Abstract
We recently reported an increase in dicentric chromosome (DIC) formation after a single computed tomography (CT) scan (5.78–60.27 mSv: mean 24.24 mSv) and we recommended analysis of 2000 metaphase cells stained with Giemsa and centromere-FISH for dicentric chromosome assay (DCA) in cases of low-dose radiation exposure. In the present study, we analyzed the frequency of chromosome translocations using stored Carnoy's-fixed lymphocyte specimens from the previous study; these specimens were from 12 patients who were subject to chromosome painting of Chromosomes 1, 2 and 4. Chromosomes 1, 2 and 4 were analyzed in ∼5000 cells, which is equivalent to the whole-genome analysis of almost 2000 cells. The frequency of chromosome translocation was higher than the number of DICs formed, both before and after CT scanning. The frequency of chromosome translocations tended to be higher, but not significantly higher, in patients with a treatment history compared with patients without such a history. However, in contrast to the results for DIC formation, the frequency of translocations detected before and after the CT scan did not differ significantly. Therefore, analysis of chromosome translocation may not be a suitable assay for detecting chromosome aberrations in cases of low-dose radiation exposure from a CT scan. A significant increase in the frequency of chromosome translocations was not likely to be detected due to the high baseline before the CT scan; the high and variable frequency of translocations was probably due to multiple confounding factors in adults.
Keywords: dicentric chromosome, translocated chromosome, chromosome painting, low-dose radiation exposure, confounding factors
INTRODUCTION
Japan is a computed tomography (CT)-rich country. Potential cancer risks associated with ionizing radiation exposure from CT scans are a concern, especially for children and young adults, because of reported increases in leukemia, brain tumors, and other cancers after CT scans [1, 2]. These studies state that CT scanning may induce chromosome aberrations. Chromosome aberrations that may cause cancer and hematological malignancies include chromosomal translocations, deletions and inversions, each of which can result from the cleavage of chromosomes, also called DNA double-strand breaks (DSBs) [3–5]. Previously, dicentric chromosome (DIC) assay (DCA) or γH2AX (phosphorylated form of H2AX histone variant) -based visualization were used to determine whether CT scanning increases chromosomal aberrations or DNA damage [6–9]. The most effective tool for estimating biological radiation doses received by individuals is the scoring of chromosomal aberrations such as DICs and ring chromosomes or translocated chromosomes in peripheral blood (PB) lymphocytes. Cells with DICs are mitotically unstable and are gradually eliminated from the body because they are unable to pass through repeated cell divisions. On the other hand, cells with translocated chromosomes are mitotically stable and are not lost by cell division [10]. We recently showed that DIC formation increases significantly in PB lymphocytes after a single CT scan (5.78–60.27 mSv: mean 24.24 mSv) (Supp. Table 1A and 1B) [11]. We therefore recommended that 2000 or more metaphase figures in Giemsa-stained samples, and 1000 or more metaphase figures in Centromere-FISH be analyzed by DCAs in studies of low-dose radiation exposure [11]. DICs and chromosome translocations are produced in about an equal ratio in human PB cells after exposure to radiation [12, 13]. Therefore, we reasoned that a single CT scan might produce chromosome translocations that could be the cause of leukemia or other cancer.
Here we analyzed the frequency of chromosome translocations after a single CT scan: we used stored Carnoy's-fixed lymphocyte specimens taken from 12 patients in a previous study [11]. Based on our review of the published literature, there are no published studies comparing the frequency of chromosome translocations before and after a CT scan.
METHODS
Ethics statement
The samples and the medical records used in this study were approved by the Ethics Committee of the Fukushima Medical University School of Medicine (Approval No. 1577). Written informed consent was obtained from all participants for analysis of the PB samples, and the protocols were carried out in accordance with guidelines approved by the Council for International Organizations of Medical Science [14].
Subjects
The study involved 12 patients (3 males and 9 females) aged 62–83 years (mean 71 years) who had medical examinations in hematological internal medicine, respiratory internal medicine and respiratory surgery at Fukushima Medical University Hospital. Data regarding past history of disease and treatment, CT scans, and smoking status of the subjects are shown in Table 1. Patients who received previous radiotherapy or chemotherapy did not undergo any treatment within the year prior to entry into this study.
