Abstract
To evaluate the outcomes and feasibility of stereotactic body radiotherapy (SBRT) for cT3 and cT4N0M0 non–small cell lung cancer (NSCLC), 25 patients with localized primary NSCLC diagnosed as cT3 or cT4N0M0, given SBRT between May 2005 and July 2013, were analyzed. All patients had inoperable tumors. The major reasons for tumors being unresectable were insufficient respiratory function for curative resection, advanced age (>80 years old) or technically inoperable due to invasion into critical organs. The median patient age was 79 years (range; 60–86). The median follow-up duration was 25 months (range: 5–100 months). The 2-year overall survival rates for T3 and T4 were 57% and 69%, respectively. The 2-year local control rates for T3 and T4 were 91% and 68%, respectively. As for toxicities, Grade 0–1, Grade 2 and Grade 3 radiation pneumonitis occurred in 23, 1 and 1 patient, respectively. No other acute or symptomatic late toxicities were reported. Thirteen patients who had no local, mediastinal or intrapulmonary progression at one year after SBRT underwent pulmonary function testing. The median variation in pre-SBRT and post-SBRT forced expiratory volume in 1 s (FEV1) values was –0.1 (–0.8–0.8). This variation was not statistically significant (P = 0.56). Forced vital capacity (FVC), vital capacity (VC), %VC and %FEV1 also showed no significant differences. SBRT for cT3 and cT4N0M0 NSCLC was both effective and feasible. Considering the favorable survival and low morbidity rate, SBRT is a potential treatment option for cT3 and cT4N0M0 NSCLC.
Keywords: stereotactic body radiotherapy, non–small cell lung cancer, T3N0M0, T4N0M0, inoperable, unresectable
INTRODUCTION
For non–small cell lung cancer (NSCLC) patients with localized disease at diagnosis, the 5-year relative survival rate was reported to be 54.0%, according to the Surveillance, Epidemiology and End Results (SEER) database [1]. Although the survival of lung cancer patients is improving, treatment outcomes are still unsatisfactory, especially for those with T3 or T4 disease.
Since the TNM system was applied to lung cancer, the surgical landmark has been set between Stages IIIA and IIIB. T3 and/or N1 tumors are universally considered to be resectable, while T4 and/or N2 tumors are generally regarded as being unresectable by surgery alone. As for patients with T3N0 and T4N0 tumors, despite local progression, if it does not show spread to lymph nodes [2], surgery is preferred [3]. This is in contrast to T1–2N2 disease, which, even in the absence of invasion of surrounding tissues from the primary lesion, has spread to lymph nodes and is usually treated with concurrent chemoradiation.
Extensive local treatment and/or major surgical procedures are often required in patients with T3N0 disease. According to a Japanese lung cancer registry study [4], the 5-year survival rate of patients with pathological T3N0 is quite high, at ∼50%. In those with T4 disease, tumors are generally regarded as being unresectable [5]. However, surgery appears to be beneficial in selected patients. According to some studies focusing on extended resection of T4 tumors [6–8], radical resection of the tumor has curative potential if there are no mediastinal lymph node metastases.
Therefore, even if the T factor is highly unfavorable, some patients without lymph node metastasis may be good candidates for complete resection. However, the major problems with surgery include patients not always being able to undergo an extensive operation and/or failure to achieve complete resection [9].
Stereotactic body radiotherapy (SBRT) has recently been regarded as a treatment option for patients with medically inoperable T1 or T2N0M0 NSCLC. It can be applied, with acceptable toxicity, even to those with comorbidities or advanced age [10]. However, SBRT has as yet not become a standardized treatment for T3 invasion or T4 extension with N0M0, and there are only a few reports [11, 12] describing the use of SBRT in such patients. We have proactively performed SBRT for cT3 or cT4N0M0 NSCLC in patients who were not good candidates for surgery. In this study, we retrospectively evaluated the feasibility and treatment outcomes of SBRT for cT3 and cT4N0M0 NSCLC.
