethics committee of the University Miguel Hernández de Elche, Alicante, Spain (record num: DF-MPA-001-11).
Molecular characterization: DNA extraction was done from the pellet obtained after centrifugation of larvae culture preserved in ethanol 70% (6.000 rpm in a bench top microcentrifuge for 10 minutes) after washing with PBS 1% twice. DNA extractions were done with the commercial kit QIAamp DNA Mini Kit (Quiagen®, Barcelona, Spain) following the manufacturer's instructions. Finally, the DNA were eluted in 50 µl of elution buffer provided with the kit. PCR were done with oligonucleotide primers NC1 and NC2 designed to regions of the 5.8S and 28S respectively, to amplifying a 450–580 bp region that found to be conserved across some helminthes groups12. Direct sequencing of the PCR products was performed with NC1 and NC2 primers using the Big-Dye Terminator Cycle Sequencing Ready Reaction Kit V3.1 and the automated sequencer “3730 DNA analyzer” (Applied Biosystems, Foster City, CA). Sequences obtained were analyzed and edited using MEGA 6 software (Molecular Evolutionary Genetic Analysis)13 and compared to GenBank® database (U.S. National Library of Medicine, Bethesda, USA).
Results
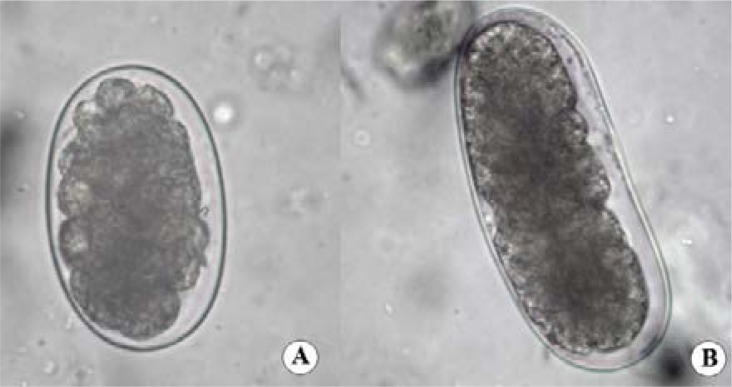
In the 497 faecal samples analyzed by Kato-Katz, in 159 samples (32%; IC95%: 0.2–0.3) we found the presence of eggs that were similar to those of hookworm (Figure 1A), named as hookworm-like eggs (Figure 1B and 1C). A total of 82 (51.1%; IC95%: 43.5–59.5) were from males and 77 (48.4%; IC95%: 40.4–56.4) were from females, mostly belonging to children who aged 9 years old (16.4%; IC95%: 10.9–23.0).
Figure 1.
Hookworm egg in Kato-Katz technique (x400) (A); Hookworm-like egg in Kato-Katz slide (x400) (B), Hookworm-like eggs in Kato-Katz slide (x100) (C).
Applying the diphasic concentration technique in the 159 samples with hookworm-like eggs, only 2 samples (1.2%; IC95%: 0.1–4.4) presented the typical oval round ends of hookworm egg, with a range of size of 62.5 to 67.2 µm × 40 to 42.5 µm (average size: 65.0x 41.6 µm) (Figure 2A).
Figure 2.
Hookworm egg (Diphasic concentration technique × 400) (A); Hookworm-like egg (Diphasic concentration technique × 400) (B).
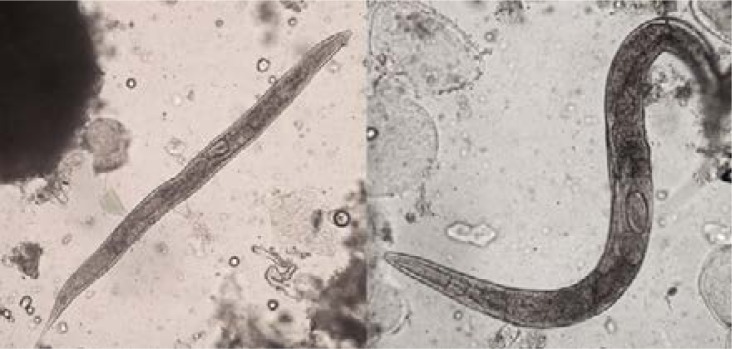
In the remaining 157samples (98.7%; IC95%: 95.5–99.8), hookworm-like eggs with a slightly concave side and with range of size of 85.0 to 92.5 µm × 35.0 to 40.0 µm (average size: 89.1 × 36.8 µm) were observed (Figure 2B). Moreover, female adult worms were also observed in two of those 157 samples (Figure 3).