Table 1.
Patient background data
| Patient no. | Disease | Part body examined in CT scan | Days from CT scan to PB collection | Treatmenta | Smoking status | Past CT examinationc | Other X-ray examinationsd |
|---|---|---|---|---|---|---|---|
| 1 | Lung cancer | Chest | 8 | (–) | (–) | (+) | Chest, UGI, PET |
| 2 | Lymphoma | Cervix, chest, abdomen, pelvis | 3 | (+) | (–) | (+) | Chest, UGI |
| 3 | Lymphoma | Chest, abdomen, pelvis | 11 | (+) | (–) | (+) | Chest, UGI, PET |
| 4 | Chest abnormal shadow | Chest | 15 | (–) | (–) | (+) | Chest, UGI |
| 5 | Chest abnormal shadow | Chest | 22 | (–) | (+)b | (+) | Chest, UGI |
| 6 | Lymphoma | Chest, abdomen, pelvis | 14 | (+) | (–) | (+) | Chest, UGI |
| 7 | Lymphoma | Cervix, chest, abdomen, pelvis | 2 | (+) | (+)#2 | (+) | Chest, UGI, PET |
| 8 | Lymphoma | Cervix, chest, abdomen, pelvis | 28 | (+) | (–) | (+) | Chest, UGI |
| 9 | Lymphoma | Chest, abdomen, pelvis | 7 | (+) | (+)#2 | (+) | Chest, UGI, PET |
| 10 | Chest abnormal shadow | Chest | 14 | (–) | (–) | (+) | Chest, UGI |
| 11 | Lung cancer | Chest | 2 | (–) | (+)#2 | (+) | Chest, PET |
| 12 | Lung cancer (suspected) | Chest | 6 | (–) | (+)#2 | (+) | Chest, UGI, PET |
aChemotherapy or radiotherapy had been performed at least five years before this study. bThese patients had given up smoking at least 10 years before this study. cAll patients except one (No. 10) underwent CT scanning more than five times during the past 5 years. dUGI = X-ray examination of the upper gastrointestinal tract, PET = positron emission tomography.
Separation of lymphocytes from PB and cell culture conditions
Heparinized PB from each patient before and after (within 3–28 days of) the CT scan, and mononuclear blood cells were isolated using BD Vacutainer CPT tubes (BD Biosciences, San Jose, CA, USA) according to the manufacturer's instructions. Cells were suspended in RPMI 1640 medium (Nacalai Tesque, Kyoto, Japan) containing 20% fetal bovine serum (Equitech Bio, Keilor East, Australia), 2% phytohemagglutinin-HA15 (Remel, Lenexa, KS, USA) and 60 μg/ml of kanamycin solution (Life Technologies, Carlsbad, CA, USA) in a 15-ml Falcon tube. Lymphocytes were cultured in a 5% humidified CO2 incubator at 37°C for 48 h. First-division metaphase cells were obtained by treating the culture with colcemid (final concentration, 0.05 μg/ml; Life Technologies) for 48 h.
Cell harvesting
After 48 h of culture, cells were harvested, treated with 0.075 M KCL, and fixed with methanol/acetic acid (3:1) according to the standard cytogenetic procedure [10, 15]. Finally, the cell pellets were suspended in 1–2 ml of fixative, depending on the size of the pellets. One drop (∼20 μl) of the suspension was dispensed onto a slide and spread on a water bath.
Chromosome painting
Each slide was first dried at 65°C for at least 1 h for hardening. Next, 6–7 μ– of a Customized XCP-Mix probe (Mix-#1R-#2G-#4RG; MetaSystems, Altlussheim, Germany) solution was applied per 22 × 22-mm area, and the slide was covered with a glass coverslip and sealed with paper bond. Subsequent operations were carried out according to the manufacturer's instructions. Nuclear DNA was denatured by incubating the slides on a hot plate at 75°C for 2 min, followed by incubation overnight at 37°C in a humidified chamber to allow for hybridization. The glass coverslips were removed and the slides were washed in 0.4 × SSC at 72°C for 2 min. After draining, the slides were then washed in 2 × SSC/0.05% Tween-20 at room temperature (RT) for 30 s. Subsequently, the slides were briefly rinsed in distilled water to avoid crystal formation and then air dried at RT. Finally, nuclei were counterstained with Vectashield Mounting Medium containing DAPI (Vector, Burlingame, USA), and the slides were covered with a glass coverslip and sealed with nail polish.
