Summary
Background
rotator cuff muscle atrophy, fibrosis and fatty infiltration are common complications after large and massive rotator cuff tears. Currently, there are no effective treatments for these muscle pathologies after injury. Furthermore, the cellular source for fibrotic and adipose tissues in rotator cuff muscle after injury remains unknown. In this study, we proposed that two groups of muscle resident progenitors, Tie2+ muscle mesenchymal progenitors and PDGFRα+ fibro/adipogenic progenitor cells (FAPs), contribute significantly to rotator cuff muscle fibrosis and fatty infiltration.
Methods
we tested our hypothesis using reporter mice. Rotator cuff muscles from Tie2-GFP and PDGFRα-GFP reporter mice were harvested at 2 and 6 weeks after unilateral massive rotator cuff tear surgeries. Immunofluorescent staining for fibroblast and adipocyte markers was conducted.
Results
our results showed significant co-localization of Tie2+ cells with fibrotic markers vimentin and αSMA. In the PDGFRα-GFP reporter mice, GFP signal was seen in only a small fraction of cells staining positive for vimentin and αSMA. However, PDGFRα showed significant co-localization with adipocyte markers, including PPAR-γ, adiponectin, and perilipin A. Oil red O staining confirmed that the mature adipocytes appearing in rotator cuff muscles after injury are also PDGFRα+.
Conclusion
these data demonstrated that the Tie2+ muscle mesenchymal progenitors are the major source of fibroblasts while PDGFRα+ FAPs are the major source of adipocytes in rotator cuff muscle fatty infiltration. Basic Science Study.
Keywords: rotator cuff tear, fatty infiltration, fibrosis, fibro-adipogenic progenitors, Tie2, muscle
Introduction
Rotator cuff (RC) tears are one of the most common tendon injuries seen in orthopedic patients. Secondary muscle degradation, which includes muscle atrophy, fibrosis, and fatty infiltration (FI), after injury is a critical factor that determines the clinical outcome of patients. Massive RC tears have been found to be associated with atrophy and FI of both the supraspinatus and infraspinatus muscles in multiple studies. Poor outcomes and high re-tear rates after surgery are thought to be due in part to these changes within the muscles1, and have been demonstrated to directly correlate with the amount of muscle atrophy and FI2–5. Although fibrosis is not well described in RC injuries, recent studies have linked poor muscle function to increased expression of fibrotic markers following RC injury6,7. Fibrosis is also found after other muscle injuries and has been shown to directly cause muscle dysfunction in muscular dystrophy. Traditionally, it is thought that atrophy and FI are markers of end-stage RC disease and do not appear after smaller tears. However, recent data suggest that smaller tears can also result in atrophy and FI and may affect long term overall prognoses8. Patients who have disruption of the anterior supraspinatus rotator cuff cable exhibit a higher prevalence of FI in the supraspinatus than with similar tears within the central portion of the supraspinatus, even with small and medium sized tears8. Thus, muscle changes including FI are likely important even in smaller and medium-sized tears.
Currently, a critical gap in our understanding of the pathophysiology of fibrosis and FI after RC tears is knowledge of the cellular source of fibroblasts and adipocytes in RC muscle after tendon tears. Multiple stem cell and progenitor cell populations, including circulating bone marrow mesenchymal stem cells (BM-MSCs) and focal muscular progenitor cells, possess fibrogenic and adipogenic potential and thus could be a potential source of rotator cuff fibrosis and FI. Recent studies also proved the adipogenic potential of a Tie2+ muscle-resident progenitor cell population9. Furthermore, a novel stem cell line, termed fibro/adipogenic progenitors (FAPs), which does not arise from the myogenic lineage, was shown to be capable of giving rise to either fibroblasts or adipocytes with the correct stimulus10,11. Although these cells have a supportive role in myogenesis during development, FAPs can give rise to ectopic fibroblasts and adipocytes that accumulate in degenerating muscles. Recent studies have demonstrated that FAPs are the major source of adipocytes in muscle FI following other injury mechanisms11,12. Additionally, it has been demonstrated that FAPs are characterized with the surface marker platelet-derived growth factor receptor alpha (PDGFRα) in both human and rodent skeletal muscles, thus making cellular tracking of these cells possible in vivo13,14.
The goal of this study was to identify the major cellular sources of fibrosis and fatty infiltration in rotator cuff muscles after injury in a mouse model. Specifically, we focused on two primary muscle progenitor cell populations that had a high likelihood of transitioning into fibroblasts and adipocytes, investigating the contribution of Tie2+ progenitors and PDGFRα+ FAPs in rotator cuff muscles using GFP reporter mice. We hypothesized that either Tie2+ mesenchymal progenitors or FAP cells would be the dominant stem cell source of fibroblasts and/or adipocytes in RC muscles after tendon injuries in a mouse model.
Materials and methods
All research was conducted ethically according to international standards15.
