Abstract
Variations in relative biological effectiveness (RBE) from a fixed value of 1.1 are critical in proton beam therapy. To date, studies estimating RBE at multiple positions relative to the spread-out Bragg peak (SOBP) have been predominantly performed using passive scattering methods, and limited data are available for spot-scanning beams. Thus, to investigate the RBE of spot-scanning beams, Chinese hamster fibroblast V79 cells were irradiated using the beam line at the Hokkaido University Hospital Proton Therapy Center. Cells were placed at six different depths, including the entrance of the proton beam and the proximal and distal part of the SOBP. Surviving cell fractions were analyzed using colony formation assay, and cell survival curves were obtained by the curve fitted using a linear–quadratic model. RBE10 and RBE37 were 1.15 and 1.21 at the center of the SOBP, respectively. In contrast, the distal region showed higher RBE values (1.50 for RBE10 and 1.85 for RBE37). These results are in line with those of previous studies conducted using passive scattering proton beams. Taken together, these data strongly suggest that variations in RBE should be considered during treatment planning for spot-scanning beams as well as for passive scattering proton beams.
Keywords: relative biological effectiveness, spot scanning, radiotherapy, proton therapy
INTRODUCTION
Proton therapy allows the delivery of high-radiation doses to tumors without damaging the surrounding healthy organs [1], and an increasing number of patients have been treated using this therapy [2]. Although most patients are treated using passive scattering methods, modern proton therapy centers often adopt pencil beam scanning because it offers more flexible and conformal dose distributions compared with passive scattering methods, and minimizes overall exposure.
Hokkaido University Hospital started spot-scanning proton therapy (SSPT), a type of pencil beam scanning proton therapy, in March 2014. During SSPT, several thousand small-sized, nearly mono-energetic proton beams are used to administer a planned dose to the target volume. The depth position of the spots are adjusted by changing the acceleration energy, whereas their lateral positions are moved using a pair of scanning magnets [3, 4]. This technique does not require collimators or compensators, and can thus reduce neutron contamination from interactions of protons with these materials.
Relative biological effectiveness (RBE) is defined as the ratio of the absorbed dose of a reference radiation to that of a test radiation that produces the same biological effect, and RBE is an essential consideration during treatment planning for proton therapy. Treatment planning in proton therapy is generally based on a constant RBE of 1.1, according to Report 78 of the International Commission on Radiation Units and Measurements (ICRU78). In line with ICRU78, determinations of RBE have been performed widely, using passive scattering methods [5, 6], and show RBE values ranging from 1.1 to 1.2 at the beginning of the flat top portion of the spread-out Bragg peak (SOBP). However, recent studies warrant cautious use of this RBE value for treatment planning because it may lead to lower estimates of biological doses in organs at risk (OARs) that are located close to the distal fall-off of SOBP [7–9].
Many studies have revealed that RBE tends to increase with increasing linear energy transfer (LET) towards the distal region of the SOBP [10, 11], and Paganetti reviewed this RBE characteristic of proton beam comprehensively [12]. However, it remains unclear whether these RBE values for passive scattering methods are relevant to spot-scanning systems. This is because, first, the proton energy spectra, the fluence, and the LET of SSPT proton beams may differ from those of conventional passive scattering systems, resulting in differences in the RBE [13]. Second, smaller neutron contamination from SSPT may contribute to differences in RBE between these systems [14].
While Gueulette et al. determined RBE for SSPT at three depth positions according to the intestinal crypt regeneration in mice and showed RBE values of 1.11 for the initial plateau, 1.16 for the center, and 1.21 for the distal region of the SOBP [15], RBE values could not be estimated at positions with high-dose gradients, such as the distal fall-off of the SOBP, reflecting poor estimates of precise positions with in vivo experiments. The goal of this study is to derive an RBE suitable for SSPT, including the value at the distal fall-off of the SOBP. We performed a clonogenic survival assay using a V79 cell line and estimated the RBE values at six different depths (a 5 mm depth point from the primary plane, three points in the SOBP plateau and two points in the distal fall-off).
MATERIALS AND METHODS
Cell culture
V79 cells were obtained from the RIKEN Cell Bank (Tsukuba, Ibaraki, Japan) and cultured in α-MEM (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (BioWest, Nuaillé, France) at 37°C in 5% CO2/95% air.
Irradiation
Cells were seeded in a chamber slide flask (Thermo Scientific/Nunc, Penfield, NY) at 1.8 × 106 cells/flask, incubated for 6 h and then filled with α-MEM immediately prior to irradiation.