Image capturing and scoring of chromosomal aberrations (Chromosome 1, 2 and 4 painting)
Soon after completion of the chromosome preparations, FISH images were captured in the AutoCapt mode using two sets of AXIO Imager Z2 microscopes (Carl Zeiss AG, Oberkochen, Germany) equipped with CCD cameras and Metafer 4 software (MetaSystems GmbH, Altlussheim, Germany), respectively. Metaphase cells were selected for scoring in manual mode. Chromosome analysis was performed according to the International Atomic Energy Agency (IAEA) manual (IAEA 2001) [15] by two trained, experienced observers. It should be emphasized that the two observers who evaluated the data were not informed of the patients' backgrounds.
Only metaphase figures with ∼44–46 chromosomes were selected for chromosomal analysis. Thus, cells with three chromosome pairs (Chromosomes 1, 2 and 4) colored in three different paintings were selected for analysis. Metaphase cells exhibiting tetraploidy were omitted from the analysis. Based on a previous report that indicated that almost all apparently one-way (non-reciprocal) translocations are usually actually two-way (reciprocal) translocations [16], we included apparently one-way translocations in the two-way translocation counts. In the case of complex chromosomal abnormalities, the numbers of translocations were determined based on the number of color junctions (NCJ) [17]. For example, an NCJ of 1 or 2 reflects one translocation, an NCJ of 3 or 4 reflects two translocations, and an NCJ of 5 or 6 reflects three translocations and so on. We also recorded other chromosomal aberrations such as DICs.
For scoring, the formula used to calculate the frequency of translocations across the whole genome (FG) was based on the formula using three colors (Chromosome 1: Red, Chromosome 2: Green, Chromosome 4: Yellow) for the detection of translocations as follows [1]:
FG: the full genome aberration frequency,
Fp: the translocation frequency detected by FISH,
fp: the fraction of genome hybridized, taking into account the gender
of the subjects (female: fp = 0.2234, male: fp = 0.2271).
The proportion of the genome occupied by Chromosomes 1, 2 and 4 is ∼23%. Therefore, FG is determined by the following formula:
In order to unify the cell numbers of the analysis, we determined FG as per 2000 cell equivalents, which were obtained according to the above respective formula for females and males, respectively.
Methods used for the DCA are described in the supplementary information.
Statistical analysis
The Student's t-test was used to compare the frequency of chromosome translocation before CT scanning in patients with or without previous treatment. The Student's paired t-test was used to compare the frequency of chromosome translocations before and after CT scanning. Analyses were performed using STATA software, Version 11.1 (StataCorp, College Station, TX, USA). P values of <0.05 were regarded as statistically significant.
RESULTS
Subject background data
Background data pertaining to the 12 patients are shown in Table 1. For the six patients with malignant lymphoma (ML) who were followed up after chemotherapy (mainly rituximab plus CHOP: cyclophosphamide, doxorubicin, vincristine and prednisolone) and/or radiotherapy (treatment group), at least 5 years had elapsed between those treatments and the present study. Two patients (Patients 3 and 7) had received both chemotherapy and radiotherapy. Patients without treatment history were individuals who had only undergone surgery for lung cancer or only taken CT examination for diagnosis. Patients with a history of smoking had ceased smoking >10 years prior to the study. All patients had undergone chest X-ray during annual medical examinations. In addition, all patients except one (Patient 10) had undergone CT scans more than five times during the previous 5 years, and three ML patients and three lung cancer patients had undergone a positron emission tomography (PET) examination before this study. Patients with lung cancer (Patients 1 and 11) had undergone surgery, and the last patient (Patient 12) had been given letrozole after surgery for breast cancer. With respect to medication, one patient (Patient 2) was given 2.5 mg of predonine every other day for hay fever, four patients (Patients 3, 4, 8 and 11) were prescribed medication for hypertension, and two patients (Patients 10 and 11) were prescribed medication for diabetes. Smoking history was deemed not to have an influence on DIC formation because 10 years had passed since smoking cessation in patients who had previously smoked.