Reporter mouse strains
Inducible Tie2-specific GFP-tagged transgenic mice were bred by mating mice expressing the tetracycline transactivator (tTA) under control of the endothelial lineage-selective Tie-2 promoter with tetO-Histone-GFP mice. Expression of GFP in Tie2+ progenitors was induced by maintaining the Tie2-GFP animals with tetracycline-free food following gestation.
PDGFRα-specific green fluorescent protein (GFP) transgenic reporter mice were purchased from Jackson Laboratories, Inc. (Stock #007669, Sacramento, CA). These mice express the H2B-eGFP fusion gene from the endogenous PDGFRα locus. Fluorescence patterns in tissues mimic the expression pattern of the endogenous gene. Previous studies have demonstrated the viability of this reporter mouse model for in vivo cell tracing for adipogenesis16,17.
Massive rotator cuff tear surgery
Twelve three-month-old PDGFRα-GFP and twelve Tie2-GFP mice (6 males and 6 females for each strain) were used in this study. In addition, 12 wild-type C57/BL6 mice were used as non-labeled controls. Massive rotator cuff tears were induced unilaterally by complete transection of the supraspinatus and infraspinatus tendons along with transection of the suprascapular nerve according to an established mouse model for massive rotator cuff tears18. Sham surgery was performed on the opposite side to serve as an internal control. All procedures were approved by our Institutional Animal Care and Use Committee.
Muscular harvesting and histology
Animals were sacrificed at two and six weeks after surgery (3 males and 3 females in each strain at each time point). Supraspinatus muscles from both shoulders were harvested and flash frozen in liquid nitrogen-cooled 2-methylbutane. The muscles were then cryosectioned at a thickness of 10μm. Immunofluorescence (IF) staining for adipogenic markers PPAR-γ, adiponectin, and perilipin A, as well as for fibrogenic markers vimentin and αSMA, was performed according to a protocol by Hemmingsen, et al.19. Sections were blocked and incubated in the respective primary antibodies overnight at a dilution range of 1:75–1:200. A rhodamine-conjugated goat-anti-rabbit secondary antibody was used at a dilution of 1:5000. After three washes in 1× PBS, sections were mounted with Vecta Shield with DAPI, a nuclear stain. Oil red O staining was performed according to a protocol by Liu et al. to identify fat deposition through presence of mature adipocytes18.
Image capture and quantification
To measure the contribution of GFP+ cells to adipogenesis and fibrosis, whole-section images for each muscle were captured after immunofluorescent staining with at 360 nm (DAPI), 480 nm (GFP), and 570 nm (rhodamine) using an Axio Observer D1 fluorescence microscope. Using image analysis software, color channels were split into blue, green, and red. DAPI+, GFP+, and rhodamine+ cells were quantified in each muscle sample. To determine GFP/rhodamine double positive cells, images for green and red channels in each sample were overlapped. GFP+, rhodamine+ and GFP+/rhodamine+ cells were quantified manually in each section by two blinded reviewers. The percentage of GFP and rhodamine double positive cells within all rhodamine+ cells in each muscle was calculated as GFP+ rhodamine+ cell number ÷ rhodamine+ cell number × 100%. Overlap between Tie2+ cells and adipogenic markers was negligible, and therefore not quantified. Data was run through a chi-squared test to confirm p<0.05 and is presented as mean ± standard error.
Results
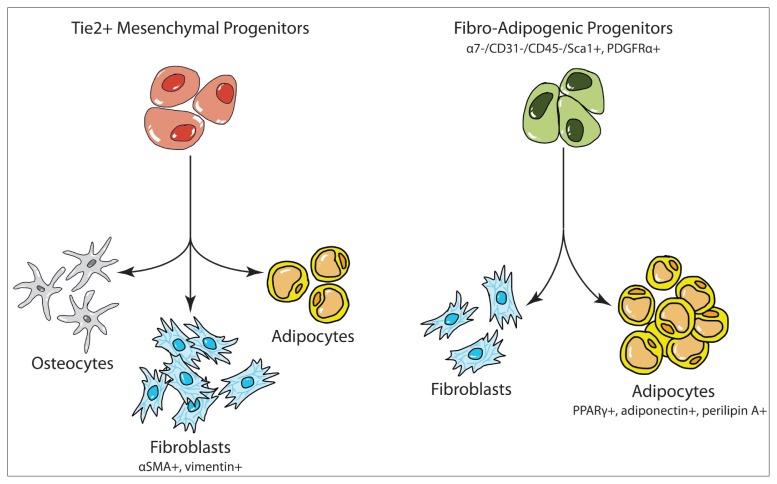
Using reporter mice, two muscle-resident progenitor cell populations, Tie2+ cells and PDGFRα+ FAP cells, were examined for their respective abilities to differentiate into adipocytes and fibroblasts after injury (Fig. 1).
Figure 1.
