Abstract
Although radon therapy is indicated for hyperuricemia, the underlying mechanisms of action have not yet been elucidated in detail. Therefore, we herein examined the inhibitory effects of radon inhalation and hot spring water drinking on potassium oxonate (PO)–induced hyperuricemia in mice. Mice inhaled radon at a concentration of 2000 Bq/m3 for 24 h or were given hot spring water for 2 weeks. Mice were then administrated PO at a dose of 500 mg/kg. The results obtained showed that serum uric acid levels were significantly increased by the administration of PO. Radon inhalation or hot spring water drinking significantly inhibited elevations in serum uric acid levels through the suppression of xanthine oxidase activity in the liver. Radon inhalation activated anti-oxidative functions in the liver and kidney. These results suggest that radon inhalation inhibits PO-induced hyperuricemia by activating anti-oxidative functions, while hot spring water drinking may suppress PO-induced elevations in serum uric acid levels through the pharmacological effects of the chemical compositions dissolved in it.
Keywords: hyperuricemia, radon inhalation, hot spring water drinking, anti-oxidative functions
INTRODUCTION
Therapy using radon hot springs (222Rn) is performed for pain- or respiratory-related diseases such as osteoarthritis [1] and bronchial asthma [2] in the Misasa Medical Center, Okayama University Hospital, Japan. Radon therapy is also performed in Europe, and speleotherapy has been reported to have endocrinological effects on respiratory diseases [3]. Although baths with low radon concentrations were previously shown to have no influence on the function of the endocrine system [4], radon therapy is known to be effective against pain-related diseases. For example, a meta-analysis of controlled clinical trials demonstrated the positive effects of radon therapy on pain in rheumatic diseases [5].
Theoretically, animals absorb radon through the lungs and skin. However, radon absorption through the skin has neither been rejected nor confirmed [3]. The mechanism of action of radon therapy has been suggested to involve radon being taken into the lungs via breathing, dissolving in the blood by gas exchange, being transported to many tissues systemically through the bloodstream, and having stimulatory effects in these tissues [6]. In an attempt to elucidate the mechanism of action of radon therapy, we previously developed a radon exposure system for small animals [7]. We subsequently demonstrated that radon inhalation induced the production of anti-oxidant substances in many organs, such as the brain, heart, lung, liver, pancreas, kidney and small intestine of mice [8], and also inhibited carbon tetrachloride (CCl4)–induced hepatopathy and renal damage [9]. These findings suggest that the anti-oxidative functions induced by radon inhalation contribute to the mitigation of reactive oxygen species (ROS)–related diseases.
Drinking treatments are effective against not only pain-related diseases, but also against hyperuricemia due to their effects on purine and uric acid metabolism, and that the pharmacological effects of chemicals dissolved in hot spring water are much greater than from radon [10–13]. The concentrations of dissolved minerals (such as sodium bicarbonate and carbonates) in European hot springs were previously found to be 10-times higher than those in Japanese hot springs; therefore, drinking treatments in Europe only require approximately half of the volume needed in Japan [14]. However, the mechanisms of action of these treatments, particularly those of radon drinking treatments, currently remain unclear.
During the metabolism of purines, hypoxanthine and xanthine are oxidized to uric acid by the enzyme xanthine oxidase (XOD) [15, 16]. XOD is distributed in various cells [17], including not only vascular cells [18, 19], but also the liver, which is one of the major sources of ROS [20–23]. The liver and kidneys are exposed to oxidative stress through increases in serum uric acid levels [24], and hyperuricemia has been found to be inhibited by the administration of anti-oxidants such as vitamin C [25].
The aim of the present study was to investigate and compare the inhibitory effects of radon inhalation and hot spring water drinking treatments on potassium oxonate (PO)–induced hyperuricemia. We focused on PO-induced oxidative stress because, as described above, the liver and kidneys are exposed to oxidative stress through increases in serum uric acid levels. We examined the following biochemical parameters in order to assess the effects of radon treatments: serum uric acid levels, XOD activity in the liver, superoxide dismutase (SOD) activity, catalase (CAT) activity, and total glutathione content (t-GSH) in the liver and kidneys.
MATERIALS AND METHODS
Animals
Male ICR mice (8 weeks of age, body weight 32–38 g) were obtained from Charles River Laboratories Japan Inc. (Yokohama, Japan) for the radon inhalation treatment and from CLEA Japan Inc. (Tokyo, Japan) for the water drinking treatment. They were maintained in plastic cages under controlled conditions of temperature (22 ± 2°C), humidity (55 ± 10%), and light (12 h of light, 12 h of dark), and were given free access to food and water. Ethics approval for all protocols and experiments was obtained from the Animal Experimental Committee of Okayama University.
