Abstract
Thiazide (TZD) diuretics are among the most commonly prescribed antihypertensives globally; however their chronic blood pressure (BP) lowering mechanism remains unclear. Herein we discuss the current evidence regarding specific mechanisms regulating the antihypertensive effects of TZDs, suggesting that TZDs act via multiple complex and interacting mechanisms, including natriuresis with short term use and direct vasodilatory effects chronically. Additionally, we review pharmacogenomics signals that have been associated with TZDs BP-response in several cohorts (i.e. NEDD4L, PRKCA, EDNRA-GNAS, and YEATS4) and discuss how these genes might be related to TZD BP-response mechanism.
Understanding the association between these genes and TZD BP mechanism might facilitate the development of new drugs and therapeutic approaches based on a deeper understanding of the determinants of BP-response.
Introduction
Hypertension (HTN) is a global public health burden affecting more than one billion individuals worldwide, and about one third of US adults [1,2]. It is a well-documented leading contributor to cardiovascular mortality, and a major modifiable risk factor for stroke, coronary heart disease, heart failure and end stage renal disease, making its management of critical importance [2]. Over the past five decades, thiazide (TZD) diuretics have been a mainstay in the treatment of HTN, and they are currently among the most commonly prescribed anti-HTN medications [3]. According to the current HTN guidelines in the US, this class of drugs is highly recommended as first line agents for most patients with uncomplicated essential HTN [4]. Despite their wide spread use, the underlying mechanism of BP lowering by TZDs has not been fully elucidated, and data have shown that only about half of TZD treated patients achieve BP control [5]. Understanding the mechanism underlying TZDs may help in identifying novel drug targets and therapeutic approaches, and have a measurable impact on the successful development of new drugs [6•], based on a deeper understanding of the determinants regulating BP-response. Thus, this review discusses the existing knowledge surrounding the BP lowering mechanisms of TZDs, the most compelling signals from TZD pharmacogenomics studies and the insights these findings may provide into TZDs mechanism of action.
BP lowering mechanisms of thiazide diuretics
Short-term BP lowering mechanism
TZDs are well known to mediate their diuretic effects via inhibiting the Na+/Cl− cotransporter (NCC) in the distal convoluted tubule, which consequently increases fluid loss, leading to a reduction in the extracellular fluid (ECF), and plasma volume and eventually a decrease in cardiac output and BP [7]. Therefore, the anti-HTN mechanism of TZDs has long been hypothesized to be attributed to their diuretic effect and enhancement of sodium excretion. In support of this hypothesis, Bennett et al. have shown that adding 20 g of salt per day to the diet of HTN patients treated with hydrochlorothiazide (HCTZ) negated the anti-HTN effect of HCTZ. Additionally, TZDs have been shown to be ineffective in end stage renal disease, which supports the importance of natriuresis for the anti-HTN action of TZD diuretics [8]. However, other evidence contradicts this hypothesis. Specifically, chlorothiazide, a TZD diuretic, lowered the BP of patients with severe renal failure [9], suggesting the diuretic effects of TZDs might not be the driving mechanism underlying their BP lowering action. Consistent with this suggestion, studies have shown that after 4–6 weeks of TZD diuretic initiation, the ECF and plasma volumes return to normal levels, yet BP reduction is maintained [10,11]. Collectively, these studies suggest TZD BP lowering effects might be initially related to sodium regulation and reduction in plasma volume and cardiac output (Figure 1). However, it seems unlikely that this is the central mechanism underlying their chronic anti-hypertensive effect.
Figure 1.
Known and theoretical blood pressure lowering mechanisms of thiazide diuretics.
Long-term BP lowering mechanism
Over the past half a century, researchers have sought to define the mechanism underlying the chronic BP lowering effects of TZDs (Figure 1). Many have suggested this long-term mechanism is mediated via reduction in total peripheral resistance (TPR) [10,12]. However, the precise mechanism and factors underlying this reduction have not been fully elucidated [13]. Several studies have suggested that TZDs reduce TPR via a vasodilation effect [14–16]; yet the mechanism by which TZDs dilate blood vessels has been perplexing and controversial [17].