Proposed differentiation pathways for Tie2+ cells and FAP cells.
Adipogenic differentiation is found only in PDGFRα+ cells
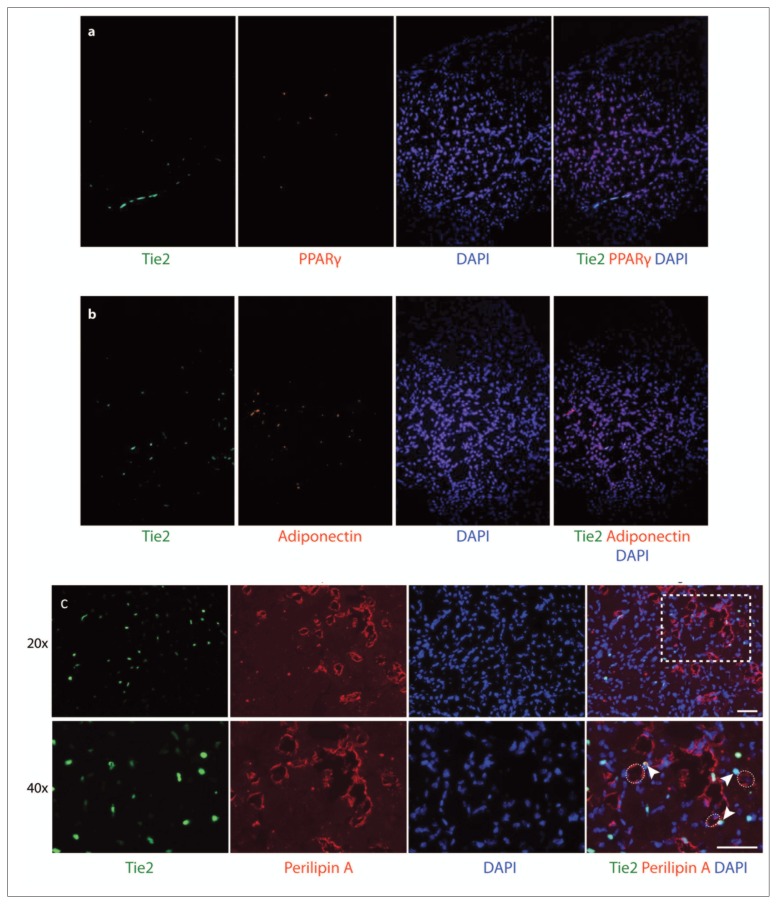
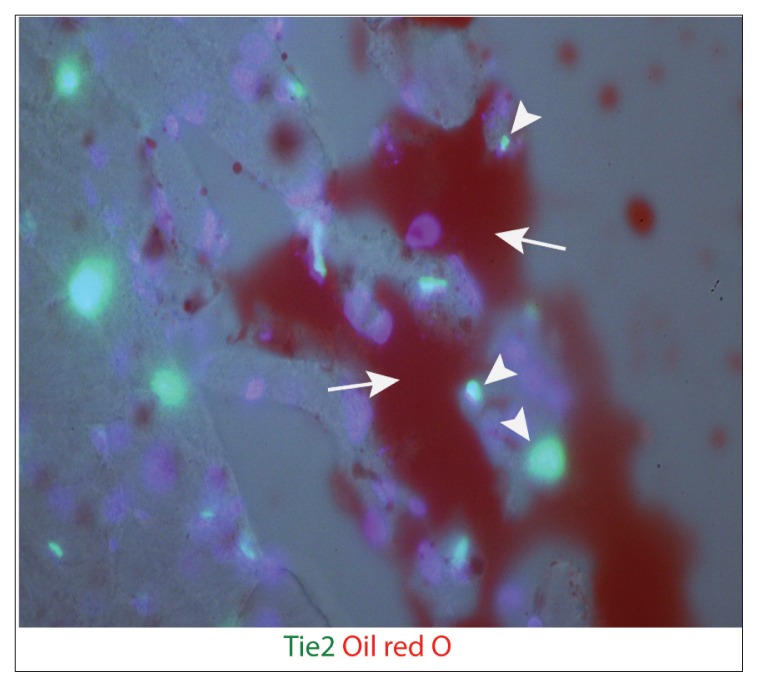
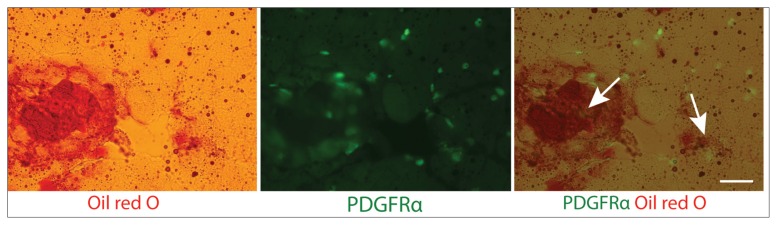
Supraspinatus muscles from both Tie2-GFP reporter mice and PDGFRα-GFP reporter mice were analyzed using immunofluorescence to assess adipogenic differentiation six weeks after a massive tendon tear and denervation. Staining of Tie2-GFP sections showed that Tie2+ cells were negative for PPAR-γ and adiponectin (Fig. 2 a, b). Staining for perilipin A, a surface marker for lipid droplets, showed localization of the Tie2 signal just adjacent to the perilipin+ fat cells (Fig. 2 c). Furthermore, oil red O stains revealed that mature adipocytes were often found adjacent to the endogenous GFP signal but did not overlap with Tie2+ cells (Fig. 3). This suggests that Tie2+ progenitor cells may contribute to the development of adipocytes in rotator cuff muscle FI, but that these cell populations are not the source of fat cells.