Experimental procedure
Induction of hyperuricemia
An animal model of hyperuricemia was induced by PO, a urate oxidase (uricase) inhibitor, because the PO model of hyperuricemia is widely perceived as a good experimental model. Briefly, mice were intraperitoneally injected with PO (500 mg/kg body weight; Wako Pure Chemical Industry Co. Ltd, Osaka, Japan) after the radon inhalation or hot spring water drinking treatment. PO was suspended in 0.5% sodium carboxymethylcellulose (CMC-Na; Azuwann Co. Ltd, Osaka, Japan), which was freshly prepared before its administration. Control mice were manipulated in parallel with a 0.5% CMC-Na injection.
Hot spring water drinking treatment
Mice were randomly divided into the following nine groups based on the treatments and time-courses (n = 8–9 for each group): distilled water drinking only (DW), radon-containing hot spring water drinking only (Water with Rn), radon-deaeration hot spring water drinking only (Water without Rn), distilled water drinking with the administration of PO (DW+PO), Rn-containing hot spring water drinking with the administration of PO (Water with Rn+PO) and Rn-deaeration hot spring water drinking with the administration of PO (Water without Rn+PO). Mice were continuously fed distilled water, hot spring water containing radon, or radon deaeration hot spring water (from which radon was removed) for 2 weeks. Radon-containing hot spring water was obtained from the Misasa Medical Center, Okayama University Hospital, with attention to the water foaming and dissipation of radon. Radon-deaeration hot spring water was obtained by bubbling Rn-containing hot spring water using an air pump for ∼20 min to dissipate radon. After 2–3 days of storage, hot spring water was supplied to mice at room temperature. Drinking water was replaced three times a week.
The pH of hot spring water was ∼7.0–7.3. Table 1 shows the principal chemical compositions of hot spring water for the drinking treatment. The radon concentration in water was measured using a liquid scintillation counter. The radon concentration in and drinking volume of water were monitored continuously at 2- or 3-day intervals (Table 2). Mean radon concentrations in Rn-containing hot spring water and Rn-deaeration hot spring water were 338 ± 11 Bq/l and 1.8 ± 0.4 Bq/l, respectively, at the initiation of the treatments (Table 2).
Table 1.
Principal chemical compositions of drinking water
| Element | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Cr | Cu | Zn | Cd | Pb | As | Se | Sb | B | Al | Ni |
| 0.11 | <0.01 | <0.005 | 0.024 | 0.011 | <0.005 | <0.005 | 0.29 | <0.005 | <0.005 | 1.6 | <0.005 | <0.005 |
All values are in milligrams per liter.
Table 2.
Radon concentrations in hot spring water and drinking volume
| Parameters | Rn-containing hot spring water | Rn-deaeration hot spring water | Distilled water |
|---|---|---|---|
| Concentrations of water (Bq/l) | |||
| at the start point of supplying | 338 ± 11 | 1.8 ± 0.4 | |
| at the end point of supplying | 62 ± 2 | N.D. | N.D. |
| Drinking volume (ml/day/capita) | 2.85 ± 0.38 | 2.32 ± 0.33 | 2.26 ± 0.18 |
N.D. = not detected.
After the drinking treatments, hyperuricemia was induced in mice via the intraperitoneal (i.p.) administration of a single dose of PO (500 mg/kg body weight) in CMC-Na. Mice were sacrificed by an overdose of ether anesthesia 1.5 and 3 h after the administration of PO. Blood was drawn from the heart for a serum analysis, and the livers and kidney were surgically excised and rinsed in 10 mM phosphate buffered saline (PBS; pH 7.4) buffer to analyze the activities of XOD, SOD and CAT, and the levels of t-GSH and proteins. Serum was separated by centrifugation at 3000 × g for 5 min for the uric acid assay. Samples were preserved at –80°C for later biochemical analyses.
Radon inhalation treatment
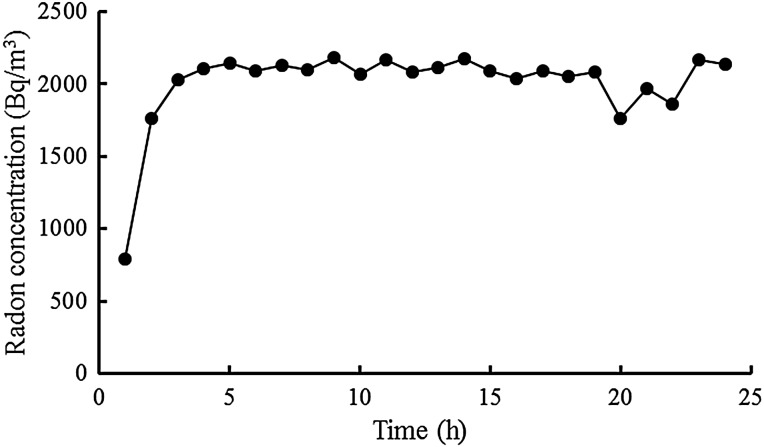
Mice were randomly divided into three groups (n = 6 for each group): sham inhalation only (Control), sham inhalation with the administration of PO only (PO), and radon inhalation with the administration of PO (Rn+PO). Mice were exposed to air only or radon for 24 h (using the radon exposure system we previously developed) and fed normal drinking water. Briefly, radon at a concentration of 2000 Bq/m3 was blown into a mouse cage [26]). The radon concentration in the cages was then determined by reference to radon therapy at the Misasa Medical Center, Okayama University Hospital [1, 2]. Radon concentrations were measured using a radon monitor (CMR-510; Femto-Tech Inc., Carlisle, OH, USA). Radon concentrations in mouse cages are shown in Fig. 1. The mean radon concentrations achieved by the inhalation treatments were ∼2000 Bq/m3 (Fig. 1).