Figure 3.
Adult worms of C. elegans (Diphasic concentration technique × 100)
Comparing the results obtained by both techniques, and considering the diphasic concentration technique more precise than the Kato-Katz, the specificity (68%), sensibility (100%) and positive (1.3%) and negative (100%) predictive values intended for Kato-Katz, revealed its limitations in the correct identification of hookworm eggs.
Finally, sequence of the 500bp band obtained by PCR, from the larvae of the 159 Kato-Katz positive-samples agar-plate culture (Figure 4), matched with Caenorhabditis elegans, a free-living nematode.
Figure 4.
Larvae of C. elegans in water drop from the coprocultive (vision through electronic loupe).
Discussion
A brief review of other studies showed frequent problems in the identification of hookworm eggs by microscopy. It is often reported as misdiagnosis of hookworm eggs with other nematode species. Some researchers14–17 have found eggs that initially were classified as hookworm eggs, but with further analysis it was proved that these eggs were of Trichostrongylus spp. Other studies15,18 also affirm that confusion between hookworms eggs and Oseophagostum bifurcum it is possible because of similarity of eggs, because of that, a study19 used a multiplex real-time PCR to achieve the accurate distinction between hookworms and O. bifurcum. In addition, another study15 affirms that hookworm eggs can also be confused with Ternidens deminutus eggs.
Also happens that eggs similar to those of hookworms could be from plant parasitic nematode eggs, as it is the case of Meloidogyne spp.20 and Heterodera spp.15,21–23
Finally, confusion may also occur with mite eggs15,23,24 and even artefacts such as plant cells22,23.
In the present work, it was observed that eggs of the free-living nematode C. elegans, which can be found in humus, compost heaps, garden soil, flowers and fruits who have fallen on the ground25, can lead to a misclassification with hookworm eggs mainly by Kato-Katz. The limitations found in this Kato-Katz technique in the correct identification of hookworm eggs, highlight the use of several diagnostic techniques, as can be the diphasic concentration and/or PCR, in order to perform an adequate diagnosis of hookworm.
Since the stool samples studied in the present work proceeds from northern Rwanda, a rural tropical area, it is important to keep in mind the high incidence of Caenorhabditis species in these wet tropical areas26.
Conclusion
The diagnosis of human hookworm eggs only by the use of Kato-Katz technique, can lead to the occurrence of false positive results, due to similarity with those of other free-living worms that may be present in the stools. Because of this, to give appropriate instructions for a correct stool sample collection, without any contact with soil, land, animal faeces, etc., it is extremely important. Moreover, the use of several diagnostic techniques, to achieve a correct diagnosis of hookworm species, results evident.
Our results, together with the brief bibliographic review, emphasize the importance not only of the correct identification of the structures found in faecal samples, but also the importance of avoiding sample contamination from soil material, even when people were instructed correctly about how to proceed with the collection of the samples.
