Abstract
The biological effect of ionizing radiation (IR) on genomic DNA is thought to be either direct or indirect; the latter is mediated by IR induction of free radicals and reactive oxygen species (ROS). This study was designed to evaluate the effect of N-acetyl-L-cysteine (NAC), a well-known ROS-scavenging antioxidant, on IR induction of genotoxicity, cytotoxicity and ROS production in mammalian cells, and aimed to clarify the conflicting data in previous publications. Although we clearly demonstrate the beneficial effect of NAC on IR-induced genotoxicity and cytotoxicity (determined using the micronucleus assay and cell viability/clonogenic assays), the data on NAC's effect on DNA double-strand break (DSB) formation were inconsistent in different assays. Specifically, mitigation of IR-induced DSBs by NAC was readily detected by the neutral comet assay, but not by the γH2AX or 53BP1 focus assays. NAC is a glutathione precursor and exerts its effect after conversion to glutathione, and presumably it has its own biological activity. Assuming that the focus assay reflects the biological responses to DSBs (detection and repair), while the comet assay reflects the physical status of genomic DNA, our results indicate that the comet assay could readily detect the antioxidant effect of NAC on DSB formation. However, NAC's biological effect might affect the detection of DSB repair by the focus assays. Our data illustrate that multiple parameters should be carefully used to analyze DNA damage when studying potential candidates for radioprotective compounds.
Keywords: DNA double-strand breaks, γH2AX, 53BP1, comet assay, radiation
INTRODUCTION
Ionizing radiation (IR) induces a variety of genomic DNA lesions, the most detrimental being DNA double-strand breaks (DSBs). The biological effects of IR are thought to be either direct or indirect effects, the latter being mediated by IR induction of free radicals and reactive oxygen species (ROS). It is generally estimated that approximately one-third of IR-induced DNA damage is cause by the former and two-thirds by the latter [1]. Thus scavenging of free radicals and reduction of ROS is critical for radioprotection. In this regard, antioxidants have long been investigated as potential radioprotective agents.
One such compound is the antioxidant N-acetyl-L-cysteine (NAC), which is a glutathione precursor containing an -SH group, the use of which leads to increased intracellular glutathione concentrations and thereby ROS scavenging. The protective effect of NAC on IR-induced DNA and cellular damage has been extensively studied using different assays, such as the comet assay (single cell gel electrophoresis) [2], micronucleus assay [3], immunochemical staining for γH2AX [4] or 53BP1 foci [5], and/or cell viability/clonogenic assays in mammalian cells; however, the data derived from these studies is inconsistent. In various studies, NAC has been observed to either suppress [6–10] IR-induced DSBs or to fail to do so [11]; similarly, NAC has been reported to succeed [12] or fail [13, 14] to protect cells from IR-induced cell death; and finally, NAC was reported to suppress IR-induced DSBs while not improving cell survival [7, 8].
Therefore, this study was designed to evaluate the effect of NAC on IR-induced genotoxicity and cytotoxicity using various methods, as well as to measure ROS production in parallel in mammalian cells.
MATERIALS AND METHODS
Cells and cell culture
Normal differentiated rat thyroid cells (PCCL3) were cultured as previously described [15]. Immortalized human fibroblast BJ cells stably expressing telomerase reverse transcriptase (BJ-hTERT) were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum.
NAC pretreatment, irradiation and H2O2 treatment
Cells were pretreated with 20 or 4 mM NAC (Sigma–Aldrich, St Louis, MD) for 15 min and X-irradiated using an ISOVOLT Titan 320 (GE Inspection, Tokyo, Japan) or treated with 100 μM H2O2 for 10 min at 37°C. X-rays were administered as a single dose of 1–5 Gy at a dose rate of 0.8903 Gy/min.