Fig. 1.
Changes in radon concentrations in the mouse cage over the period of radon inhalation using a radon inhalation system.
Hyperuricemia was induced in mice after inhalation by the same method as that for the drinking treatment experiment. Blood was drawn from the heart 3 h after the administration of PO for a serum analysis, the livers and kidneys were surgically excised, and specimens were treated using similar procedures to those described for the drinking treatment experiment. Samples were preserved at –80°C for later biochemical analyses. Samples were obtained from mice treated without PO immediately after inhalation, using the same procedures.
Biochemical assays
Serum uric acid levels were measured according to Takagi's modification of the phosphotungstic acid method described by Caraway et al. [27]. Briefly, serum was deproteinized by a mixture of phosphotungstic acid and sodium hydroxide solutions and centrifuged at 3000 × g at 4°C for 5 min. Deproteinized serum was collected, mixed with phosphotungstic acid and sodium carbonate solutions, and then incubated at 25°C for 25 min. The optical density of the colored product, tungsten blue, was read at 710 nm using a spectrophotometer. All reagents and chemicals used for the uric acid assay were from Wako Pure Chemical Industries Co. Ltd (Osaka, Japan).
XOD activity in the liver was measured using the Xanthine Oxidase Activity Colorimetric/Fluorometric Assay Kit (BioVision Inc., Milpitas, CA, USA) according to the manufacturer's recommendations. Briefly, the liver was homogenized in XOD assay buffer and the homogenate was centrifuged at 16 000 × g at 4°C for 10 min in order to obtain a clear XOD extract. Supernatants were collected, mixed with the XOD enzyme mix, substrate mix, OxiRed™ Probe, and reaction mix, and the absorbance was then read at 570 nm using a spectrophotometer at 10-min intervals by incubating at 25°C for 30 min. In the XOD assay, protein concentrations were measured using the Lowry method with the Bio-Rad DC protein assay kit (Bio-Rad Laboratories Inc., Tokyo, Japan).
Livers and kidneys were homogenized on ice in 10 mM PBS. The homogenates were centrifuged at 12 000 × g at 4°C for 45 min and the supernatants were used to measure SOD and CAT activities.
SOD activity was measured by the nitroblue tetrazolium (NBT) reduction method [28] using the Wako-SOD test (Wako Pure Chemical Industry Co. Ltd, Osaka, Japan) according to the manufacturer's recommendations. Briefly, inhibition of the reduction of NBT was measured at 560 nm using a spectrophotometer. One unit of enzyme activity was defined as 50% inhibition of NBT reduction.
CAT activity was measured as the rate of hydrogen peroxide (H2O2) reduction at 37°C at 240 nm using a spectrophotometer [29]. The assay mixture consisted of 50 μl of 1 M Tris (tris-hydroxymethyl-aminomethane)-HCl buffer containing 5 mM ethylenediaminetetraacetic acid (pH 7.4), 900 μl of 10 mM H2O2, 30 μl of deionized water, and 20 μl of the supernatant. CAT activity was calculated using a molar extinction coefficient of 7.1 × 10−3 M−1cm−1.
The t-GSH content was measured using the Bioxytech GSH-420 assay kit (Oxis Health Products Inc., Portland, OR, USA) according to the manufacturer's recommendations. Briefly, liver and kidney samples were suspended in 10 mM PBS, and mixed with an ice-cold 7.5% trichloroacetic acid solution and homogenized. The homogenates were centrifuged at 3000 × g for 10 min. The supernatants were then used in the assay.
Protein concentrations were measured using the Bradford method [30] with the Protein Quantification Kit-Rapid (Dojindo Molecular Technologies Inc., Kumamoto, Japan).
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Each experimental group consisted of samples from six to nine animals. The significance of differences was determined by using Tukey's test and Dunnet's test for multiple comparisons. P < 0.05 was considered significant.
RESULTS
Changes in drinking volume
No significant differences were observed in drinking volumes throughout the experimental period between any of the treatment groups (Table 2).