One hypothesized mechanism is that TZDs’ vasodilatory effects might be mediated via the endothelium. This hypothesis was supported by an in vitro study showing that methaclothiazide, a TZD diuretic, inhibited the vasoconstrictive effect of norepinephrine and vasopressin in the aorta of spontaneously HTN rats, but not in Wistar Kyoto (non-HTN) rats [18]. Additionally, this effect was abolished by the removal of the endothelium or by using a nitric oxide synthase inhibitor, suggesting that TZDs’ hypotensive effects might be mediated via a nitric oxide endothelium-dependent mechanism. On the contrary, another study showed that TZDs, at clinically therapeutic concentrations, inhibit the vasoconstriction effects of norepinephrine and angiotensin II in the presence or the absence of the endothelium [19]. This study also reported that TZD induced vasodilation was associated with a significant reduction in RhoA and Rho Kinase expression in the vascular smooth muscle, but no changes in cellular calcium levels were observed. The changes in expression observed were independent of endothelium, suggesting that TZDs act directly on the vascular smooth muscle and not the endothelium. The authors of this study suggested that the chronic anti-HTN effects of TZDs might be mediated via calcium desensitization that occurs over long term use of these medications. However, this hypothesis is based only on this study, and more research is needed to confirm this hypothesis.
Others suggest that TZD diuretics cause vasodilation via opening the calcium activated potassium channels (KCA). This hypothesis was supported by results from an in vitro study showing that HCTZ dilates guinea pigs mesenteric arteries, and this effect was abolished by using charbdotoxin, an inhibitor of the KCA [20]. Additionally, an in vivo study has also shown that HCTZ caused a vasodilatory effect when injected into human brachial artery, and this effect was abolished by using tetraethylammonium, a KCA inhibitor [21]. Although this in vivo study has shown that the vasodilatory effects of TZDs may be mediated via KCA, the TZD plasma concentrations measured in this study were ~10 times the plasma concentrations seen clinically in TZD treated patients [22], which brings into question if this vasodilatory effect underlies the BP lowering in the clinical setting.
Other researchers have proposed that the long term anti-HTN effects of TZDs are due to their carbonic anhydrase inhibiting properties, which produce alkalosis in the vascular smooth muscle cells. Consequently, this activates the pH sensitive KCA channels, reduces voltage gated calcium-channels and causes calcium fall, and eventually vasorelaxation. This hypothesis might be intriguing, nevertheless the carbonic anhydrase inhibiting potency of TZDs varies, and we cannot generalize this mechanism to all of them. Additionally, this study was also conducted using very high plasma concentrations of TZD, considerably higher than those achieved therapeutically [23,24]. It is possible that TZDs accumulate in vascular tissues during chronic use, which could account for the inconsistency between achieved TZD diuretic plasma concentration and those reported for vasorelaxation; however, further studies are needed to support this hypothesis.
More recently, a study conducted by Ma et al. [25••] shed light on epoxyeicosatrienoic acids (EETs) as an important mediator of TZDs’ hypotensive effects. EETs are known endothelium derived factors that promote vasodilation via activation of the KCA, leading to hyperpolarization of vascular smooth muscle and eventually BP reduction. EETs are known to be catalyzed primarily by an enzyme called soluble epoxide hydrolase (sEH) to a less active vicinal diol called dihydroxyeicosatrienoic acid (DHET). Ma et al. have shown that indapamide, a TZD-like diuretic, and HCTZ decreased the protein expression of sEH in HTN rats after 8 weeks of treatment. They have also reported that indapamide increased the production of EETs by increasing the mRNA and protein expression levels of CYP2C23, an enzyme involved in the synthesis of EETs. Although this hypothesis aligns with previously proposed mechanisms claiming the involvement of the KCA in the long term mechanism underlying TZD, nevertheless, more evidence is needed to confirm the involvement of EETs in the mechanism underlying TZD BP-response.