Figure 2.
Behavior of Tie2+ cells in fatty infiltration of muscle six weeks post-injury. Tie2-GFP reporter mouse supraspinatus muscle sections were subjected to immunofluorescence staining for (a) middle-stage adipogenesis marker PPAR-g, (b) late-stage adipogenesis marker adiponectin, and (c) perilipin A. Circles indicate area of lipid droplet as defined by perilipin A stain and arrowheads note adjacent Tie2+ stains. Dashed rectangle indicates area magnified at 40x; scale bar: 50 μm.
Figure 3.
Mature adipocytes in Tie2-GFP reporter mouse supraspinatus muscle six weeks post-TT+DN operation stained with oil red O. Arrows indicate fat cells. Arrowheads indicate Tie2+ ELCs.
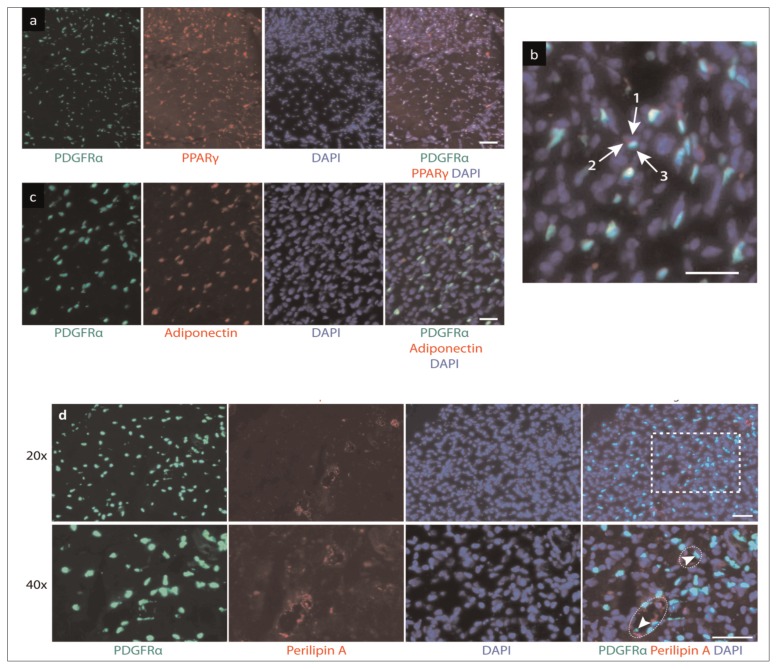
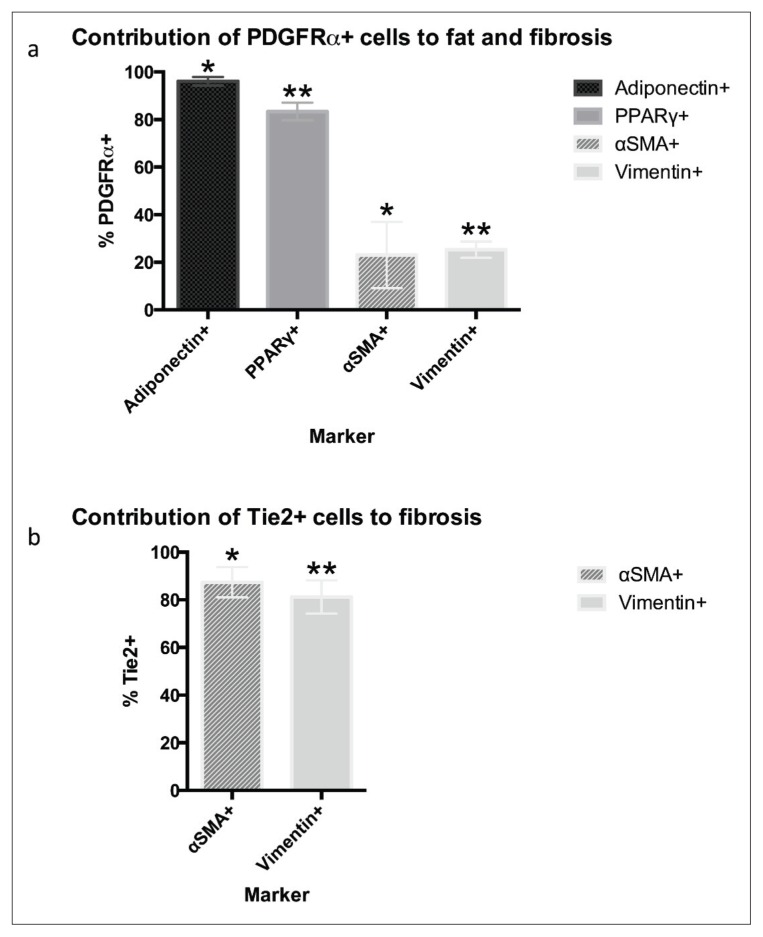
When sections from PDGFRα-GFP reporter mice were analyzed under the same conditions, they displayed strong overlap between the endogenous GFP signal and stains for adipogenic markers (Fig. 4 a–c). Quantification of fluorescence showed that 96.10 ± 3.27% of all cells expressing the late-stage adipogenic marker adiponectin also expressed PDGFRα (Fig. 5 a). Similarly, 83.40 ± 3.67% of cells expressing PPAR-γ were positive for PDGFRα. There was also considerable accumulation of PDGFRα within perilipin+ fat cells (Fig. 4 d). Furthermore, oil red O staining showed strong overlap between mature adipocytes and PDGFRα-GFP+ cells (Fig. 6). These results suggest FAPs are the main source of adipocytes.
Figure 4.