Frequency of chromosome translocation before and after CT scanning
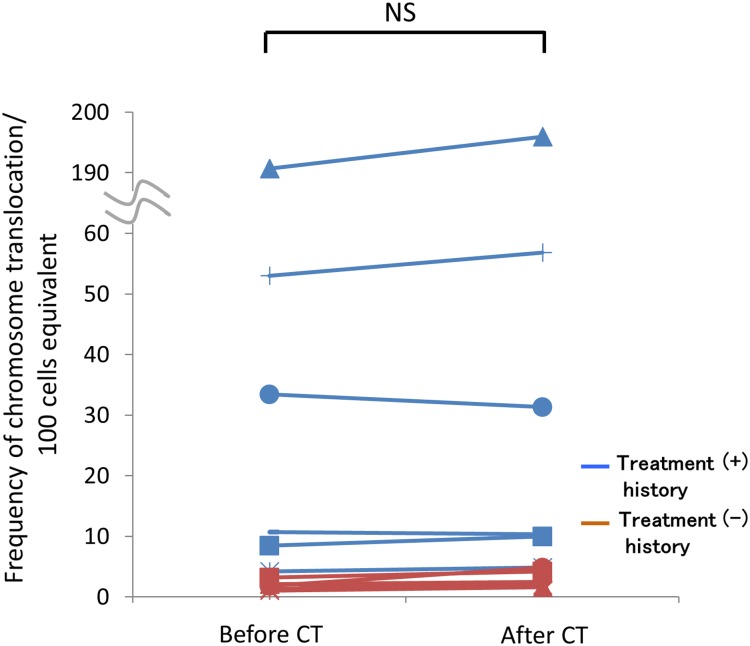
In our previous study in which we analyzed DIC formation using Giemsa staining and centromere-FISH, we analyzed 2000 metaphases per patient [11]. Subsequently, to match the number of cells analyzed between the DIC and translocation analysis, we analyzed ∼5000 cells, which was equivalent to whole-genome analysis of almost 2000 cells (Table 2). We compared the frequency of chromosome translocation before the CT scan for patients with and without previous chemotherapy and/or radiotherapy, and the frequency of chromosome translocation tended to be higher in patients with previous treatment, but this difference was not significant (Fig. 1). Notably, the frequency of translocations detected after the CT scan was not significantly higher than that detected before the CT scan (Fig. 2). Therefore, the frequency of chromosome translocations did not show the same tendency to increase after a CT scan as did the level of DIC formation, as found in our earlier research.
Table 2.
Increase in chromosome translocation after a single CT scan (chromosome 1, 2, 4-painting)
| Patient no. | Timing | Number of cells scored |
Number of translocations | Increment | Frequency of observed translocationb |
|
|---|---|---|---|---|---|---|
| Cell count of analysis | Cell equivalenta | |||||
| 1 | Before CT | 5131 | 2011 | 43 | 7 | 2.14 |
| After CT | 5126 | 2009 | 50 | 2.49 | ||
| 2 | Before CT | 5184 | 2031 | 172 | 29 | 8.47 |
| After CT | 5134 | 2012 | 201 | 9.99 | ||
| 3 | Before CT | 5128 | 2010 | 3089 | 128 | 189.50 |
| After CT | 5139 | 2014 | 3937 | 195.48 | ||
| 4 | Before CT | 5126 | 2009 | 26 | 13 | 1.29 |
| After CT | 5106 | 2001 | 39 | 1.95 | ||
| 5 | Before CT | 5118 | 2006 | 21 | 12 | 1.05 |
| After CT | 5138 | 2014 | 33 | 1.64 | ||
| 6 | Before CT | 5120 | 2007 | 671 | −40 | 33.43 |
| After CT | 5135 | 2012 | 631 | 31.36 | ||
| 7 | Before CT | 5133 | 2012 | 1067 | 82 | 53.03 |
| After CT | 5156 | 2021 | 1149 | 56.85 | ||
| 8 | Before CT | 5121 | 2007 | 215 | −7 | 10.71 |
| After CT | 5134 | 2012 | 208 | 10.34 | ||
| 9 | Before CT | 5122 | 2008 | 85 | 12 | 4.23 |
| After CT | 5104 | 2001 | 97 | 4.85 | ||
| 10 | Before CT | 5139 | 2014 | 37 | 60 | 1.84 |
| After CT | 5128 | 2010 | 97 | 4.83 | ||
| 11 | Before CT | 5134 | 2012 | 65 | 19 | 3.23 |
| After CT | 5111 | 2003 | 84 | 4.19 | ||
| 12 | Before CT | 5137 | 2014 | 43 | −11 | 2.14 |
| After CT | 5120 | 2007 | 32 | 1.59 | ||
aCell count 196/500 (see Methods for formula). bPer 100 cells equivalent.