Detection of DSBs by 53BP1 and γH2AX stainings
At the indicated time-points after irradiation, the cells were fixed with 3.7% formaldehyde for 10 min followed by permeabilization with 0.5% Triton-X and immunofluorescent analysis. The primary antibodies used were rabbit anti-p53-binding protein 1 (53BP1) (1:200; Bethyl, Montgomery, TX) and mouse anti-phospho-H2AX (γH2AX) (1:200; Millipore, Darmstadt, Germany). Secondary antibodies used were Alexa Fluor488 goat anti-rabbit IgG and anti-mouse IgG (Life Technologies, Tokyo, Japan). The cells were embedded with VECTASHIELD Mounting Medium containing DAPI (Vector Laboratories, Burlingame, CA). The slides were analyzed using an All-in-One BZ-9000 Fluorescence Microscope (Keyence, Osaka, Japan) and the number of nuclear foci per cell was counted in 300 cells. Alternatively, fluorescent intensity of γH2AX staining per cell was quantified using a BZ-II Analyzer (Keyence, Osaka, Japan) in 300 cells.
Detection of DSBs by the neutral comet assay
The neutral comet assay was performed using the Trevigen Comet Assay kit (Trevigen, Gaithersburg, MD). At the indicated time-points after irradiation, the cells were suspended in cold PBS, and an aliquot of cells (103/10 μl) was added to 100 μl of molten LMA agarose maintained at 39°C. An aliquot of 10 μl was immediately spread onto each comet slide at 37°C. The slide was incubated at 4°C for 10 min to accelerate gelling of the agarose and then transferred to cold lysis solution for 60 min at 4°C. A denaturation step was performed in 50 mM Tris base, 150 mM CH3COONa3H2O, pH 9, for 30 min at 4°C. The slides were then subjected to electrophoresis with cold TAE buffer, pH 8.2 at 25 V for 30 min at 4°C, and immersed in DNA precipitation solution (100 mM NH4Ac in 95% ethanol) and then in 100% ethanol for 30 min and air dried. DNA was stained with 100 μl SYBR Gold (Trevigen, 1:30,000) for 20 min and immediately rinsed with dH2O and air dried. The slides were analyzed using an All-in-One BZ 9000 fluorescence microscope and CometScore free ware v1.5 (TriTek, Niigata, Japan).
Detection of intracellular ROS
Cells in 96-well plates (1 × 105 cells/well) were washed twice with PBS, incubated with 5 μM 2′,7′-dichlorofluorescin diacetate (DCFDA) (Life Technologies) for 30 min at 37°C and washed twice with PBS. Immediately after radiation exposure, the plates were read with a 2030 Multilabel Plate Reader ARVO X3 (PerkinElmer, Branchburg, NJ) using excitation and emission wavelengths of 485 and 528 nm, respectively.
Clonogenic assay
Cells in 6-well plates (4 × 103 cells/well) were treated with NAC and X-irradiation. Ten days later, the cells were fixed for 10 min, and stained with 1% crystal violet (Wako, Osaka, Japan) in 10% ethanol for 10 min. The number of colonies containing 50 or more cells was calculated.
Cell viability assays
Cells in 96 well plates (2 × 103 cells/well) were treated with NAC and X-irradiation. After a 7-day incubation, cell numbers were determined with the water-soluble tetrazolium salt (WST) assay using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer's protocol.
Micronucleus assay
Cells in 12-well plates (4 × 105 cells/well) were treated with NAC and X-irradiated. Immediately after irradiation, the cells were cultured with 0.5 μg/ml of cytochalasin B (a cytokinesis inhibitor; Wako) for 48 h. The cells were fixed for 10 min, stained with DAPI and analyzed using an All-in-One BZ 9000 fluorescence microscope at a magnification of ×400. Micronuclei were scored as having a diameter that was less than one-third of the main nuclei. The number of micronuclei in 100 binucleated cells was determined.
Statistical analysis
All data are expressed as mean ± S.E. and differences between groups were examined for statistical significance using the Student's t-test. P < 0.05 was used to identify statistically significant differences.