Behavior of PDGFRα+ cells in fatty infiltration of muscle six weeks post-injury. Muscle sections were subjected to immunofluorescence staining for middle-stage adipogenesis marker PPAR-γ at (a) 20× and (b) 40x, scale bar: 25 μm; (c) late-stage adipogenesis marker adiponectin, scale bar: 50 μm; and (d) perilipin A, scale bar: 50 μm. Arrows in (b) indicate (1) nucleus, (2) PPAR-γ, and (3) PDGFRα. Circles in (d) highlight lipid droplets and arrowheads show PDGFRα signals within the fat cell. Dashed rectangle indicates area magnified at 40x.
Figure 5.
Contribution of Tie2+ and PDGFRα+ cells to formation of fatty infiltration and fibrosis. Data gathered as cell counts from two blinded independent reviewers across three samples (*p<0.05; **p<0.001).
Figure 6.
Colocalization of mature adipocytes and PDGFRα+ cells six weeks post-injury. Mouse supraspinatus muscle sections from PDGFRα-GFP reporter mice were subjected to oil red O staining. Arrows indicate overlap of the PDGFRα signal with fat cells. Scale bar: 100 μm.
Fibrogenic differentiation is found mainly in Tie2+ cells
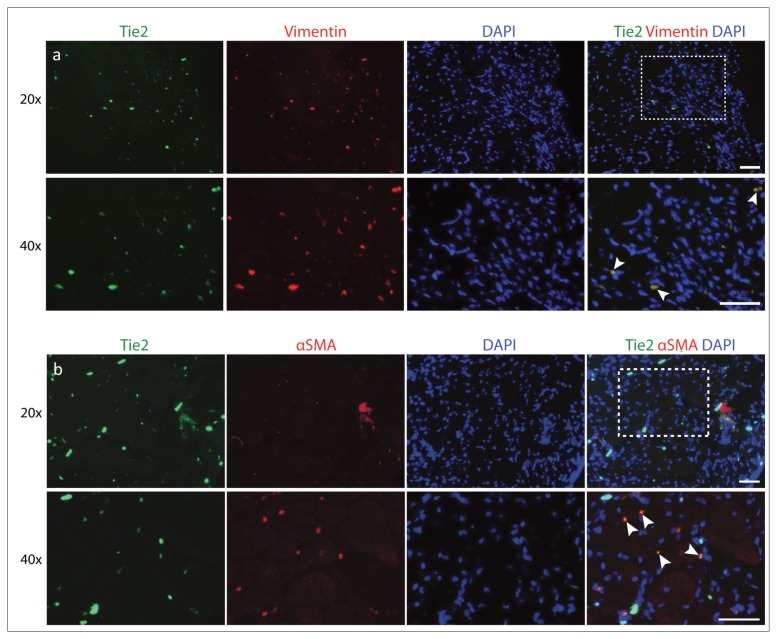
Similar methods of analysis were used to investigate fibrogenic differentiation. In our Tie2-GFP reporter mice, cells expressing Tie2-GFP overlapped strongly with vimentin (Fig. 7 a). Cells that were positive for vimentin showed 81.19 ± 7.04% colocalization with Tie2 (Fig. 5 b). Staining for αSMA, a marker of myofibroblast formation, showed that 87.42 ± 6.37% of αSMA+ cells also expressed Tie2 (Fig. 7 b).