Proton beams were generated using a ProBeat RT (Hitachi, Tokyo, Japan), with an SOBP width of 6 cm, an energy range of 156.7–182.8 MeV, spot spacing of 5 mm and a field size of 10 × 10 cm. The isocenter plane was matched with the center of the SOBP, and dose flatness of the SOBP was ±2.5% compared with the center of the SOBP in the depth direction. The average dose rate was 2.68 Gy/min. As reference photon beams, 6 MV X-rays were generated using a linear accelerator (Mitsubishi Electric Co., Tokyo, Japan) with a field size of 10 × 10 cm, and the dose rate was ∼2.5 Gy/min. According to the protocol of Japanese Standard Dosimetry 12 [16], the measurement of the dose and dose-rate was conducted with a PTW Markus Chamber (Type 34045; PTW, Freiburg, Germany) and an electrometer (Type 10021; PTW, Freiburg, Germany).
To position cells during irradiation, we designed a high-density polyethylene block with a water equivalent ratio = 1.03, ρ = 0.98 g/cm3 and varied the thickness of the up-front block to set depths of cells in the beams. To investigate the depth dependency of the RBE, proton beam irradiation was performed at the following six points: (a) at a 5 mm depth from the primary plane, (b) at the proximal 95% physical dose point to the center of the SOBP, (c) at the center of the SOBP, (d) at the distal 95% physical dose point to the SOBP center, (e) at the distal 73% physical dose point to the SOBP center and (f) at the distal 33% physical dose point to the SOBP center (Table 1). The high-density polyethylene block was also used during X-ray irradiation, and the buildup was 1.5 cm in water. In order to compensate back and side scatter, the blocks werelocated in the back or at the side of the block in which the slide flask was inserted. The flatness of the irradiated field was ±0.72%.
Table 1.
Survival parameters and biological equivalent doses for V79 cells irradiated with X-rays or proton beams at various depths
| Position | Dose fraction | Depth (mm) | α (Gy−1) | β (Gy−2) | α/β (Gy) | D10 (Gy) | RBE10 | D37 (Gy) | RBE37 | RBE2Gy | RBE4Gy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.66 | 5 | 0.18 | 0.0191 | 9.42 | 7.19 | 1.24 | 3.87 | 1.35 | 1.44 | 1.32 |
| B | 0.95 | 165 | 0.16 | 0.0203 | 7.88 | 7.42 | 1.2 | 4.09 | 1.28 | 1.54 | 1.37 |
| C | 1 | 190 | 0.14 | 0.0200 | 7.00 | 7.73 | 1.15 | 4.32 | 1.21 | 1.46 | 1.33 |
| D | 0.95 | 220 | 0.32 | 0.0117 | 27.35 | 5.94 | 1.5 | 2.83 | 1.85 | 2.52 | 2.04 |
| E | 0.73 | 222.5 | 0.24 | 0.0191 | 12.57 | 6.42 | 1.39 | 3.32 | 1.58 | 1.96 | 1.68 |
| F | 0.33 | 224 | 0.22 | 0.0209 | 10.53 | 6.15 | 1.45 | 3.3 | 1.58 | 1.86 | 1.65 |
| X-rays | 0.09 | 0.0185 | 4.86 | 8.91 | 5.23 |
Clonogenic survival assay
The clonogenic survival assay was performed according to the previous study by Matsumoto et al. [17]. Briefly, cells were trypsinized by incubating with 0.25% trypsin/1 mM EDTA solution at 37°C in 5% CO2/95% air for 3 min and harvested immediately after irradiation. After washing with PBS, cells were diluted with α-MEM, and were seeded on 6 cm dishes at densities from 100 to 50 000 cells per dish to yield ∼100 colonies per dish depending on the radiation dose, followed by culturing for 7 days. Colonies were fixed with methanol and stained with Giemsa solution. Colonies containing >50 cells were recorded as surviving cells. Surviving fractions at each dose were calculated with respect to plating efficiencies of non-irradiated controls and were plotted for physical doses. Survival curves were fitted using the following linear–quadratic (LQ) model: SF = exp (−αD − βD 2), where SF is the surviving fraction and D is the physical dose. At D10 and D37, cell survival was reduced to 10% and 37%, respectively, and RBE10, RBE37, RBE2Gy and RBE4Gy values were calculated as the ratio of the D10, D37 and isosurviving fraction at 2 Gy and 4 Gy to that of 6 MV X-rays. All experiments were performed at least three times.