RESULTS AND DISCUSSION
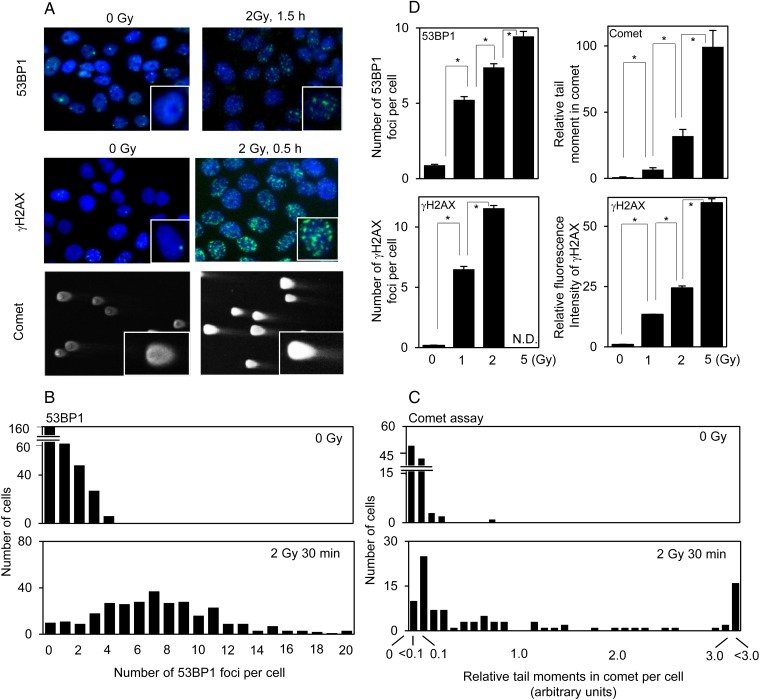
As shown in Fig. 1A, 2 Gy IR induced distinct 53BP1 and γH2AX immunofluorescent nuclear foci, as well as the typical ‘head and tail’ pattern (representing intact DNA and DSBs, respectively) in the neutral comet assay in PCCL3 cells. Figure 1B and C shows representative quantitation of the distributions of 53BP1 foci per cell and comet assay tail moments, which is thought to be the most sensitive parameter for this assay [11], in control and irradiated cells. Quantification of γH2AX foci resulted in data similar to that of 53BP1 (data not shown). A clear dose dependency was observed in the comet assay, 53BP1 foci, and fluorescence intensity of γH2AX between 0 and 5 Gy; however, 5 Gy was not evaluated with γH2AX foci because of the previously reported problem with focus overlap at 5 Gy [16] (Fig. 1D; Supplementary Fig. 1).
Fig. 1.
DSBs determined by 53BP1/γH2AX staining and the neutral comet assay in control PCCL3 cells and cells irradiated with 2 Gy IR: (A) representative photographs of 53BP1/γH2AX staining and the comet assay; (B and C), the distribution of the number of 53BP1 foci and tail moments in the comet assay, respectively, in control and irradiated cells. (D) effect of IR dose (0 to 5 Gy) on 53BP1/γH2AX staining and the comet assay. *P < 0.01.