Effects of hot spring water drinking on PO-induced hyperuricemia
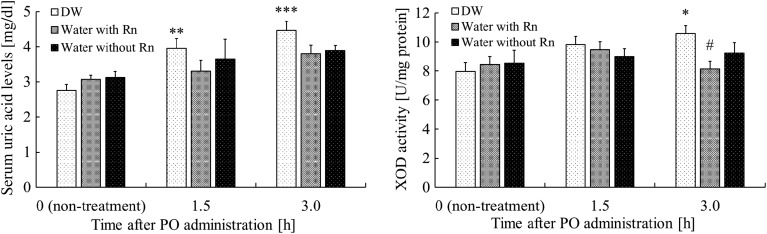
Serum uric acid levels were significantly higher (P < 0.01) in the DW+PO group than in the DW group 1.5 h after the administration of PO, whereas no significant differences were noted between the DW+PO group and the other groups (Water with Rn+PO or Water without Rn+PO groups). Serum uric acid levels were significantly higher (P < 0.001) in the DW+PO group than in the DW group 3 h after the administration of PO. However, serum uric acid levels were slightly lower in the Water with Rn+PO and Water without Rn+PO groups (Fig. 2).
Fig. 2.
Effects of drinking hot spring water on serum uric acid levels and xanthine oxidase (XOD) activity in the liver of mice with potassium oxonate (PO)–induced hyperuricemia. Each value is the mean ± SEM. The number of mice per experimental point is 8–9. *P < 0.05, **P < 0.01, ***P < 0.001, distilled water (DW) drinking with the administration of PO vs DW drinking only. #P < 0.05, Rn-containing hot spring water (Water with Rn) drinking with the administration of PO, or Rn-deaeration hot spring water (Water without Rn) drinking with the administration of PO vs DW drinking with the administration of PO.
Effects of hot spring water drinking on XOD activity in mice with PO-induced hyperuricemia
XOD activity in the liver was higher in the DW+PO group than in the DW group 1.5 h after the administration of PO; however, no significant differences were observed between the DW+PO group and the other groups (Water with Rn+PO or Water without Rn+PO groups). XOD activity in the liver was significantly higher (P < 0.05) in the DW+PO group than in the DW group 3 h after the administration of PO. However, XOD activity in liver was significantly lower (P < 0.05) in the Water with Rn+PO group than in the DW+PO group (Fig. 2).
Effects of hot spring water drinking on antioxidant-associated substances in the liver and kidney of mice with PO-induced hyperuricemia
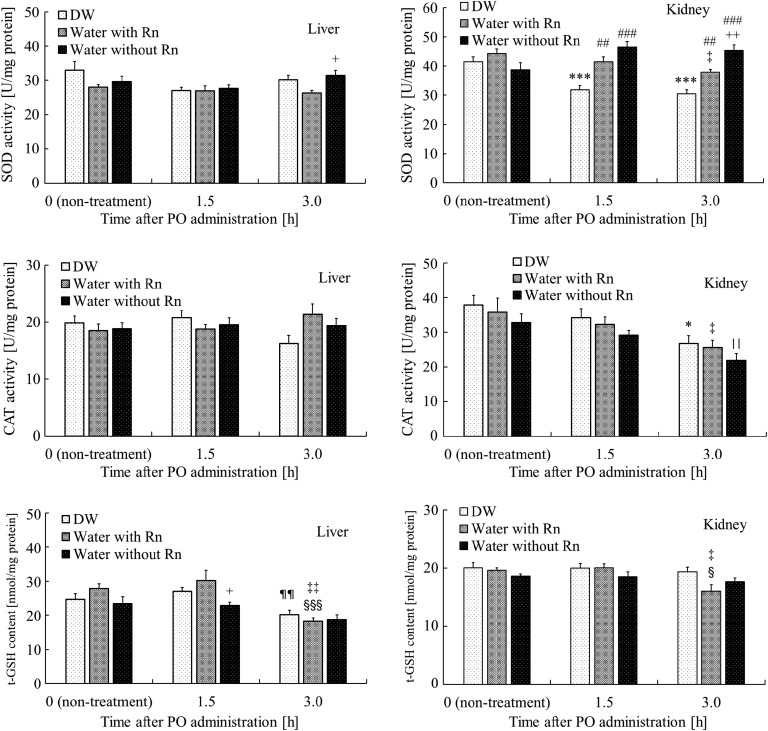
SOD activity in the liver at 3 h after the administration of PO was significantly higher (P < 0.05) in the Water without Rn+PO group than in the Water with Rn+PO group. The t-GSH content in the liver at 1.5 h after the administration of PO was significantly lower (P < 0.05) in the Water without Rn+PO group than in the Water with Rn+PO group. Furthermore, t-GSH content in the DW+PO group was significantly lower (P < 0.05) at 3 h after the administration of PO than that of the group at 1.5 h after the administration of PO; however, no significant differences were noted between the DW+PO group and the other groups (Water with Rn+PO or Water without Rn+PO groups). In addition, the t-GSH content in the Water with Rn+PO group was significantly lower at 3 h after the administration of PO than that in the Water with Rn group (P < 0.01) and significantly lower (P < 0.01) than that of the Rn+PO group at 1.5 h after the administration of PO (P < 0.001). SOD activity in the kidney was significantly lower (P < 0.001) in the DW+PO group 1.5 and 3 h after the administration of PO than in the DW group. However, it was significantly higher in the Water with Rn+PO (1.5 h: P < 0.01, 3 h: P < 0.001) and in the Water without Rn+PO (P < 0.001) groups than in the DW+PO group. Furthermore, at 3 h after the administration of PO, it was significantly higher (P < 0.01) in the Water without Rn+PO group than in the Water with Rn+PO group. In addition, SOD activity in the Water with Rn+PO group was significantly lower (P < 0.05) at 3 h after the administration of PO than in the Water with Rn group. CAT activity in the kidney was significantly lower (P < 0.05) in the DW+PO group than in the DW group 3 h after the administration of PO; however, no significant differences were noted between the DW+PO group and the other groups (Water with Rn+PO or Water without Rn+PO groups). In addition, at 3 h after the administration of PO, CAT activity was significantly lower (P < 0.05) in the Water with/without Rn+PO groups than in the Water with/without Rn groups. The t-GSH content in the kidney was significantly lower (P < 0.05) at 3 h after the administration of PO in the Water with Rn+PO group than in Water with Rn and that of the group at 1.5 h after the administration of PO (Fig. 3).