Fig. 1.
Frequency of chromosome translocations before the CT scan. The frequency of chromosome translocations prior to CT scanning in patients with (+) (n = 6) or without (–) (n = 6) chemotherapy and/or radiotherapy treatment history is shown. The line indicates the mean value. There was no significant difference in translocation frequency between patients with or without treatment history. NS = not significant.
Fig. 2.
Comparison of the frequency of chromosome translocations before and after the CT scan. No significant difference was found between the number of translocations before and after the CT scan in patients either with (+) (n = 6) or without (–) (n = 6) chemotherapy and/or radiotherapy treatment history. NS = not significant.
DISCUSSION
Our previous finding that there is an increase in DIC formation [11] (i.e. evidence of cleavage of double-stranded DNA) after a single CT scan prompted us to subsequently analyze the frequency of chromosome translocation in the same samples. After adding two samples to the samples set used for the previous study, we first compared the number of DICs formed before the CT scan in patients with and without previous chemotherapy and/or radiotherapy. The number of DICs formed tended to be higher (but not significantly) in patients with a treatment history than in patients without such a history, as determined using both Giemsa staining (Supp. Fig. 1A) and centromere-FISH (Supp. Fig. 1B). Most notably, the number of DICs formed after the CT scan was significantly higher than the number formed prior to the scan, as determined with both Giemsa staining (P < 0.01) (Supp. Fig. 1C) and centromere-FISH (P < 0.01) (Supp. Fig. 1D). Chemotherapy and radiotherapy induce DNA damage and cause chromosome instability [18, 19]. We speculate that DIC formation would be apt to occur after a CT scan in patients with a treatment history. However, we could not compare the number of DICs formed after the CT scan in patients with and without previous treatment because of the small number of patients.
Based on the results of previous studies, which showed that DICs and chromosome translocations are produced in about an equal ratio [12, 13], and on our findings of a significant increase in DIC formation after a single CT scan [11], we speculated that there could also be an increase in chromosome translocation in our samples. However, we did not detect a significant increase in chromosome translocation in the sample set used for our study. One possible reason for this result is that significant additional increments of chromosome translocation could not be detected because the baseline number of translocations increases with aging in adults, and the frequency of chromosome translocations per 2000 cells equivalent both before and after the CT scan was higher than the respective number of DICs formed per 2000 metaphases. Another possible reason is that the frequency of chromosome translocation was calculated based on changes in Chromosomes 1, 2 and 4, which are considered representative of all chromosomes. Therefore, more accurate translocation frequency would be detected by performing chromosome painting for all of the chromosomes, using a method such as M-FISH. However, this method is expensive and very complex; consequently, it is not usually performed for translocation assay.
Smoking habits have an impact on the frequency of chromosome translocation [20–23], and exposure to natural radiation has also been suggested as playing a role in the formation of chromosome translocations. Study of residents in a high-background radiation area (HBRA) in China indicates that the frequency of chromosome translocations is higher than the number of DICs and ring chromosomes formed [24]. However, the increase in chromosome translocation in a HBRA was within the range of individual variation in the control samples [24]. Subsequently, these authors demonstrated that smoking plays a more significant role than elevated natural radiation exposure in bringing about the induction of chromosome translocation frequency in those HBRAs [25]. Because cells with chromosome translocations are mitotically stable and able to pass through repeated cell division, the frequency of chromosome translocations is thought to reflect accumulation of translocations due to exposure to chemicals or cigarette smoking over a long time, or due to treatment for various diseases or medical radiation exposure. Therefore, the increment of chromosome translocations induced by a CT scan could be hidden in the frequency of chromosome translocation due to such confounding factors. Indeed, in our study, the frequency of chromosome translocations was higher than the number of DICs formed both before and after CT scan, and the frequency of chromosome translocations tended to be higher in patients with a treatment history than in patients without such a history before CT scanning; this tendency was especially pronounced in two patients (Patients 3 and 7), who underwent both chemotherapy and radiotherapy (Table 1, Fig. 1). We, therefore, suggest that it could be difficult to distinguish additional chromosome translocations that are due to a CT scan from translocations accumulated over a long time. In contrast, because DICs are unstable types of aberrations, they gradually decrease after one month, and they are less affected by smoking and by past treatment history. These factors could be reasons why the DCA is a suitable assay for biological dose assessment for acute radiation exposure. However, there was a linear increase between the number of DICs formed and increasing exposure dose in HBRAs in China [26], suggesting that, following chronic low-dose radiation exposure, lymphocytes with DICs might accumulate rather than be eliminated at cell division.