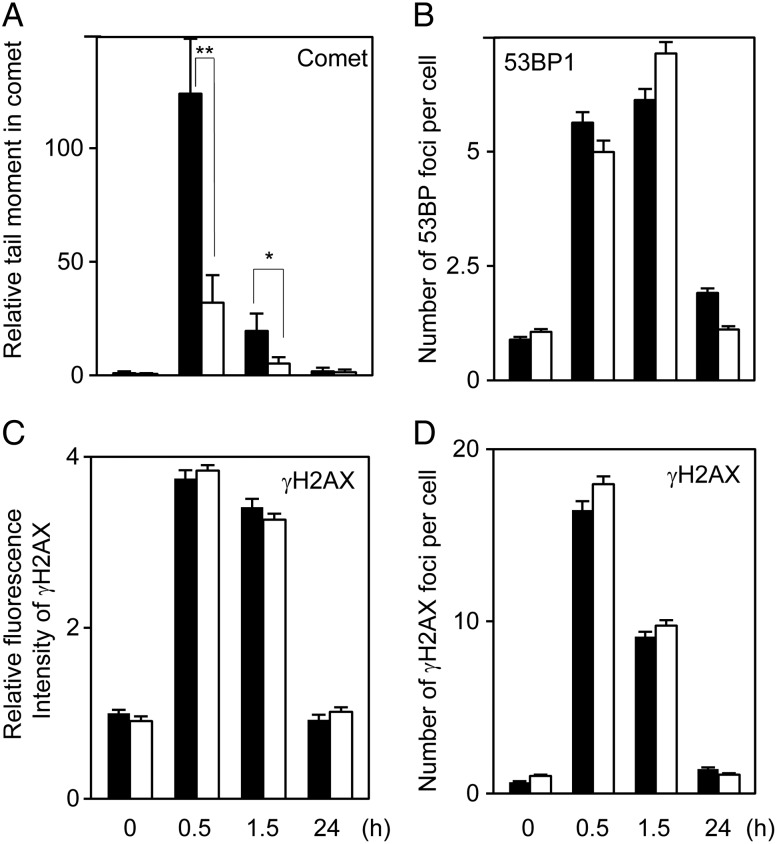
The temporal effects of NAC (20 mM) on DSBs were examined from 30 min to 24 h after 2 Gy irradiation (Fig. 2). The 2 Gy IR doubled intracellular ROS levels, and NAC almost completely suppressed IR-induced ROS production (Fig. 3A). In the comet assay (Fig. 2A) the tail moments were significantly ameliorated by NAC pretreatment at 0.5 and 1.5 h post-IR and almost completely reverted to basal levels at 24 h post-IR, irrespective of the presence/absence of NAC. Unexpectedly, the numbers of foci in the 53BP1 and γH2AX focus formation assays and the fluorescent intensity of γH2AX at 0.5 and 1.5 h post-IR were unchanged after NAC pretreatment (Fig. 2B–D). It should be noted here that peak γH2AX fluorescence was observed at 0.5 h post-IR, while peak 53BP1 was observed at 1.5 h post-IR in our study; this is consistent with previous reports [17, 18]. Thus, the data on NAC's effects on DSBs detected with the comet assay versus the focus/fluorescence assays are contradictory. Results that were essentially the same were observed with the lower concentration of NAC (4 mM), with DSB prevention being much weaker despite the complete suppression of ROS generation (Supplementary Fig. 2).
Fig. 2.
The temporal effects of 20 mM NAC on IR-induced DSBs. DSBs were determined by the neutral comet assay (A), 53BP1 foci (B), γH2AX foci (C) and γH2AX fluorescent intensity (D) from 0.5 to 24 h after 2 Gy irradiation. The open and solid bars indicate the data obtained with/without NAC, respectively. *P < 0.05; **P < 0.01.
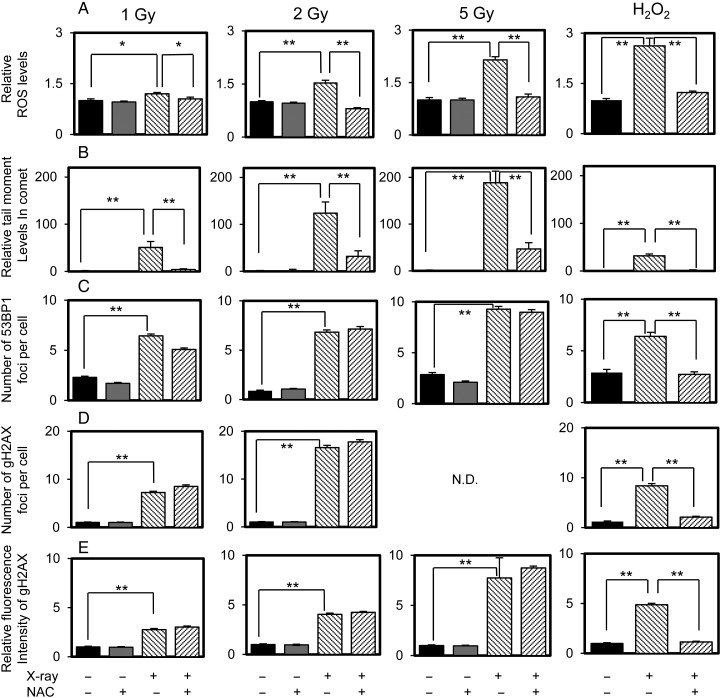
Fig. 3.