Pharmacogenetics of thiazide diuretics BP response
Identifying genetic signals with differential response to TZD holds the potential for optimizing the use of this class of drugs, but more importantly to the topic of this review, may also provide insights in to TZDs’ BP-lowering mechanisms. Hence, in this section, we will highlight genetic signals that have been associated with differences in the BP-response of TZDs, and have been replicated in several independent cohorts, with few or no studies showing no association between the genotype and BP lowering. Insights from these pharmacogenomics signals may provide insight into BP lowering mechanism of TZDs, identify potential new targets for antihypertensive drug development and be a tool for precision medicine approaches to treatment.
NEDD4L (neural precursor cell expressed, developmentally down-regulated 4-like)
NEDD4L is known to encode a ubiquitin ligase enzyme that interferes with sodium excretion in the kidneys via reducing the renal tubular expression of epithelial sodium channel (ENAC) [26]. In knockout mouse models, NEDD4L has been associated with higher levels of ENaC expression, and salt-sensitive HTN [27]. A common synonymous single nucleotide polymorphism (SNP) within NEDD4L, rs4149601G>A, has been reported as an important predictor of HTN, salt sensitivity, and TZD-BP response [28–30,31•]. This SNP causes alternative splicing, which was associated with A-allele carriers having down-regulated ENaC expression compared to G-allele carriers [32]. In consequence, one would expect that individuals carrying the G-allele of rs4149601 would respond better to TZDs because they would have higher ENaC expression, and more salt sensitivity. Data from the NORDIL (Nordic Diltiazem) study were able to confirm this hypothesis and demonstrated that European Americans (Whites) G-allele carriers treated with either a TZD diuretic or a β-blocker, for 6 months, had better SBP and DBP reduction compared to AA carriers in both groups (SBP: −19.5 ± 16.8 versus −15.0 ± 19.3 mmHg, P < 0.001, DBP: −15.4 ± 8.3 versus −14.1 ± 8.4, P = 0.02, respectively) [33]. This signal was further confirmed to be a TZD specific signal by replicating it in Whites treated with a TZD diuretic for 9 weeks in the Pharmacogenetic Evaluation of Antihypertensive Responses (PEAR) study [34••] (Figure 2). On the contrary, no association was observed between rs4149601 and β-blocker treated patients within PEAR Whites or diltiazem treated White patients within NORDIL, suggesting that this SNP is specially influencing response to TZDs. This is not surprising given that ENaC is central to the diuretic effects of TZDs.
Figure 2.

Blood pressure response to hydrochlorothiazide by NEDD4L rs4149601 genotype for White participants in PEAR study. Solid gray bars indicate change in systolic blood pressure (SBP), gray and white lined bars indicate change in diastolic blood pressure (DBP). Values are shown as mean ± standard error. Add; additive, DOM; dominant. Used with permission [34].
In NORDIL, it was also shown that rs4149601 G-allele carriers, treated with both β-blocker and TZD diuretic, had better cardiovascular outcomes compared to those with the AA genotype (OR = 0.52, 95% CI (0.36–0.74), P < 0.0001) [33]. Additionally, in the INVEST (International Verapamil SR Trandolapril) study, White G-allele carriers, not treated with HCTZ, had significantly worse cardiovascular outcomes compared to non-carriers (OR = 10.65, 95% CI (1.18–96.25)) [34••]. Taken together, these data highlight the importance of NEDD4L as an important predictor for TZD diuretic BP response and associated outcomes. Moreover, it suggests that ENaC and sodium regulation in fact play a role in the long-term BP-lowering mechanism seen in TZDs. It also suggests that the NEDD4L protein may represent a novel protein target for an antihypertensive drug. Whether NEDD4L genotype might be used in the future to guide selection of antihypertensive therapy remains to be seen, but the data in that regard are promising, particularly since it associates not only with BP-lowering but also long-term cardiovascular outcomes.