Fig. 3.
Effects of drinking hot spring water on antioxidant-associated substances in the liver and kidney of mice with potassium oxonate–induced hyperuricemia. The data and number of mice are as described in Fig. 2. *P < 0.05, ***P < 0.001, distilled water (DW) drinking with the administration of PO vs DW drinking only. ##P < 0.01, ###P < 0.001, Rn-containing hot spring water (Water with Rn) drinking with the administration of PO, or Rn-deaeration hot spring water (Water without Rn) drinking with the administration of PO vs DW drinking with the administration of PO. ¶¶P < 0.01, distilled water (DW) drinking with the administration of PO at 3 h after administration vs distilled water (DW) drinking with the administration of PO at 1.5 h after administration. ‡P < 0.05, ‡‡P < 0.01, Rn-containing hot spring water (Water with Rn) drinking with the administration of PO at 3 h after administration vs Rn-containing hot spring water (Water with Rn) drinking only. §P < 0.05, §§§P < 0.001, Rn-containing hot spring water (Water with Rn) drinking with the administration of PO at 3 h after administration vs Rn-containing hot spring water (Water with Rn) drinking with the administration of PO at 1.5 h after administration. ||P < 0.05, Rn-deaeration hot spring water (Water without Rn) drinking with the administration of PO at 3 h after administration vs Rn-deaeration hot spring water (Water without Rn) drinking only. +P < 0.05, ++P < 0.01, Rn-deaeration hot spring water (Water without Rn) drinking with the administration of PO vs Rn-containing hot spring water (Water with Rn) drinking with the administration of PO.
Effects of radon inhalation on PO-induced hyperuricemia
Since serum uric acid levels were higher in mice 3 h after the administration of PO than 1.5 h after, the former were subsequently used in radon inhalation experiments.
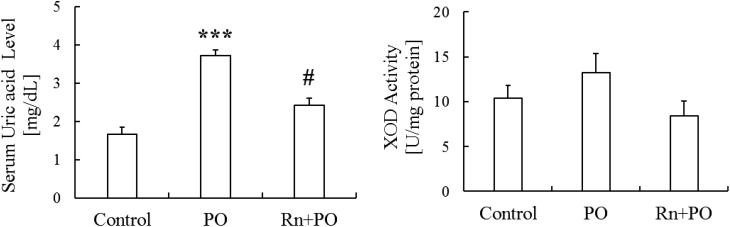
Serum uric acid levels were significantly higher (P < 0.001) in the PO group than in the Control group. However, they were significantly lower (P < 0.05) in the Rn+PO group than in the PO group (Fig. 4).
Fig. 4.
Effects of radon inhalation on serum uric acid levels and xanthine oxidase (XOD) activity in the liver of mice with potassium oxonate–induced hyperuricemia. Each value is the mean ± SEM. The number of mice per experimental point was six. ***P < 0.001, sham inhalation with the administration of PO vs control, #P < 0.05 radon inhalation with the administration of PO vs PO alone.
Effects of radon inhalation on XOD activity in mice with PO-induced hyperuricemia
XOD activity in the liver was higher in the PO group than in the Control group, and was lower in the Rn+PO group than in the PO group (Fig. 4).