Intracellular ROS levels (A), and DSBs determined by the neutral comet assay (B) and 53BP1/γH2AX staining (C to E) in control PCCL3 cells and cells pretreated with 20 mM NAC and/or irradiated or exposed with H2O2. The data were obtained 0.5 h (the comet assay and γH2AX) or 1.5 h (53BP1) after irradiation or 10 min after addition of H2O2. *P < 0.05; **P < 0.01.
Next, experiments were carried out with 1–5 Gy IR to determine the dose dependency of IR. Figure 3 shows the comet assay and γH2AX staining results at 0.5 h post-IR, and 53BP1 staining at 1.5 h post-IR. IR increased intracellular ROS levels in a dose-dependent manner with a significant, yet trivial, increase observed at 1 Gy (an increase of ∼×1.2); NAC almost completely suppressed IR-induced ROS production within the range of 1–5 Gy (Fig. 3A). In parallel, DSBs determined using the comet assay were also decreased by NAC, although its effect was almost complete at 1 Gy and it was less effective at higher irradiation doses (Fig. 3B), which is consistent with previous reports [7, 8]. However, a similar inhibitory effect of NAC on DSB formation was not observed with 53BP1 and γH2AX staining (Fig. 3C and E). The possible metabolism of ROS by catalase in the culture medium [19] was excluded by confirming the absence of an effect of NAC on 53BP1 foci and ROS levels using serum-free media (data not shown). In contrast, NAC-suppression of H2O2-induced ROS production and DSB formation detected by using the comet assay and 53BP1 or γH2AX staining was clearly demonstrated (Fig. 3A–E). Further, data that was essentially equivalent was obtained with fibroblasts (Supplementary Fig. 3), indicating that the contradictory data between 53BP1/γH2AX staining and the comet assay are not cell-type-specific.
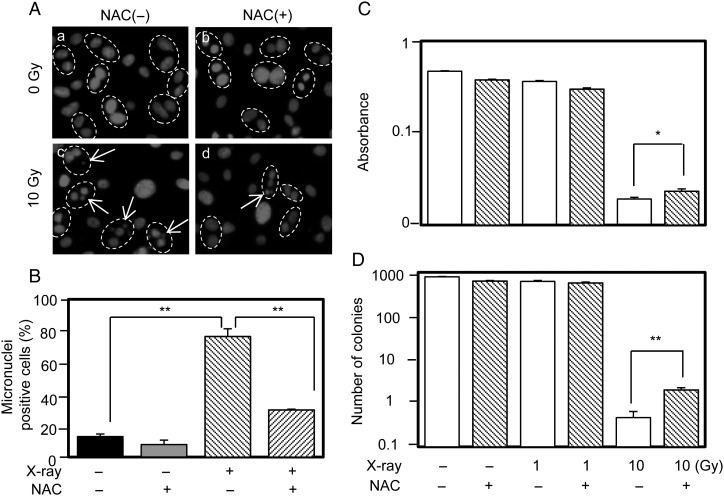
Next, experiments were performed to evaluate the effect of NAC on IR-mediated genotoxicity and cytotoxicity using the micronucleus assay (performed 48 h post-IR) and a cell survival assay (performed 7–10 days post-IR). It was observed that 10 Gy IR increased the number of micronuclei, which was significantly decreased by NAC (Fig. 4A and B). Similarly, 10 Gy IR killed most of the cells and significantly suppressed cell/colony numbers as determined by the WST and clonogenic assays, respectively, with NAC clearly improving cell survival in both assays (Fig. 4C and D).