PRKCA (protein kinase C-alpha)
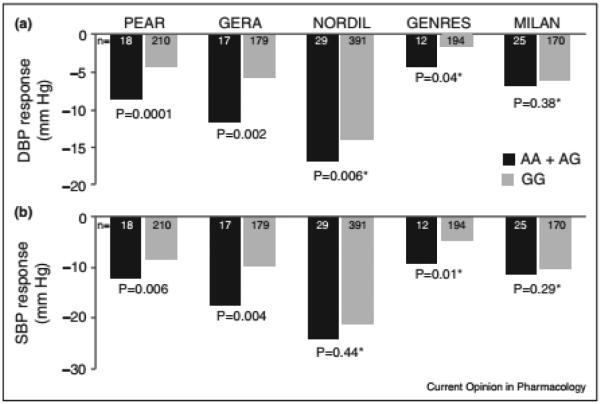
PRKCA is a member of the PKC family of serine–threonine specific protein kinases that have been shown to be fundamental regulators of cardiac contractility and calcium handling in myocytes [35], and involved in diverse cellular signaling pathways such as vascular smooth muscle contraction and vascular endothelial growth factor signaling pathways [36]. A GWAS meta-analysis between PEAR and GERA (Genetic Epidemiology of Responses to Antihypertensive) White HTN participants treated with HCTZ revealed an intronic SNP, rs16960228, in PRKCA as an important predictor of HCTZ BP response [37••]. The results of this study revealed that rs16960228 A-allele carriers had a greater BP response compared to GG carriers, which was further replicated in two other studies, NORDIL and GENRES (Genetics of Drug Responsiveness in Essential Hypertension Study) (Figure 3). The meta-analysis p-value of rs16960228 from the combined four studies achieved GWAS significance (P = 3.3 × 10−8). Further functional analysis revealed that rs16960228 A-allele carriers (with better HCTZ BP-response) had significantly higher baseline expression levels compared to GG carriers in PEAR Whites (P = 0.028). Moreover, rs16960228 was also significantly associated with DBP response in White PEAR participants treated with a β-blocker, in an opposite direction to its association with HCTZ BP response. Given the differences in response patterns between TZDs and β-blocker (which tend to have opposite effects), this finding can be considered further validation of this genetic signal. Collectively, rs16960228 replication evidence along with biological and functional validation of the PRKCA gene suggests the potential importance of PRKCA as an important predictor for TZD BP response. Additionally, the involvement of the PRKCA gene in calcium handling and vascular smooth muscle contraction pathway suggest that TZDs’ long-term BP-lowering mechanism might be mediated by acting on the vascular smooth muscles and/or interfering with calcium handling or sensitivity, as previously hypothesized [19], via
Figure 3.
Blood pressure response to hydrochlorothiazide by PRKCA rs16960228 genotype for White participants from five independent studies. (a) Diastolic blood pressure response; (b) systolic blood pressure response. The blood pressure responses are adjusted for pretreatment blood pressure levels, age, and sex; P-values are for contrast of adjusted means between genotype groups.
Used with permission [37].
PRKCA. More work on this candidate gene and pathway might open new avenues for drug discoveries and new therapeutic approaches for better clinical outcomes.
GNAS-EDN3 (G-protein alpha subunit-endothelian-3)
The GNAS-EDN3 region has been shown in GWAS meta-analyses to be associated with HTN and BP [38]. A SNP within this region, rs2273359, has been reported with a consistent significant association to HCTZ SBP-response in Whites within PEAR, GERA and NORDIL [37••] (Figure 4). The combined meta-analysis P-value of this SNP across the three studies almost reached GWAS significant level (P = 5.5 × 10−8). These data suggest the importance of this region as a potential determinant of TZD BP-response, however, more functional and biological evidence is needed to better elucidate the link between this region and TZD BP-response. The association between this genetic region and TZD BP lowering further emphasize the notion that TZDs’ action is mediated via vascular smooth muscle, given the fact that both GNAS and EDN3 are involved in the vascular smooth muscle contraction pathway [36]. Nevertheless, more work on this region is needed, which might provide us with valuable insights into the complex pathophysiological mechanism underlying HTN, and BP lowering mechanisms of TZDs.