An increase in the risk of cancer in children and young adults because of CT scanning is a cause for concern [1, 2]. However, there are no published studies comparing the frequency of chromosome translocations before and after a CT scan. Usually there are various confounding factors other than medical exposure that contribute to chromosome aberrations in adults; therefore, it is unclear whether chromosome translocation has been due to radiation exposure or to other events. Evidence of an increase in the number of DICs formed after a single CT scan led to speculation that chromosome translocation could occur at the same time. It is still a possibility that chromosome translocation following CT scanning might be a cause of disease, especially in children. On the other hand, a recent study suggests that cancer risk from CT scans is biased by cancer-predisposing factors, such as congenital chromosomal abnormalities and immune deficiencies [27]. Notably, Nakamura proposed a similar hypothesis [28]; specifically, he mentioned that radiation exposure is a critical cause of acute lymphocytic leukemia in individuals who carry pre-existing clonal pre-leukemic cells because radiation exposure induces additional mutations in those cells. Chromosomal analysis after CT scanning should be performed in children to clarify these speculations.
Taken together, our findings indicated that analysis of chromosome translocations may not be a suitable assay of chromosome aberrations in cases of low-dose radiation exposure due to the influence of confounding factors in adults.
SUPPLEMENTARY DATA
Supplementary data is available at the Journal of Radiation Research online.
FUNDING
This work and the Open Access publication charges for this article were supported in part by a Grant-in-Aid for Scientific Research (C), No. 25460694 and by funds for the development of methods for monitoring exposure to low-dose radioactivity from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Kenneth E. Nollet, Director, Department of International Cooperation Radiation Medical Science Center, Fukushima Medical University School of Medicine, for reviewing the manuscript, and we thank radiologists of the Fukushima Medical University Hospital for their cooperation. This work was carried out at the Joint Usage/Research Center (RIRBM), Hiroshima University. The results of this research were presented at the 15th International Congress of Radiation Research in Kyoto, Japan, 25–29 May 2015.
REFERENCES
- 1.Pearce MS, Salotti JA, Little MP et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012;380:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathews JD, Forsythe AV, Brady Z et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanjangud G, Amarillo I, Rao N. Solid tumor cytogenetics: current perspectives. Clin Lab Med 2011;31:785–811. [DOI] [PubMed] [Google Scholar]
- 4.Bochtler T, Frohling S, Kramer A. Role of chromosomal aberrations in clonal diversity and progression of acute myeloid leukemia. Leukemia 2015;29:1243–52. [DOI] [PubMed] [Google Scholar]
- 5.Krem MM, Press OW, Horwitz MS et al. Mechanisms and clinical applications of chromosomal instability in lymphoid malignancy. Br J Haematol 2015;171:13–28. [DOI] [PubMed] [Google Scholar]
- 6.Löbrich M, Rief N, Kühne M et al. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A 2005;102:8984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephan G, Schneider K, Panzer W et al. Enhanced yield of chromosome aberrations after CT examinations in paediatric patients. Int J Radiat Biol 2007;83:281–7. [DOI] [PubMed] [Google Scholar]
- 8.Rothkamm K, Balroop S, Shekhdar J et al. Leukocyte DNA damage after multi-detector row CT: a quantitative biomarker of low-level radiation exposure. Radiology 2007;242:244–51. [DOI] [PubMed] [Google Scholar]
- 9.Geisel D, Zimmermann E, Rief M et al. DNA double-strand breaks as potential indicators for the biological effects of ionising radiation exposure from cardiac CT and conventional coronary angiography: a randomised, controlled study. Eur Radiol 2012;22:1641–50. [DOI] [PubMed] [Google Scholar]