Fig. 4.
Micronuclei and cell survival assays in control PCCL3 cells and cells irradiated and/or pretreated with NAC. (A) Representative photographs of micronuclei. The dashed circles indicate binucleated cells and the arrows indicate micronuclei. The effects of NAC on IR-induced micronuclei-positive cells, cell viability with the WST assay, and the clonogenic assay are shown in B, C and D, respectively. *P < 0.05; **P < 0.01.
Thus, we obtained a complex dataset on the effect of NAC on IR-induced DSBs. The ameliorating effect of NAC was clearly detected by the neutral comet assay (that detects DSBs) and the micronucleus and cell viability/clonogenic assays (both assays reflect not only the degree of DNA damage, but also the efficacy of recovery), but not using 53BP1/γH2AX staining (which also detects DSBs).
As mentioned previously, the protection of DNA from IR-induced DSBs by NAC has been demonstrated in previous reports; for instance, in cultured microvascular endothelial cells and human lymphoblastoid cell lines using the γH2AX focus assay [6–8] and in human lymphocytes using the micronucleus and alkali comet assays (which detect DSBs and single-strand breaks) [10] and using the 53BP1/γH2AX focus assay [9]. In contrast, there are some controversial data stating that NAC does not efficiently mitigate IR-induced DNA damage determined using the alkali comet assay in human lymphocytes [11]. Regarding cell survival/cytotoxicity, NAC improved the survival of irradiated granulocyte/macrophage colony-forming cells [12], but not squamous lung cancer cells (SW-1573) [13] or lymphoblastoid cells [14]. Furthermore, it was reported that NAC protected cells from DNA damage determined using γH2AX foci but not from cell death determined using the clonogenic assay; therefore, caution must be exercised when using γH2AX as a surrogate marker in radioprotection studies [6–8].
The comet assay reflects the physical status of genomic DNA, while 53BP1/γH2AX staining represents processes related to the biological response to DNA damage (e.g. phosphorylation/dephosphorylation and recruitment/release of DSB repair–related molecules such as 53BP1 and γH2AX). Our data indicates that NAC suppresses IR-induced DSBs in the comet assay but not in the focus assays, indicating that the antioxidant NAC exerts its protective effect on IR-induced genomic damage, but may additionally affect cellular responses to DNA damage. Indeed, it has been reported that NAC possesses other biological activities, such as protein stabilization and support of the DNA repair process [12], as well as altering the phosphorylation status of proteins by affecting the activity of receptor-type tyrosine kinases (MAP kinase and IκB kinase) [20]. Furthermore, it has been reported that γH2AX can be induced by other factors, such as UV-induced nucleotide excision [21] and altered osmolarity [22]. Therefore, our data call attention to the fact that multiple parameters should be used carefully for analyzing DNA damage when studying potential radioprotective compound candidates.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Radiation Research online.
FUNDING
This work was supported in part by a Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan [26870440 to TK]. Funding to pay the Open Access publication charges for this article was provided by [26870440].