Figure 7.
Colocalization of Tie2+ cells with fibrotic markers. Tie2-GFP reporter mouse muscle sections were analyzed for (a) vimentin and (b) αSMA. Arrowheads indicate regions of overlap. Scale bar: 50 μm.
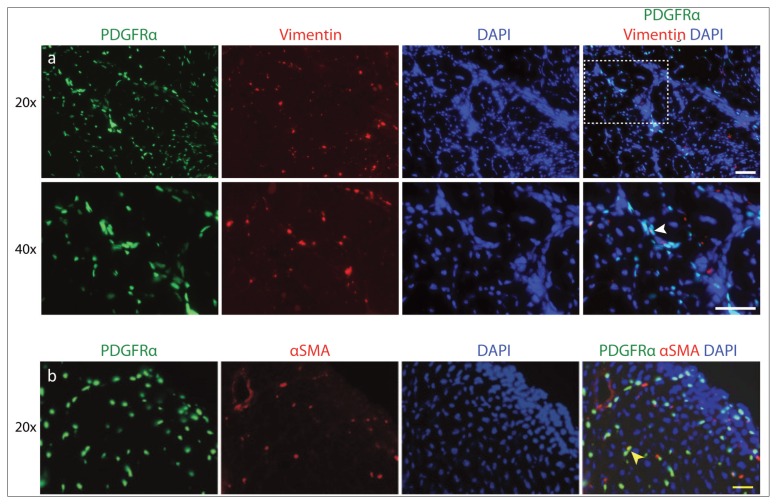
In the PDGFRα-GFP reporter mice, signal from endogenous PDGFRα-GFP was seen in only 25.28 ± 3.44% of cells staining positive for vimentin (Fig. 8a, b). Quantification of fluorescence showed that 23.07 ± 2.35% of the PDGFRα+ cells colocalized with stains for αSMA (Fig. 7 b). Results from stains for both αSMA and vimentin suggest that cells expressing fibrotic markers mainly develop from a Tie2+ progenitor cell origin, although PDGFRα+ FAPs are also capable of differentiating into fibroblasts.
Figure 8.
Colocalization of PDGFRα+ cells with fibrotic markers. Reporter mouse muscle sections were stained for (a) vimentin and (b) αSMA. Arrowheads show overlap with PDGFRα. Scale bar: 50 μm.
Discussion
The purpose of this study was to evaluate possible cellular sources of fibroblasts and adipocytes in RC muscle fibrosis and FI after tendon tears using reporter mouse models. Rotator cuff FI is one of the most common muscle pathologies seen in orthopaedic patients, originally described and classified by Goutalier et al.3, 4. FI has been a topic of significant interest in the orthopaedic community as it appears to strongly correlate with poor clinical outcomes and success rates of rotator cuff repairs. The degree of fatty infiltration is important not only for its effects on decreased muscle function, but also because there is a level of fatty infiltration that is irreversible even following repair. Patients graded with pathologic levels of fatty infiltration exhibit limited muscle strength of external rotation compared to those who maintain more rotator cuff musculature than fat. Thus, FI is an important predictor of disease progression and may potentially predict surgical outcome.
While much of muscle research has focused on intra-cellular changes that occur within the myocytes, the extracellular matrix (ECM) has a vital role in maintaining muscle homeostasis20. Injury to muscle often results in a dramatic increase in the amount of extracellular matrix production, resulting in muscle fibrosis. The ECM is a dynamic scaffold for the muscle and is made primarily of collagens. Type I and III collagen are the major forms, with type IV collagen surrounding the muscle cells in a basement membrane and adhering to a focal integrin adhesion complex. The omplex association of the ECM to the intracellular components of muscle acts in most cases as an excellent transducer of mechanical activity, but is very sensitive to alterations in patterns of use. In most muscle injury models, including spaceflight, sarcopenia, denervation, and spinal cord injury, fibrosis is a central outcome of the injury process20_ENREF_14. In previous studies using a mouse model of massive RC tears, consistent fibrosis is seen18, 21. It is postulated that increased fibrosis results in higher tension in the muscle in RC tears, thus making repair more challenging in chronic, retracted tears. In a clinical setting, fibrosis has been found in human RC tissue at time of repair and has been correlated with decreased force production and increased disruption of myofibril architecture 6, 22–24. Fibrosis has also been identified as a direct cause of muscle dysfunction in muscular dystrophy. Since fibrosis is not traditionally able to be imaged on advanced CT and MR scans, its influence on outcomes of RC repair may be under-represented.