Effects of radon inhalation on antioxidant-associated substances in the liver and kidney of mice with PO-induced hyperuricemia
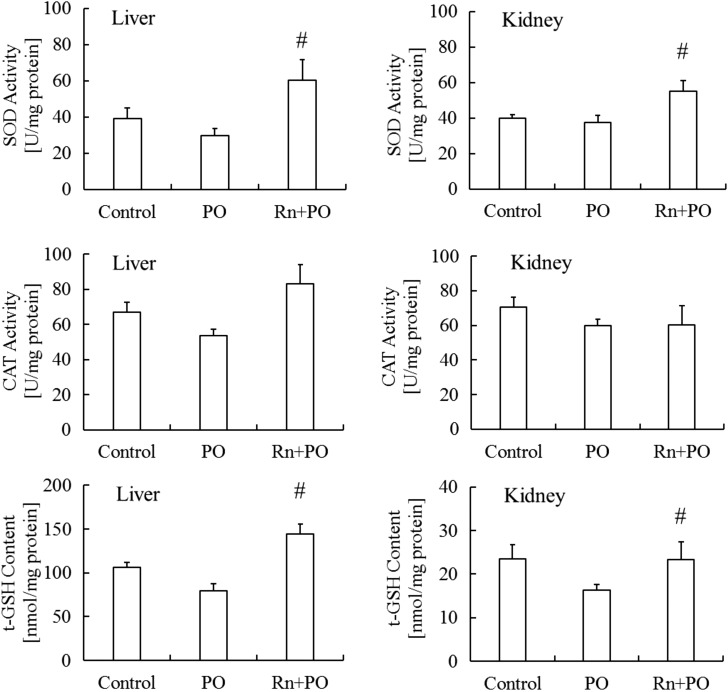
SOD and CAT activities and t-GSH content in the liver and kidney were lower in the PO group than in the control group. SOD activity (P < 0.05) and t-GSH content (P < 0.05) in the liver were significantly higher in the Rn+PO group than in the PO group. No significant differences were observed in CAT activity in the liver among these groups.
SOD activity and t-GSH content in the kidney were significantly higher (P < 0.05) in the Rn+PO group than in the PO group. No significant differences were detected in CAT activity in the kidney between these groups (Fig. 5).
Fig. 5.
Effects of radon inhalation on antioxidant-associated substances in the liver and kidney of mice with potassium oxonate–induced hyperuricemia. The data and number of mice are as described in Fig. 4. #P < 0.05 radon inhalation with the administration of PO vs PO.
DISCUSSION
Radon inhalation activates anti-oxidative functions in various organs, such as the brain, heart, lung, liver, pancreas and kidneys in mice and, thus, may contribute to the inhibition of oxidative stress-related diseases in these organs [8]. We previously demonstrated that radon inhalation protected tissues from chemically induced oxidative damage [9, 31], indicating that radon inhalation activates a biological defense system in mouse tissues that inhibits oxidative stress-related diseases. However, the mechanisms of action of radon inhalation and hot spring water drinking on hyperuricemia currently remain unclear. Therefore, we herein examined the effects of radon inhalation and hot spring water drinking on PO-induced hyperuricemia. The results obtained showed that the administration of PO elevated serum uric acid levels. With the inhalation treatments, serum uric acid levels were significantly lower in the Rn+PO group than in the PO group. With the drinking treatments, serum uric acid levels were slightly lower in the Water with Rn+PO and Water without Rn+PO groups than in the DW+PO group. Furthermore, no significant differences were observed in the volume of water consumption between any of the groups. Therefore, radon inhalation or water with/without radon drinking suppressed PO-induced hyperuricemia. These are probably due to the pharmacological effects of chemical compositions dissolved in hot spring water are much greater than the those of radon, because serum uric acid levels were similar between mice treated with Water with Rn and those treated with Water without Rn.
In an attempt to clarify the mechanisms suppressing hyperuricemia, XOD activity in the liver and antioxidant substances were investigated. Excessive elevations in serum uric acid levels have been shown to produce ROS and oxidative damage [24, 32], which further increases xanthine and hypoxanthine levels as well as XOD activity in the liver. During the metabolism of purines, the production of ROS such as the superoxide radical (O2 •–) and H2O2 occurs through the formation of metabolic by-products from the oxidation of hypoxanthine and xanthine to uric acid by XOD [33]. Therefore, an increase in ROS or XOD activity contributes to elevations in uric acid levels. SOD plays an important role in protecting cells from oxidative damage by converting O2 •– into H2O2. CAT transforms H2O2 into H2O as well as GSH. Our results showed that the administration of PO increased XOD activity in the liver, which may have, in turn, enhanced oxidative stress and suppressed anti-oxidative functions. Radon inhalation decreased XOD activity in the liver, indicating the inhibition of uric acid biosynthesis. SOD activity and the t-GSH content in the liver and kidney were significantly higher in the Rn+PO group than in the PO group, suggesting that radon inhalation suppresses PO-induced hyperuricemia by enhancing anti-oxidative functions and inhibiting uric acid biosynthesis in the liver. In the case of the drinking treatments, regardless of the suppression of XOD activity, elevations in uric acid levels were suppressed in both groups treated with water in the presence or absence of radon. Moreover, even in the same organ or group, no certain tendency for increase or decrease in the anti-oxidant-associated substances was observed to be dependent on the presence or absence of radon. Previous studies reported that drinking and bathing in hot spring water promotes the excretion of uric acid in the urine. These findings indicate that various compositions dissolved in hot spring water play an important role in decreasing uric acid levels. However, further studies are needed in order to clarify the involvement of these compositions.