Figure 4.
Blood pressure response to hydrochlorothiazide by GNAS-EDN3 rs2273359 genotype for White participants from five independent studies. (a) Diastolic blood pressure response; (b) systolic blood pressure response. The blood pressure responses are adjusted for pretreatment blood pressure levels, age, and sex; P-values are for contrast of adjusted means between genotype groups.
Used with permission [37].
YEATS4 (YEATS domain containing 4)
Using a GWAS approach, Turner and colleagues identified a 3-SNP haplotype signal (constructed from rs317689, rs315135, and rs7297610 near LYZ, YEATS4, and FRS genes on chromosome 12q) associated with TZD DBP response in Blacks within the GERA study (P = 2.39 × 10−7) [39]. They showed that the ATC haplotype was more prevalent in Black good responders (P = 2 × 10−4), whereas the ACT and ATT were more prevalent among Black poor responders (P = 0.0018 and 0.0219, respectively). This haplotype signal was further replicated in hypertensive Blacks treated with HCTZ monotherapy in PEAR [40•]. Additionally, single SNP analysis in PEAR Blacks revealed that this haplotype association is driven by rs7297610, which has been shown to affect the expression levels of YEATS4 gene [40•]. Data showed that carriers of the CC genotype (associated with better HCTZ BP response) had a higher base-line expression compared to T-allele carriers (P = 0.024). However, whether this expression difference plays a role in the reduced BP-response observed with HCTZ therapy is still unknown. The potential role of YEATS, FRS or LYZ on BP regulation or the effects of TZDs is not apparent. Thus, the lack of functional and biological evidence associated with this signal makes it hard to interpret how it might be associated with TZDs’ BP lowering mechanism. So in contrast to the previous three signals that were discussed, future work to unravel the role of this genetic signal may be more complicated.
Conclusion
TZDs remain one of the most widely prescribed medications worldwide. Evidence presented herein suggests that the diuretic effect of TZD might not be the only mediator of their BP lowering mechanism; however, the precise mechanisms for TZD mediated vascular effect remain poorly understood. Until now, most of the evidence supporting different BP lowering mechanisms underlying this class of drugs comes from small in vitro studies using animal rather than human tissues, and in many cases these studies used TZD concentrations that are not clinically relevant. Well powered and designed in vivo studies are still needed to better elucidate the long term mechanisms underlying TZD BP response. Understanding this mechanism might provide more insight in the mechanism underlying BP regulation, and facilitate the development of new drugs and therapeutic approaches based on a deeper understanding of the molecular determinants of the BP response.
Identifying pharmacogenetic signals associated with TZD BP response can also provide mechanistic insights that have not been previously investigated, and hold the promise for drug development and improving patient care. Herein, we presented several compelling genetic signals for TZD BP-response, with replication evidence in several studies. We showed that the functional and biological effect of NEDD4L gene suggest that ion transport and sodium regulation might be playing an important role in the long term BP-lowering mechanism underlying TZDs. Additionally, both PRKCA and GNAS-EDN3 association with TZD BP-response, and their biological function, suggest that TZDs long term action might also be mediated via vascular smooth muscle relaxation by interacting with these signals or within pathways where these signals are located. These examples further emphasize the importance of pharmacogenetics research for drug development and for providing mechanistic leads that have not been previously investigated.
Identifying additional genetic signals associated with TZD drug response and replicating these signals across multiple, appropriately designed and well powered studies might open new avenues for understanding the complex mechanism of BP regulation and discovery of new antihypertensive medications with better efficacy and safety profiles. International collaborative consortiums, such as the International Consortium for Antihypertensive Pharmacogenomics Studies (ICAPs; https://icaps-htn.org/), hold the promise to advance the field of HTN pharmacogenomics and provide more insight into TZD BP response, and other antihypertensive medications, by validating current signals and identifying additional novel genetic predictors of antihypertensive BP response. Perhaps moving forward, more signals will be added to the list, thus, a clear and robust strategic plan should be set up to validate the functional and biological plausibility of these signals and the pathways where these signals act, to better understand the mechanism underlying BP regulation of TZD and other anti-hypertensives.