Despite the importance of these pathologies, little is known about the underlying etiology of both fibrosis and FI in rotator cuff muscles on a cellular and molecular mechanistic level. Previous studies have shown that the TGF-b pathway regulates fibrosis and that downstream components of the mTOR pathway mediate FI25, 26; however, the sources of these pathologies were not investigated. Our findings using Tie2-GFP reporter mice indicated that Tie2+ muscle mesenchymal progenitor cells are the major source of fibroblasts in RC muscle fibrosis. Tie2 was previously considered to be a surface marker specific for endothelial cells, leading Tie2+ cells to be regarded as endothelial lineage cells (ELCs). However, a recent study demonstrated that Tie2 is also expressed in a separate population of muscle-resident progenitor cells9. In that study, the Authors showed that Tie2+ mesenchymal progenitors are the major source of osteocytes seen in muscle heterotopic ossification and have the potential to differentiate into multiple cell types, possibly including fibroblasts and adipocytes. Results from our study suggest that these Tie2+ progenitor cells do not form adipocytes but are the major cellular source of fibroblasts in rotator cuff muscle after injury.
Furthermore, we identified PDGFRα+ FAPs as the major cellular source of adipocytes in rotator cuff muscle after tendon tears in our mouse model. The FAP cell population was first discovered by two separate groups in 201011, 12. Using different cell surface markers, these two groups discovered a muscle-resident non-myogenic progenitor population that did not differentiate into myocytes in vivo or in vitro. Instead, these FAP cells were found to differentiate into fibroblasts and adipocytes under certain circumstances, such as chemical-induced muscle injury. One group further demonstrated that FAPs are characterized by the surface marker platelet-derived growth factor receptor alpha (PDGFRα) in both human and rodent skeletal muscles13, 14. Our immunofluorescence staining and histology suggest that FAPs are the major cellular source of adipocytes in rotator cuff muscle FI.
Identification of the major cellular sources of rotator cuff muscle fibrosis and FI may help in the development of cellular targeted treatment for these diseases. FAP cells have recently been studied in a mouse model of muscular dystrophy, and results of this study provide insight into the plasticity of this cell line and the potential to drive this stem cell population to promote myogenesis in RC repair. A previous study found that HDAC inhibition induced core components of myogenic transcriptional machinery (MyoD and BAF60C) and upregulated multiple myogenic miRNAs to direct pro-myogenic differentiation and suppress the fibro-adipogenic phenotype27. Similar approaches may be considered in rotator cuff tear repair surgeries to promote Tie2+ cells and FAPs away from fibrogenic and adipogenic fates towards a more myogenic phenotype.
There are several potential limitations in this study. First, the PDGFRα-GFP reporter mice used in this study were not an inducible reporter mouse strain. An advantage of inducible reporter mice compared to non-inducible reporter mice is that the expression of the reporter protein (here, GFP) can be turned on and off at will. The PDGFRα-GFP reporter mouse strain used in this study has been used previously for in vivo cell tracing for adipogenesis16, 17. PDGFRα is not an adipocyte cell surface marker. Thus, it is unlikely that GFP-tagged PDGFRα is being produced by adipocytes from cellular origins other than FAPs. Second, due to the limited source of transgenic reporter mice, a relatively small sample size (n=6) was used in this study. However, our results showed very similar staining patterns between samples. Thus, we believe our result is representative. Third, the Tie2-GFP reporter mice do not distinguish between endothelial and non-endothelial Tie2+ progenitors in muscle. However, previous studies showed that non-endothelial Tie2+ cells, rather than endothelial Tie2+ cells, possess multiple differentiation potentials. Though further work is needed, we believe Tie2+ muscle mesenchymal progenitor cells, rather than Tie2+ endothelial cells, are the major source of fibroblasts in RC muscles after tendon tears. Lastly, our mouse model involves transection of the suprascapular nerve. In humans, it is theorized that injury to the rotator cuff creates increased tension on the suprascapular nerve, which places the nerve at risk for injury28. This is a different mechanism of injury to the nerve than is seen clinically in rotator cuff tears; however, this mouse model has been shown to mimic pathophysiologic changes seen after a rotator cuff injury in previous studies18, 29.
In summary, results from this study suggest that Tie2+ progenitor cells are the major source of fibroblasts and FAPs are the major cellular source of adipocytes in RC muscle following tendon tears. Future work will be needed to further examine the key pathways regulating fibrogenesis and adipogenesis of these progenitor cell populations in rotator cuff muscles, ultimately leading to the development of novel targeted treatments for secondary muscle degeneration after RC tears.
Conclusion
Rotator cuff tears are among the most common injuries seen by orthopedic surgeons and can result in secondary muscle degeneration that persists even after repair. The development of pathologies such as fatty infiltration and fibrosis can affect the outcome of repair; however, the cellular source of these complications has not previously been explored. Results from this study suggest that Tie2+ progenitor cells are the major source of fibroblasts and FAPs are the major cellular source of adipocytes contributing to degeneration in rotator cuff muscle following injury. This study takes the first step towards the development of targeted treatments to prevent secondary muscle degeneration after rotator cuff injury. Having identified these two populations, future work will be needed to further examine the key pathways regulating fibrogenesis and adipogenesis of these progenitor cells.