Supplementary Material
REFERENCES
- 1.Hall E, Giaccia A. Radiobiology for the Radiologist. 7th edn. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2012. [Google Scholar]
- 2.Singh NP, McCoy MT, Tice RR et al. Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988;175:184–91. [DOI] [PubMed] [Google Scholar]
- 3.Fenech M. The in vitro micronucleus technique. Mutat Res 2000;455:81–95. [DOI] [PubMed] [Google Scholar]
- 4.Rogakou EP, Pilch DR, Orr AH et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998;273:5858–68. [DOI] [PubMed] [Google Scholar]
- 5.Ward IM, Minn K, Jorda KG et al. Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J Biol Chem 2003;278:19579–82. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka Y, Bindokas VP, Duggan RC et al. Flow cytometric analysis of phosphorylated histone H2AX following exposure to ionizing radiation in human microvascular endothelial cells. J Radiat Res 2006;47:245–57. [DOI] [PubMed] [Google Scholar]
- 7.Kataoka Y, Murley JS, Baker KL et al. Relationship between phosphorylated histone H2AX formation and cell survival in human microvascular endothelial cells (HMEC) as a function of ionizing radiation exposure in the presence or absence of thiol-containing drugs. Radiat Res 2007;168:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reliene R, Pollard JM, Sobol Z et al. N-acetyl cysteine protects against ionizing radiation–induced DNA damage but not against cell killing in yeast and mammals. Mutat Res 2009;665:37–43. [DOI] [PubMed] [Google Scholar]
- 9.Kuefner MA, Brand M, Ehrlich J et al. Effect of antioxidants on X-ray–induced gamma-H2AX foci in human blood lymphocytes: preliminary observations. Radiology 2012;264:59–67. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari P, Kumar A, Balakrishnan S et al. Radiation-induced micronucleus formation and DNA damage in human lymphocytes and their prevention by antioxidant thiols. Mutat Res 2009;676:62–8. [DOI] [PubMed] [Google Scholar]
- 11.Abt G, Vaghef H, Gebhart E et al. The role of N-acetylcysteine as a putative radioprotective agent on X-ray–induced DNA damage as evaluated by alkaline single-cell gel electrophoresis. Mutat Res 1997;384:55–64. [DOI] [PubMed] [Google Scholar]
- 12.Selig C, Nothdurft W, Fliedner TM. Radioprotective effect of N-acetylcysteine on granulocyte/macrophage colony-forming cells of human bone marrow. J Cancer Res Clin Oncol 1993;119:346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanamarta AH, van Rijn J, Blank LE et al. Effect of N-acetylcysteine on the antiproliferative action of X-rays or bleomycin in cultured human lung tumor cells. J Cancer Res Clin Oncol 1989;115:340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuni AM, DeGraff W, Cook JA et al. The effects of antioxidants on radiation-induced apoptosis pathways in TK6 cells. Free Radic Biol Med 2004;37:1648–55. [DOI] [PubMed] [Google Scholar]
- 15.Mitsutake N, Knauf JA, Mitsutake S et al. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res 2005;65:2465–73. [DOI] [PubMed] [Google Scholar]
- 16.Turner HC, Brenner DJ, Chen Y et al. Adapting the gamma-H2AX assay for automated processing in human lymphocytes. 1. Technological aspects. Radiat Res 2011;175:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leatherbarrow EL, Harper JV, Cucinotta FA et al. Induction and quantification of gamma-H2AX foci following low and high LET-irradiation. Int J Radiat Biol 2006;82:111–18. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Nakashima M, Yamashita S. Dynamics of ionizing radiation-induced DNA damage response in reconstituted three-dimensional human skin tissue. Radiat Res 2010;174:415–23. [DOI] [PubMed] [Google Scholar]
- 19.Ameziane-El-Hassani R, Boufraqech M, Lagente-Chevallier O et al. Role of H2O2 in RET/PTC1 chromosomal rearrangement produced by ionizing radiation in human thyroid cells. Cancer Res 2010;70:4123–32. [DOI] [PubMed] [Google Scholar]
- 20.Kamata H, Manabe T, Kakuta J et al. Multiple redox regulation of the cellular signaling system linked to AP-1 and NFkappaB: effects of N-acetylcysteine and H2O2 on the receptor tyrosine kinases, the MAP kinase cascade, and IkappaB kinases. Ann N Y Acad Sci 2002;973:419–22. [DOI] [PubMed] [Google Scholar]
- 21.Marti TM, Hefner E, Feeney L et al. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc Natl Acad Sci U S A 2006;103:9891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baure J, Izadi A, Suarez V et al. Histone H2AX phosphorylation in response to changes in chromatin structure induced by altered osmolarity. Mutagenesis 2009;24:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.