Although XOD inhibitor drugs (e.g. allopurinol) that inhibit uric acid biosynthesis or uricosuric drugs (e.g. benzbromarone) that enhance the excretion of uric acid are typically used in the treatment of patients with hyperuricemia [34, 35], allopurinol is the only XOD inhibitor [36–38]. However, allopurinol causes side effects in some patients with hepatic diseases, renal diseases, and allergic reactions [39–42]. In the present study, we demonstrated that radon inhalation or hot spring water drinking exerted the same effects as allopurinol. Therefore, radon inhalation or hot spring water drinking may assist the functions of allopurinol.
In conclusion, radon inhalation activated anti-oxidative functions and inhibited XOD activity in the liver. As a result, radon inhalation suppressed PO-induced elevations in serum uric acid levels via respiratory organs. On the other hand, hot spring water drinking suppressed PO-induced elevations in serum uric acid levels via digestive organs. The pharmacological effects are probably much larger due to the chemicals dissolved in hot spring water than from radon.
Radon therapy is performed for various diseases at the Misasa Medical Center, Okayama University Hospital, Japan. However, the mechanisms underlying the health effects achieved have not been investigated in detail. Our results suggest the potential of radon therapy for the prevention of hyperuricemia. Moreover, the mechanisms responsible for the inhibitory effects of radon inhalation and drinking water on hyperuricemia may differ.
FUNDING
This work was supported by Okayama University and Japan Atomic Energy Agency.
ACKNOWLEDGEMENTS
The authors thank the staff at the Departments of Animal Resources and Radiation Research, Shikata Laboratory, Advanced Science Research Center, Okayama University for their technical support.
REFERENCES
- 1.Yamaoka K, Mitsunobu F, Hanamoto K et al. Study on biologic effects of radon and thermal therapy on osteoarthritis. J Pain 2004;5:20–5. [DOI] [PubMed] [Google Scholar]
- 2.Mitsunobu F, Yamaoka K, Hanamoto K et al. Elevation of antioxidant enzymes in the clinical effects of radon and thermal therapy for bronchial asthma. J Radiat Res 2003;44:95–9. [DOI] [PubMed] [Google Scholar]
- 3.Nagy K, Berhés I, Kovács T et al. Study on endocrinological effects of radon speleotherapy on respiratory diseases. Int J Radiat Biol 2009;85:281–90. [DOI] [PubMed] [Google Scholar]
- 4.Nagy K, Berhés I, Kovács T et al. Does balneotherapy with low radon concentration in water influence the endocrine system? A controlled non-randomized pilot study. Radiat Environ Biophys 2009;48:311–5. [DOI] [PubMed] [Google Scholar]
- 5.Falkenbach A, Kovacs J, Franke A et al. Radon therapy for the treatment of rheumatic diseases—review and meta-analysis of controlled clinical trials. Rheumatol Int 2005;25:205–10. [DOI] [PubMed] [Google Scholar]
- 6.Sakoda A, Ishimori Y, Kawabe A et al. Physiologically based pharmacokinetic modeling of inhaled radon to calculate absorbed doses in mice, rats, and humans. J Nucl Sci Technol 2010;47:731–8. [Google Scholar]
- 7.Nakagawa S, Kataoka T, Sakoda A et al. Basic study on activation of antioxidation function in some organs of mice by radon inhalation using new radon exposure device. Radioisotopes 2008;57:241–51 (in Japanese). [Google Scholar]
- 8.Kataoka T, Sakoda A, Ishimori Y et al. Study of the response of superoxide dismutase in mouse organs to radon using a new large-scale facility for exposing small animals to radon. J Radiat Res 2011;52:775–81. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka T, Nishiyama Y, Toyota T et al. Radon inhalation protects mice from carbon-tetrachloride–induced hepatic and renal damage. Inflammation 2011;34:559–67. [DOI] [PubMed] [Google Scholar]
- 10.Nishida Y. Influences of hot spring waters on the metabolism of oxypurine and uric acid. J Jpn Soc Balneol Climatol Phys Med 1973;33:98–131 (in Japanese). [Google Scholar]
- 11.Ni AN, Popova VV, Luchaninova VN. Bicarbonate calcium mineral water with carbon dioxide in rehabilitation of children with dismetabolic nephropathies complicated by renal inflammation. Vopr Kurortol Fizioter Lech Fiz Kult 2004;4:32–5. [PubMed] [Google Scholar]
- 12.Takahasi N, Jin M, Ohtsuka Y et al. The influence of oxidation reduction potential of spa spring water on the human body. J Jpn Soc Balneol Climatol Phys Med 2007;70:94–102 (in Japanese). [Google Scholar]
- 13.Nagy K, Kávási N, Kovács T et al. Radon therapy and speleotherapy in Hungary. Press Therm Climat 2008;145:219–25. [Google Scholar]