Acknowledgements
This work was supported in part by grant from the National Institutes of Health Pharmacogenomics Research Network (U01 GM074492), and the American Heart Association (Predoctoral fellowship # 14PRE20460115).
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics — 2015 update. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.IMS Institute for Healthcare Informatics Medicines Use and Spending Shifts: A Review of the Use of Medicines in the U.S. in. 2014 accessed 30.09.15. [Google Scholar]
- 4.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CV, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 5.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328:914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 6 •.Nelson MR, Tipney H, Painter JL, Shen J, Nicoletti P, Shen Y, Floratos A, Sham PC, Li MJ, Wang J, et al. The support of human genetic evidence for approved drug indications. Nat Genet. 2015;47:856–860. doi: 10.1038/ng.3314. The authors of this study tested the connection between gene associations and drug targeting in which they found that drug targets with genetic support would succeed twice as often as those without it. Authors claimed that using the growing wealth of human genetic data to select the best targets and indications should have a measurable impact on the successful development of new drugs. [DOI] [PubMed] [Google Scholar]
- 7.Hropot M, Fowler N, Karlmark B, Giebisch G. Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney Int. 1985;28:477–489. doi: 10.1038/ki.1985.154. [DOI] [PubMed] [Google Scholar]
- 8.Bennett WM, McDonald WJ, Kuehnel E, Hartnett MN, Porter GA. Do diuretics have antihypertensive properties independent of natriuresis? Clin Pharmacol Ther. 1977;22:499–504. [PubMed] [Google Scholar]
- 9.Jones B, Nanra RS. Double-blind trial of antihypertensive effect of chlorothiazide in severe renal failure. Lancet. 1979;2:1258–1260. doi: 10.1016/s0140-6736(79)92278-5. [DOI] [PubMed] [Google Scholar]
- 10.van Brummelen P, Man in ’t Veld AJ, Schalekamp MA. Hemodynamic changes during long-term thiazide treatment of essential hypertension in responders and nonresponders. Clin Pharmacol Ther. 1980;27:328–336. doi: 10.1038/clpt.1980.44. [DOI] [PubMed] [Google Scholar]
- 11.Tarazi RC, Dustan HP, Frohlich ED. Long-term thiazide therapy in essential hypertension. Evidence for persistent alteration in plasma volume and renin activity. Circulation. 1970;41:709–717. doi: 10.1161/01.cir.41.4.709. [DOI] [PubMed] [Google Scholar]
- 12.Wilson IM, Freis ED. Relationship between plasma and extracellular fluid volume depletion and the antihypertensive effect of chlorothiazide. Circulation. 1959;20:1028–1036. doi: 10.1161/01.cir.20.6.1028. [DOI] [PubMed] [Google Scholar]
- 13.Hughes AD. How do thiazide and thiazide-like diuretics lower blood pressure? J Renin Angiotensin Aldosterone Syst. 2004;5:155–160. doi: 10.3317/jraas.2004.034. [DOI] [PubMed] [Google Scholar]
- 14.Shah S, Khatri I, Freis ED. Mechanism of antihypertensive effect of thiazide diuretics. Am Heart J. 1978;95:611–618. doi: 10.1016/0002-8703(78)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Aleksandrow D, Wysznacka W, Gajewski J. Influence of chlorothiazide upon arterial responsiveness to nor-epinephrine in hypertensive subjects. N Engl J Med. 1959;261:1052–1055. doi: 10.1056/NEJM195911192612103. [DOI] [PubMed] [Google Scholar]