RESULTS AND DISCUSSION
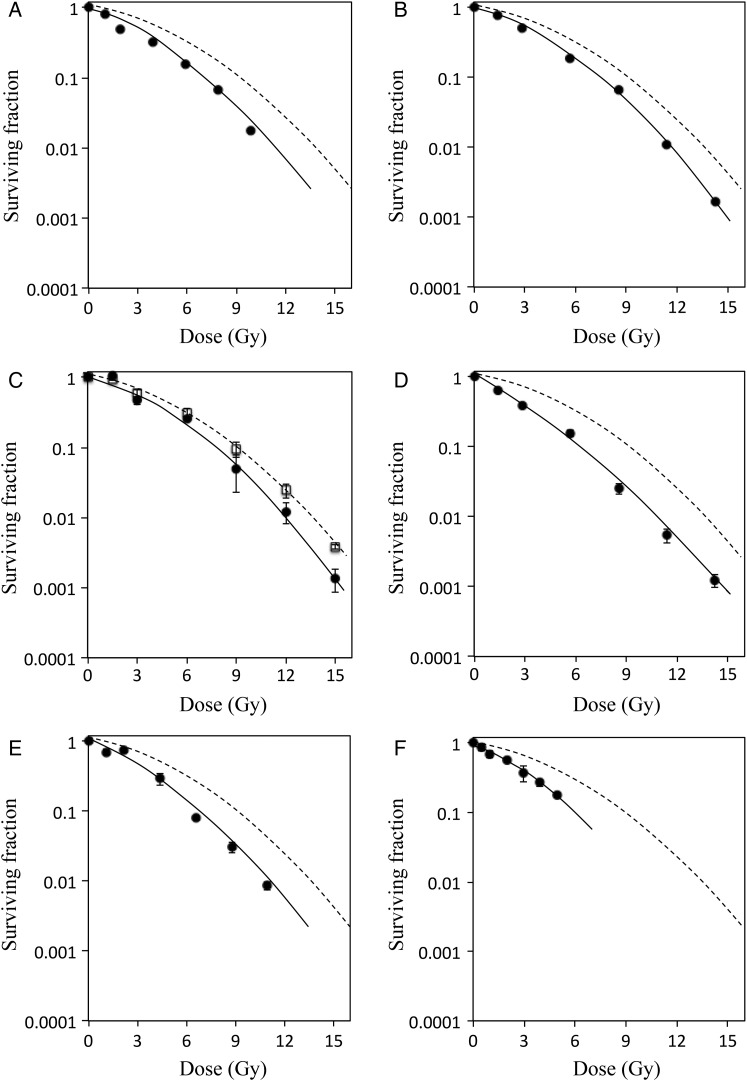
V79 cells were irradiated at multiple irradiation positions with X-rays or proton beams and survival curves were calculated (Fig. 1). Dose–response curves of proton irradiation were lower than those of X-rays at all positions. Although positions (b) and (d) received the same 95% physical doses relative to that at the center of the SOBP, the shapes of survival curves differed and the curve of (d) was close to linear compared with that of (b), indicating greater cell death at this position.
Fig. 1.
Survival curves of V79 cells irradiated with X-rays or with protons at different depths. Clonogenic survival of V79 cells irradiated with X-rays (white square, dashed line) or protons (closed circle, solid line) at each depth (A–F as shown in Table 1); surviving colonies were stained and counted after 7 days of incubation. Dose–response curves were fitted using linear–quadratic models as described in Materials and Methods, and RBE10 and RBE37 values were calculated from D10 and the D37 values. With regard to spatial resolution of dosimetry system, the dose error was <0.5%.
As shown in Table 1, RBE10 and RBE37 values were 1.15 and 1.21, respectively, at the center of the SOBP (c). The measured RBE10 and RBE37 values were in close agreement with previous studies of V79 cells that were performed in other proton beam facilities with passive scattering systems [18, 19]. In addition to V79 cells, RBE values were determined at the center of the SOBP in human salivary gland (HSG) cells, with RBE10 of 1.02 and RBE37 of 1.04 (data not shown). These values of HSG cells were also similar to those made at facilities employing passive scattering methods [6], suggesting that different irradiation systems produce similar RBE values.