References
- 1.Brooker S, Clements ACA, Bundy DAP. Global epidemiology, ecology and control of soil-transmitted helminth infections. Advances in Parasitology. 2006;62:221–261. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staudacher O, Heimer J, Steiner F, et al. Soil-transmitted helminths in southern highland Rwanda: associated factors and effectiveness of school-based preventive chemotherapy. Tropical Medicine and International Health. 2014;19(7):812–824. doi: 10.1111/tmi.12321. [DOI] [PubMed] [Google Scholar]
- 3.Goodman D, Haji HJ, Bickle QD, et al. A Comparison of methods for detecting the eggs of Ascaris, Trichuris, and Hookworm in infant stool, and the epidemiology of infection in Zanzibari infants. The American Journal of Tropical Medicine and Hygiene. 2007;76(4):725–731. [PubMed] [Google Scholar]
- 4.Knopp S, Mgeni AF, Khamis IS, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Neglected Tropical Diseases. 2008;2(11):e331. doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarafder MR, Carabin H, Joseph L, Balolong E, Olveda R, McGarvey ST. Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a ‘gold standard’. International Journal for Parasitology. 2010;40(4):399–404. doi: 10.1016/j.ijpara.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO), author Bench aids for the diagnosis of intestinal parasites. 1994. [Google Scholar]
- 7.Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli LA. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: World Health Organization; 1998. pp. 1–48. [Google Scholar]
- 8.Dacombe RJ, Crampin AC, Floyd S, et al. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101(2):140–145. doi: 10.1016/j.trstmh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Kato K, Miura M. Comparative examinations. Japanese Journal of Parasitology. 1954;3(5) [Google Scholar]
- 10.Arakaki T, Iwanga M, Kinjo F, Saito A, Asato R, Ikeshiro T. Efficacy of agar-plate culture in detection of Strongyloides stercoralis infection. The Journal of Parasitology. 1990;76:425–428. [PubMed] [Google Scholar]
- 11.Jozefzoon LM, Oostburg BF. Detection of hookworm and hookworm-like larvae in human fecocultures in Suriname. The American Journal of Tropical Medicine and Hygiene. 1994;4:501–505. [PubMed] [Google Scholar]
- 12.Gasser RB, Chilton NB, Hoste H, Beveridge I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Research. 1993;21(10):2525–2526. doi: 10.1093/nar/21.10.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular biology and Evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ralph A, O'Sullivan MVN, Sangster NC, Walker JC. Abdominal pain and eosinophilia in suburban goat keepers-trichostrongylosis. Medical Journal of Australia. 2006;184(9):467–469. doi: 10.5694/j.1326-5377.2006.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 15.Thibert JB, Guiguen C, Gangneux JP. Diagnostic microscopique différentiel des oeufs de type Ankylostomidé: à propos d'un cas deTrichostrongyloïdose. Annales de Biologie Clinique. 2006;64(3):281–285. [PubMed] [Google Scholar]
- 16.Yong TS, Lee JH, Sim S, et al. Differential diagnosis of Trichostrongylus and Hookworm eggs via PCR using ITS-1 sequence. The Korean Journal of Parasitology. 2007;45(1):69–74. doi: 10.3347/kjp.2007.45.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradbury RS, Males C, Goldmisd JM. An unusual helminth infection in a South East Asian refugee. Annals of the Australasian College of Tropical Medicine. 2010;11(1):16–18. [Google Scholar]
- 18.Goldsmid JM. The differentiation of Ternidens deminutus and hookworm ova in human infections. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1968;62(1):109–116. doi: 10.1016/0035-9203(68)90038-2. [DOI] [PubMed] [Google Scholar]
- 19.Verweij JJ, Brienen EAT, Ziem J, Yelifari L, Polderman AM, Van Lieshout L. Simultaneous detection and quantification of Ancylostoma Duodenale, Necator Americanus, and Oesophagostomum bifurcum in fecal samples using multiplex Real-Time PCR. The American Journal of Tropical Medicine and Hygiene. 2007;77(4):685–690. [PubMed] [Google Scholar]
- 20.Cantos GA, Dutra RL, Benedet NS. Estudo de ovos de Meloidogyne Spp em fezes humanas. News Lab. 2004;65:132–136. [Google Scholar]
- 21.Sandground JH. Oxyuris incognita Or Heterodera radicicola? The Journal of Parasitology. 1923;10(2):92–94. [Google Scholar]
- 22.Colmer-Hamood JA. Fecal microscopy artifacts mimicking ova and parasites. Lab Medicine. 2001;32(2):80–84. [Google Scholar]
- 23.Ash LR, Orihel TC. Atlas de Parasitología Humana. 5th ed. Argentina: Editorial Médica Panamericana; 2010. [Google Scholar]
- 24.Werneck JS, Carniato T, Gabriel A, Tufik S, Andrade SS. Mites in clinical stool specimens: potential misidentification as helminth eggs. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101(11):1154–1156. doi: 10.1016/j.trstmh.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Barrière A, Félix MA. Isolation of C. elegans and related nematodes. WormBook: The Online Review of C. elegans Biology. 2014. [August 2014]. Available at: http://www.ncbi.nlm.nih.gov/books/NBK19764/. [DOI] [PubMed]
- 26.Félix MA, Jovelin R, Ferrari C, et al. Species richness, distribution and genetic diversity of Caenorhabditis nematodes in a remote tropical rainforest. BMC Evolutionary Biology. 2013;13:10. doi: 10.1186/1471-2148-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]