- 16.Freis ED, Wanko A, Schnaper HW, Frohlich ED. Mechanism of the altered blood pressure responsiveness produced by chlorothiazide. J Clin Invest. 1960;39:1277–1281. doi: 10.1172/JCI104143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duarte JD, Cooper-DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Rev Cardiovasc Ther. 2010;8:793–802. doi: 10.1586/erc.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colas B, Slama M, Collin T, Safar M, Andrejak M. Mechanisms of methyclothiazide-induced inhibition of contractile responses in rat aorta. Eur J Pharmacol. 2000;408:63–67. doi: 10.1016/s0014-2999(00)00704-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Z, Zhu S, Liu D, Cao T, Wang L, Tepel M. Thiazide-like diuretics attenuate agonist-induced vasoconstriction by calcium desensitization linked to Rho kinase. Hypertension. 2005;45:233–239. doi: 10.1161/01.HYP.0000152701.97426.5f. [DOI] [PubMed] [Google Scholar]
- 20.Calder JA, Schachter M, Sever PS. Potassium channel opening properties of thiazide diuretics in isolated guinea pig resistance arteries. J Cardiovasc Pharmacol. 1994;24:158–164. doi: 10.1097/00005344-199407000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Pickkers P, Hughes AD, Russel FG, Thien T, Smits P. Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension. 1998;32:1071–1076. doi: 10.1161/01.hyp.32.6.1071. [DOI] [PubMed] [Google Scholar]
- 22.Beermann B, Groschinsky-Grind M. Pharmacokinetics of hydrochlorothiazide in man. Eur J Clin Pharmacol. 1977;12:297–303. doi: 10.1007/BF00607430. [DOI] [PubMed] [Google Scholar]
- 23.Pickkers P, Hughes AD. Relaxation and decrease in [Ca2+]i by hydrochlorothiazide in guinea-pig isolated mesenteric arteries. Br J Pharmacol. 1995;114:703–707. doi: 10.1111/j.1476-5381.1995.tb17195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickkers P, Garcha RS, Schachter M, Smits P, Hughes AD. Inhibition of carbonic anhydrase accounts for the direct vascular effects of hydrochlorothiazide. Hypertension. 1999;33:1043–1048. doi: 10.1161/01.hyp.33.4.1043. [DOI] [PubMed] [Google Scholar]
- 25 ••.Ma F, Lin F, Chen C, Cheng J, Zeldin DC, Wang Y, Wang DW. Indapamide lowers blood pressure by increasing production of epoxyeicosatrienoic acids in the kidney. Mol Pharmacol. 2013;84:286–295. doi: 10.1124/mol.113.085878. The authors of this paper reported that thiazide and thiazide-like diuretics might be acting on lowering blood pressure by increasing the production of epoxyeicosatrienoic acids (EETs). They have also shown that indapamide and hydrochlorothiazide decreased the protein expression of soluble epoxide hydrolase in hypertensive rats after 8 weeks of treatment. Additionally, they reported that indapamide increased the production of EETs by increasing the mRNA and protein expression levels of CYP2C23, an enzyme involved in the synthesis of EETs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey KF, Dinudom A, Cook DI, Kumar S. The Nedd4-like protein KIAA0439 is a potential regulator of the epithelial sodium channel. J Biol Chem. 2001;276:8597–8601. doi: 10.1074/jbc.C000906200. [DOI] [PubMed] [Google Scholar]
- 27.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, et al. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol. 2008;295:F462–F470. doi: 10.1152/ajprenal.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo F, Wang Y, Wang X, Sun K, Zhou X, Hui R. A functional variant of NEDD4L is associated with hypertension, antihypertensive response, and orthostatic hypotension. Hypertension. 2009;54:796–801. doi: 10.1161/HYPERTENSIONAHA.109.135103. [DOI] [PubMed] [Google Scholar]
- 29.Russo CJ, Melista E, Cui J, DeStefano AL, Bakris GL, Manolis AJ, Gavras H, Baldwin CT. Association of NEDD4L ubiquitin ligase with essential hypertension. Hypertension. 2005;46:488–491. doi: 10.1161/01.HYP.0000178594.63193.c0. [DOI] [PubMed] [Google Scholar]