Footnotes
Conflict of interests
The Authors declare that they have no conflict of interests regarding the publication of this paper.
References
- 1.Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 Suppl):1S–7S. doi: 10.1016/j.jse.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 3.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78–83. [PubMed] [Google Scholar]
- 4.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty infiltration of disrupted rotator cuff muscles. Rev Rhum Engl Ed. 1995;62(6):415–422. [PubMed] [Google Scholar]
- 5.Osti L, Buda M, Del Buono A. Fatty infiltration of the shoulder: diagnosis and reversibility. Muscles Ligaments Tendons Journal. 2014;3(4):351–354. [PMC free article] [PubMed] [Google Scholar]
- 6.Davis ME, Korn MA, Gumucio JP, et al. Simvastatin reduces fibrosis and protects against muscle weakness after massive rotator cuff tear. J Shoulder Elbow Surg. 2015;24(2):280–287. doi: 10.1016/j.jse.2014.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oak NR, Gumucio JP, Flood MD, et al. Inhibition of 5-LOX, COX-1, and COX-2 increases tendon healing and reduces muscle fibrosis and lipid accumulation after rotator cuff repair. Am J Sports Med. 2014;42(12):2860–2868. doi: 10.1177/0363546514549943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HM, Dahiya N, Teefey SA, Keener JD, Galatz LM, Yamaguchi K. Relationship of tear size and location to fatty degeneration of the rotator cuff. J Bone Joint Surg Am. 2010;92(4):829–839. doi: 10.2106/JBJS.H.01746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27(5):1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natarajan A, Lemos DR, Rossi FM. Fibro/adipogenic progenitors: a double-edged sword in skeletal muscle regeneration. Cell Cycle. 2010;9(11):2045–2046. doi: 10.4161/cc.9.11.11854. [DOI] [PubMed] [Google Scholar]
- 11.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature cell biology. 2010;12(2):143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 13.Uezumi A, Ito T, Morikawa D, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124(Pt 21):3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 14.Uezumi A, Fukada S, Yamamoto N, et al. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research. MLTJ. 2013;3(4):250–252. [PMC free article] [PubMed] [Google Scholar]
- 16.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15(3):302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18(3):355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Laron D, Natsuhara K, Manzano G, Kim HT, Feeley BT. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am. 2012;94(7):e41. doi: 10.2106/JBJS.K.00620. [DOI] [PubMed] [Google Scholar]
- 19.Hemmingsen M, Vedel S, Skafte-Pedersen P, et al. The role of paracrine and autocrine signaling in the early phase of adipogenic differentiation of adipose-derived stem cells. PLoS One. 2013;8(5):e63638. doi: 10.1371/journal.pone.0063638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieber RL, Ward SR. Cellular Mechanisms of Tissue Fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol. 2013;305(3):C241–252. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kan L, Lounev VY, Pignolo RJ, et al. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011;112(10):2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choo A, McCarthy M, Pichika R, et al. Muscle gene expression patterns in human rotator cuff pathology. J Bone Joint Surg Am. 2014;96(18):1558–1565. doi: 10.2106/JBJS.M.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato EJ, Killian ML, Choi AJ, et al. Skeletal muscle fibrosis and stiffness increase after rotator cuff tendon injury and neuro-muscular compromise in a rat model. J Orthop Res. 2014;32( 9):1111–1116. doi: 10.1002/jor.22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chillemi C, Petrozza V, Garro L, et al. Rotator cuff re-tear or non-healing: histopathological aspects and predictive factors. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1588–1596. doi: 10.1007/s00167-011-1521-1. [DOI] [PubMed] [Google Scholar]
- 25.Joshi SK, Liu X, Samagh SP, et al. mTOR regulates fatty infiltration through SREBP-1 and PPARgamma after a combined massive rotator cuff tear and suprascapular nerve injury in rats. J Orthop Res. 2013;31(5):724–730. doi: 10.1002/jor.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Joshi SK, Ravishankar B, Laron D, Kim HT, Feeley BT. Upregulation of transforming growth factor-beta signaling in a rat model of rotator cuff tears. J Shoulder Elbow Surg. 2014;23( 11):1709–1716. doi: 10.1016/j.jse.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mozzetta C, Consalvi S, Saccone V, et al. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med. 2013;5(4):626–639. doi: 10.1002/emmm.201202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albritton MJ, Graham RD, Richards RS, 2nd, Basamania CJ. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. Journal of Shoulder and Elbow Surgery. 2003;12:497–500. doi: 10.1016/s1058-2746(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Joshi S, Ravishankar B, Laron D, Kim HT, Feeley BT. Bone morphogenic protein signaling in rotator cuff muscle atrophy and fatty infiltration. Muscles, Ligaments and Tendons Journal. 2015;5(2):113–119. [PMC free article] [PubMed] [Google Scholar]