- 10.IAEA. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies, EPR-Biodosimetry. International Atomic Energy Agency, Vienna, 2011. [Google Scholar]
- 11.Abe Y, Miura T, Yoshida MA et al. Increase in dicentric chromosome formation after a single CT scan in adults. Sci Rep 2015;5:13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanda R, Hayata I. Comparison of the yields of translocations and dicentrics measured using conventional Giemsa staining and chromosome painting. Int J Radiat Biol 1996;69:701–5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Hayata I. Preferential reduction of dicentrics in reciprocal exchanges due to the combination of the size of broken chromosome segments by radiation. J Hum Genet 2003;48:531–4. [DOI] [PubMed] [Google Scholar]
- 14.Council for International Organizations of Medical Sciences. International guidelines for ethical review of epidemiological studies. Law Med Health Care 1991;19:247–58. [PubMed] [Google Scholar]
- 15.IAEA. Cytogenetic analysis for radiation dose assessment. A manual. Technical Reports Series No. 405. International Atomic Energy Agency, Vienna, 2001. [Google Scholar]
- 16.Fomina J, Darroudi F, Boei JJ et al. Discrimination between complete and incomplete chromosome exchanges in X-irradiated human lymphocytes using FISH with pan-centromeric and chromosome specific DNA probes in combination with telomeric PNA probe. Int J Radiat Biol 2000;76:807–13. [DOI] [PubMed] [Google Scholar]
- 17.Nakano M, Kodama Y, Ohtaki K et al. Detection of stable chromosome aberrations by FISH in A-bomb survivors: comparison with previous solid Giemsa staining data on the same 230 individuals. Int J Radiat Biol 2001;77:971–7. [DOI] [PubMed] [Google Scholar]
- 18.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in human cancers. Nature 1998;396:643–9. [DOI] [PubMed] [Google Scholar]
- 19.Tian H, Gao Z, Li H, Zhang B et al. DNA damage response—a double-edged sword in cancer prevention and cancer therapy. Cancer Lett 2015;358:8–16. [DOI] [PubMed] [Google Scholar]
- 20.Ramsey MJ, Moore DH II, Briner JF et al. The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutat Res 1995;338:95–106. [DOI] [PubMed] [Google Scholar]
- 21.Tucker JD, Tawn EJ, Holdsworth D et al. Biological dosimetry of radiation workers at the Sellafield nuclear facility. Radiat Res 1997;148:216–26. [PubMed] [Google Scholar]
- 22.Moore DH II, Tucker JD, Jones IM et al. A study of the effects of exposure on cleanup workers at the Chernobyl nuclear reactor accident using multiple end points. Radiat Res 1997;148:463–75. [PubMed] [Google Scholar]
- 23.Littlefield LG, McFee AF, Salomaa SI et al. Do recorded doses overestimate true doses received by Chernobyl cleanup workers? Results of cytogenetic analyses of Estonian workers by fluorescence in situ hybridization. Radiat Res 1998;150:237–49. [PubMed] [Google Scholar]
- 24.Hayata I, Wang C, Zhang W et al. Effect of high-level natural radiation on chromosomes of residents in southern China. Cytogenet Genome Res 2004;104:237–9. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Wang C, Chen D et al. Effect of smoking on chromosomes compared with that of radiation in the residents of a high-background radiation area in China. J Radiat Res 2004;45:441–6. [DOI] [PubMed] [Google Scholar]
- 26.Jiang T, Hayata I, Wang C et al. Dose–effect relationship of dicentric and ring chromosomes in lymphocytes of individuals living in the high background radiation areas in China. J Radiat Res 2000;41 Suppl:63–8. [DOI] [PubMed] [Google Scholar]
- 27.Journy N, Rehel JL, Ducou Le Pointe H et al. Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer 2015;112:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura N. A hypothesis: radiation-related leukemia is mainly attributable to the small number of people who carry pre-existing clonally expanded preleukemic cells. Radiat Res 2005;163:258–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.