- 30.Dahlberg J, Nilsson LO, von Wowern F, Melander O. Polymorphism in NEDD4L is associated with increased salt sensitivity, reduced levels of P-renin and increased levels of Nt-proANP. PLoS ONE. 2007;2:e432. doi: 10.1371/journal.pone.0000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31 •.Dahlberg J, Sjogren M, Hedblad B, Engstrom G, Melander O. Genetic variation in NEDD4L, an epithelial sodium channel regulator, is associated with cardiovascular disease and cardiovascular death. J Hypertens. 2014;32:294–299. doi: 10.1097/HJH.0000000000000044. The authors of this study identified a significant association between NEDD4L and blood pressure. Additionally, they also revealed that NEDD4L is associated with cardiovascular morbidity and mortality. [DOI] [PubMed] [Google Scholar]
- 32.Dunn DM, Ishigami T, Pankow J, von Niederhausern A, Alder J, Hunt SC, Leppert MF, Lalouel JM, Weiss RB. Common variant of human NEDD4L activates a cryptic splice site to form a frameshifted transcript. J Hum Genet. 2002;47:665–676. doi: 10.1007/s100380200102. [DOI] [PubMed] [Google Scholar]
- 33.Svensson-Farbom P, Wahlstrand B, Almgren P, Dahlberg J, Fava C, Kjeldsen S, Hedner T, Melander O. A functional variant of the NEDD4L gene is associated with beneficial treatment response with beta-blockers and diuretics in hypertensive patients. J Hypertens. 2011;29:388–395. doi: 10.1097/HJH.0b013e3283410390. [DOI] [PubMed] [Google Scholar]
- 34 ••.McDonough CW, Burbage SE, Duarte JD, Gong Y, Langaee TY, Turner ST, Gums JG, Chapman AB, Bailey KR, Beitelshees AL, et al. Association of variants in NEDD4L with blood pressure response and adverse cardiovascular outcomes in hypertensive patients treated with thiazide diuretics. J Hypertens. 2013;31:698–704. doi: 10.1097/HJH.0b013e32835e2a71. The authors of this study confirmed the association between NEDD4L and hydrochlorothiazide blood pressure response. Additionally, they also revealed that rs4149601 have a significant association with increased risk for adverse cardiovascular outcomes in Whites not treated with hydrochlorothiazide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, et al. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 36.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37 ••.Turner ST, Boerwinkle E, O’Connell JR, Bailey KR, Gong Y, Chapman AB, McDonough CW, Beitelshees AL, Schwartz GL, Gums JG, et al. Genomic association analysis of common variants influencing antihypertensive response to hydrochlorothiazide. Hypertension. 2013;62:391–397. doi: 10.1161/HYPERTENSIONAHA.111.00436. The authors of this study conducted a GWAS meta-analysis between White participants treated with hydrochlorothiazide in PEAR and GERA studies, in which they identified two replicated signals, in PRKCA and EDN3-GNAS genetic regions, with clinically relevant effects on hydrochlorothiazide blood pressure response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner ST, Bailey KR, Fridley BL, Chapman AB, Schwartz GL, Chai HS, Sicotte H, Kocher JP, Rodin AS, Boerwinkle E. Genomic association analysis suggests chromosome 12 locus influencing antihypertensive response to thiazide diuretic. Hypertension. 2008;52:359–365. doi: 10.1161/HYPERTENSIONAHA.107.104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40 •.Duarte JD, Turner ST, Tran B, Chapman AB, Bailey KR, Gong Y, Gums JG, Langaee TY, Beitelshees AL, Cooper-Dehoff RM, et al. Association of chromosome 12 locus with antihypertensive response to hydrochlorothiazide may involve differential YEATS4 expression. Pharmacogenomics J. 2013;13:257–263. doi: 10.1038/tpj.2012.4. This study confirms the association between a haplotype (from rs317689/rs315135/rs7297610) on Chromosome 12q15 and blood pressure response to hydrochlorothiazide in PEAR African-Americans participants. The authors of this study also reported that this haplotype association is driven by rs7297610, which has been shown to affect the expression levels of YEATS4 gene. [DOI] [PMC free article] [PubMed] [Google Scholar]