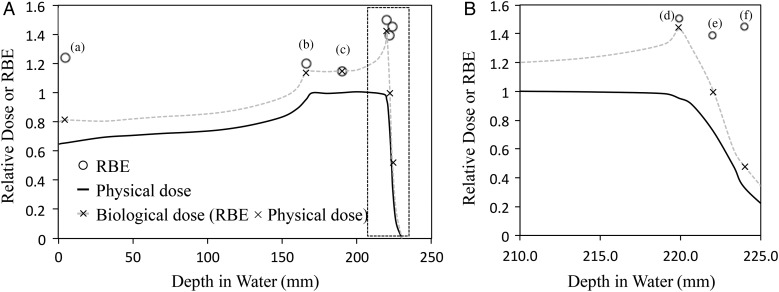
Biological doses (RBE × physical doses) are critical for treatment planning and those obtained in this study were 0.81, 1.2, 1.15, 1.4, 1.01 and 0.52 (×; Fig. 2) at positions (a), (b), (c), (d), (e) and (f), respectively. The biological dose was enhanced at the distal edge of the SOBP, and the biological proton range (90% position of the biological dose) was extended by 2.2 mm compared with the physical range. This result is in good agreement with the theoretical report by Wilkens et al. [20] and the experimental data using the passive scattering method by Matsumoto et al. [17]. Therefore, these results indicated that treatment planning should take into consideration the biological dose distribution, although whether the variation in RBE is directly linked to clinical results has not been clarified [21, 22]. Parameters of α, β and α/β were calculated at each depth (Table 1). The α values in proton beams ranged from 0.14 to 0.32 and were higher than those of X-rays. Moreover, α values of protons tended to increase with depth of the SOBP, with a maximum value of 0.32 at the distal edge (d). However, the β values showed smaller variation, with values of 0.02 ± 0.01 at all positions. These observations suggest that RBE values reflect α coefficients rather than β coefficients.
Fig. 2.
Relative dose–depth distributions in V79 cells. Relative physical doses (black solid line), RBE10 values calculated at D10 (white circle) and RBE10 × physical doses (×) at each depth (a–f as shown in Fig. 2) of the proton beam; the physical dose was normalized at the SOBP center. Data are presented for (A) all positions (a–f) from 0 to 245 mm depth from water and (B) the distal region (d–f) from 210.0 to 225.0 mm depth from water.
Wouters et al. [23] reported that cells with low α/β values, such as V79 cells, showed low a dose-dependent increase in RBE, especially below 4 Gy, and 4 Gy was the minimum dose indicating the stable RBE. To test this dose dependence of RBE, we calculated RBE4Gy and RBE2Gy values at each irradiation point from the survival curves (Table 1). The RBE4Gy values obtained in the SOBP were >1.37 and higher than the RBE10 and RBE37 values at all positions, but the increase in RBE was modest. On the other hand, RBE2Gy values were significantly higher than RBE4Gy values, indicating the low-dose dependence of RBE in accordance with the results of Wouters et al. This result suggested that radiation oncologists or medical physicists should give consideration to the increase in biological effect at the distal region of the SOBP in the treatment planning of practical fractionated SSPT, especially if the normal tissue consists of cells with low α/β values positioned near the target region. Biological optimization, which has been proposed by several authors [24], may be a promising approach that incorporates the variable RBE into the inverse planning process of SSPT treatment planning.
In summary, we measured the RBE of V79 cells in the SSPT system at the Hokkaido University Hospital Proton Therapy Center. In agreement with previous studies of passive scattering systems, RBE10 and RBE37 values at the center of the SOBP were 1.15 and 1.21, respectively. Moreover, RBE values at the distal region increased with increasing α values. The present data suggest close attention should be paid to variable RBE values during treatment planning for humans.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
FUNDING
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 15H04768 (HS), 15K09984 (TM), 2646187504 (TY) and15K09983 (HY). Funding to pay the Open Access publication charges for this article was provided by the JSPS KAKENHI Grant Number 15H04768 (HS).