- 14.Tanaka J, Seno T, Matsumdto S et al. Re-evaluation of spa-drink therapy for digestive diseases. Institute for Environmental Medicine, Okayama University Medical School 1990;61:73–8 (in Japanese). [Google Scholar]
- 15.Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med 1993;14:615–31. [DOI] [PubMed] [Google Scholar]
- 16.Ramallo IA, Zacchino SA, Furlan RL. A rapid TLC autographic method for the detection of xanthine oxidase inhibitors and superoxide scavengers. Phytochem Anal 2006;17:15–19. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto S. A new spectrophotometric assay method of xanthine oxidase in crude tissue homogenate. Anal Biochem 1974;62:426–35. [DOI] [PubMed] [Google Scholar]
- 18.Berry C, Hamilton CA, Brosnan MJ et al. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation 2000;101:2206–12. [DOI] [PubMed] [Google Scholar]
- 19.Hellsten-Westing Y. Immunohistochemical localisation of xanthine oxidase in human cardiac and skeletal muscle. Histochemistry 1993;100:215–22. [DOI] [PubMed] [Google Scholar]
- 20.Parks DA, Granger DN. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol 1983;245:285–9. [DOI] [PubMed] [Google Scholar]
- 21.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 1985;312:159–63. [DOI] [PubMed] [Google Scholar]
- 22.Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol 1996;174:288–91. [DOI] [PubMed] [Google Scholar]
- 23.Harrison R. Physiological roles of xanthine oxidoreductase. Drug Metab Rev 2004;36:363–75. [DOI] [PubMed] [Google Scholar]
- 24.Kang DH, Ha SK. Uric acid puzzle: dual role as anti-oxidant and pro-oxidant. Electrolyte Blood Press 2014;12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biniaz V, Tayebi, Ebadi A et al. Effect of vitamin C supplementation on serum uric acid in patients undergoing hemodialysis: a randomized controlled trial. Iran J Kidney Dis 2014;8:401–7. [PubMed] [Google Scholar]
- 26.Kataoka T, Teraoka J, Sakoda A et al. Protective effects of radon inhalation on carrageenan-induced inflammatory paw edema in mice. Inflammation 2012;35:713–22. [DOI] [PubMed] [Google Scholar]
- 27.Caraway WT. Determination of uric acid in serum by a carbonate method. Am J Clin Pathol 1955;7:840–5. [DOI] [PubMed] [Google Scholar]
- 28.Baehner RL, Murrmann SK, Davis J et al. The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest 1975;56:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aebi H, Wyss SR, Scherz B et al. Properties of erythrocyte catalase from homozygotes and heterozygotes for Swiss-type acatalasemia. Biochem Genet 1976;14:791–807. [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 31.Nishiyama Y, Kataoka T, Teraoka J et al. Inhibitory effects of pre and post radon inhalation on carbon tetrachloride-induced oxidative damage in mouse organs. Radioisotopes 2012;61:231–41. [Google Scholar]
- 32.Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk. Nutr Metab Cardiovasc Dis 2007;17:409–14. [DOI] [PubMed] [Google Scholar]
- 33.Maia L, Duarte RO, Ponces-Freire A et al. NADH oxidase activity of rat and human liver xanthine oxidoreductase: potent role in superoxide production. J Biol Inorg Chem 2007;12:777–87. [DOI] [PubMed] [Google Scholar]
- 34.Sinclair DS, Fox IH. The pharmacology of hypouricemic effect of benzbromarone. J Rheumatol 1974;2:437–45. [PubMed] [Google Scholar]
- 35.Perez-Ruiz F, Alonso-Ruiz A, Calabozo M et al. Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis 1998;57:545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das DK, Engelman RM, Clement R et al. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun 1987;148:314–19. [DOI] [PubMed] [Google Scholar]
- 37.Fields M, Lewis CG, Lure MD et al. Allopurinol, an inhibitor of xanthine oxidase, reduces uric acid levels and modifies the signs associated with copper deficiency in rats fed fructose. Free Radic Biol Med 1996;20:595–600. [DOI] [PubMed] [Google Scholar]
- 38.Wortmann RL. Recent advances in the management of gout and hyperuricemia. Curr Opin Rheumatol 2005;17:319–24. [DOI] [PubMed] [Google Scholar]
- 39.Elion GB, Yü TF, Gutman AB et al. Renal clearance of oxipurinol, the chief metabolite of allopurinol. Am J Med 1968;45:69–77. [DOI] [PubMed] [Google Scholar]
- 40.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med 1984;76:47–56. [DOI] [PubMed] [Google Scholar]
- 41.Cameron JS, Simmonds HA. Use and abuse of allopurinol. Br Med J 1987;294:1504–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagugli RM, Gentile G, Ferrara G et al. Acute renal and hepatic failure associated with allopurinol treatment. Clin Nephrol 2008;70:523–6. [DOI] [PubMed] [Google Scholar]