REFERENCES
- 1.van de Water TA, Bijl HP, Shilstra C et al. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist 2011;16:366–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jermann M. Particle Therapy Statistics in 2014. Int J Particle Ther 2015;2:50–4. [Google Scholar]
- 3.Pedroni E, Bacher R, Blattmann H et al. The 200-MeV proton therapy project at the Paul Scherrer Institute: conceptual design and practical realization. Med Phys 1995;22:37–53. [DOI] [PubMed] [Google Scholar]
- 4.Gillin MT, Sahoo N, Bues M et al. Commissioning of the discrete spot scanning proton beam delivery system at the University of Texas M.D. Anderson Cancer Center, Proton Therapy Center, Houston. Med Phys 2010;37:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol 1999;50:135–42. [DOI] [PubMed] [Google Scholar]
- 6.Aoki-Nakano M, Furusawa Y, Uzawa A et al. Relative biological effectiveness of therapeutic proton beams for HSG cells at Japanese proton therapy facilities. J Radiat Res 2014;55:812–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilly N, Johansson J, Isacsson U et al. The influence of RBE variations in a clinical proton treatment plan for a hypopharynx cancer. Phys Med Biol 2005;50:2765–77. [DOI] [PubMed] [Google Scholar]
- 8.Gensheimer MF, Yock TI, Liebsch NJ et al. In vivo proton beam range verification using spine MRI changes. Int J Radiat Oncol Biol Phys 2010;78:268–75. [DOI] [PubMed] [Google Scholar]
- 9.Jones B, Underwood TS, Dale RG. The potential impact of relative biological effectiveness uncertainty on charged particle treatment prescriptions. Br J Radiol 2011;84 Spec No 1:S61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek HJ, Kim TH, Shin D et al. Radiobiological characterization of proton beam at the National Cancer Center in Korea. J Radiat Res 2008;49:509–15. [DOI] [PubMed] [Google Scholar]
- 11.Paganetti H, Niemierko A, Ancukiewicz M et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407–21. [DOI] [PubMed] [Google Scholar]
- 12.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose and linear energy transfer. Phys Med Biol 2014;59:419–72. [DOI] [PubMed] [Google Scholar]
- 13.Paganetti H, Schmitz T. The influence of the beam modulation method on dose and RBE in proton radiation therapy. Phys Med Biol 1996;41:1649–63. [DOI] [PubMed] [Google Scholar]
- 14.Schneider U, Agosteo S, Pedroni E et al. Secondary neutron dose during proton therapy using spot scanning. Int J Radiat Oncol Biol Phys 2002;53:244–51. [DOI] [PubMed] [Google Scholar]
- 15.Gueulette J, Blattmann H, Pedroni E et al. Relative biologic effectiveness determination in mouse intestine for scanning proton beam at Paul Scherrer Institute, Switzerland. Influence of motion. Int J Radiat Oncol Bio Phys 2005;62:838–45. [DOI] [PubMed] [Google Scholar]
- 16.JSMP. Standard Dosimetry of Absorbed Dose in External Beam Radiotherapy (Standard Dosimetry 12). Tokyo: Tsusho Sangyo Kenkyu Sha, 2012. [Google Scholar]
- 17.Matsumoto Y, Matsuura T, Wada M et al. Enhanced radiobiological effects at the distal end of a clinical proton beam: in vitro study. J Radiat Res 2014;55:816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slabbert JP, Jones DTL, Schreuder N et al. Variations in biological effectiveness with depth in a 200 MeV proton beam. National Accelerator Centre (NAC) Annual Report 1994;103–06.
- 19.Robertson JB, Eaddy JM, Archambeau JO et al. Relative biological effectiveness and microdosimetry of a mixed energy field of protons up to 200 MeV. Adv Space Res 1994;14:271–5. [DOI] [PubMed] [Google Scholar]
- 20.Wilkens JJ, Oelfke U. A phenomenological model for the relative biological effectiveness in therapeutic proton beams. Phys Med Biol 2004;49:2811–25. [DOI] [PubMed] [Google Scholar]
- 21.Sethi RV, Giantsoudi D, Raiford M et al. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int J Radiat Oncol Biol Phys 2014;88:655–63. [DOI] [PubMed] [Google Scholar]
- 22.Jones B. The case for particle therapy. Br J Radiol 2006;79:24–31. [DOI] [PubMed] [Google Scholar]
- 23.Wouters BG, Skarsgard LD, Gerweck LE et al. Radiobiological intercomparison of the 160 MeV and 230 MeV proton therapy beams at the Harvard Cyclotron Laboratory and at Massachusetts General Hospital. Radiat Res 2015;183:174–87. [DOI] [PubMed] [Google Scholar]
- 24.Wilkens J, Oelfke U. Optimization of radiobiological effects in intensity modulated proton therapy. Med Phys 2005;32:455–65. [DOI] [PubMed] [Google Scholar]