METHODS
Patients
We retrospectively identified consecutive patients with localized primary NSCLC diagnosed as cT3 or cT4N0M0 using the 7th lung cancer TNM classification and staging system and treated with SBRT at Ofuna Chuo Hospital between May 2005 and July 2013. The patients satisfied the following criteria: performance status 0–2; the patients were inoperable, or refused surgery; and the maximum doses to the esophagus, spinal cord and trachea were within 25 Gy. In total, 25 patients were identified. Of these, 23 patients were pathologically diagnosed as having NSCLC. Another 2 patients were clinically diagnosed as having NSCLC, based only on clinical information such as elevated tumor marker levels, an increase in the maximum standardized uptake value on [18F] fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), and successive enlargements on CT images. All of the patients were considered to be inoperable. The major reasons for assessment as inoperable were advanced age (>80 years old; 10 patients), insufficient respiratory function for curative resection due to chronic obstructive pulmonary disease (COPD; 8 patients) and cardiovascular comorbidities (3 patients). Each patient's T and N stage was evaluated on CT images by two experienced radiologists (K.Y. and T.M.). Lymph node metastasis was diagnosed when a lymph node was more than 1 cm in diameter, or a lymph node was <1 cm but had an increase in the maximum standardized uptake value on 18F-FDG PET/CT. Mediastinoscopy for diagnosis of lymph node metastasis was not performed. The NSCLC diagnosis and treatment policy were discussed and assessments were made at a Lung Cancer Board consultation with a pulmonologist, thoracic surgeons and a radiation oncologist at our hospital. We excluded patients with T3 (separate tumor nodules in the same lobe) and T4 (separate tumor nodules in a different ipsilateral lobe) from the present study. Patient and tumor characteristics are shown in Table 1. The median age was 79 years (range; 60–86). All patients provided written informed consent. The Ofuna Chuo Hospital Review Board approved this study (No. 2014–06).
Table 1.
Patient characteristics
| Median age (range) | 79 (60–86) |
| Sex: male/female | 17/8 |
| Median follow up duration (month)(range) | 25 (5–100) |
| Clinical T stage | |
| T3: | |
| <2 cm carina | 2 |
| chest wall | 10 |
| mediastinal pleura | 2 |
| pericardium | 1 |
| T4: | |
| mediastinum | 3 |
| major blood vessel | 7 |
| Location: central/peripheral | 19/6 |
| Histology | |
| Squamous cell carcinoma | 12 |
| Adenocarcinoma | 7 |
| Non–small cell carcinoma | 4 |
| Pathologically unproven | 2 |
| Reasons for not fit for surgerya | |
| Old age | 10 |
| COPD | 8 |
| Cardiovascular disease | 3 |
| Postoperative impaired pulmonary function | 3 |
| Technically unresectable | 2 |
| Dementia | 1 |
| Cerebral infarction | 1 |
| Idiopathic pulmonary fibrosis | 1 |
| Median tumor diameter (cm) (range) | 4.0 (2.3–8.5) |
| Median ITV (cm3) (range) | 29 (0.9–314) |
| Median PTV (cm3) (range) | 85 (9.6–363) |
| Dose fractionation: | |
| SBRT | |
| 40 Gy/5 fr | 9 |
| 50 Gy/5 fr | 10 |
| SBRT after 3D-CRT (30 Gy/15 fr∼50 Gy/25 fr) | |
| 25 Gy/5 fr | 4 |
| 30 Gy/5 fr | 1 |
| 40 Gy/10 fr | 1 |
SBRT = stereotactic body radiotherapy, COPD = chronic obstructive pulmonary disease, ITV = internal target volume, PTV = planning target volume, 3D-CRT = three-dimensional conformal radiotherapy. aThe numbers in these items overlap.
Treatment
Our SBRT methods have been previously described in detail [13]. Briefly, long-scan-time CT was used to directly visualize the internal target volume (ITV) after immobilizing the patient with a vacuum pillow. The planning target volume (PTV) was determined by adding a margin of 6–8 mm to the ITV. Dynamic conformal multiple-arc therapy or volumetric-modulated arc therapy was used for SBRT. The total dose prescribed was 40 or 50 Gy per 5 fractions to the 60–80% isodose of the maximum dose, which covered at least 95% of the PTV during five consecutive days. We kept the doses delivered to the esophagus, spinal cord and trachea below a maximum dose of 25 Gy, and to minimize the doses for the bronchus, brachial plexus, pulmonary artery, and left ventricle as low as reasonably achievable. There were no dose constraints on the aorta or the vena cava. For tumors adjacent to critical organs, especially the esophagus, SBRT combined with conventional radiation therapy of 30 Gy/15 fr – 50 Gy/25 fr [with a median total BED10 of 84.8 Gy (73.5–104 Gy) at the margin of the PTV, and 100.2 Gy (86.8–159.1 Gy) at the maximum dose point in the PTV] was adopted (Table 1).
Follow-up
For all patients, follow-up CT scans were performed at 1 and 3 months after SBRT and then at 3-month intervals during the first 2 years. Subsequently, follow-up CT scans were obtained at 4- to 6-month intervals. Pulmonary function tests were performed at ∼1 year after SBRT. 18F-FDG PET/CT was performed at ∼1 year after SBRT and when local, regional and/or distant recurrences were highly suspected. Acute and chronic toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0.
Statistical analysis
The control and survival rates were derived using the Kaplan–Meier method. Survival curves were compared using log-rank tests. The Wilcoxon signed-rank test was used for analysis of the pulmonary function test results. Data were analyzed with IBM SPSS Statics 22.0 (IBM Corp., Armonk, NY, USA).
RESULTS
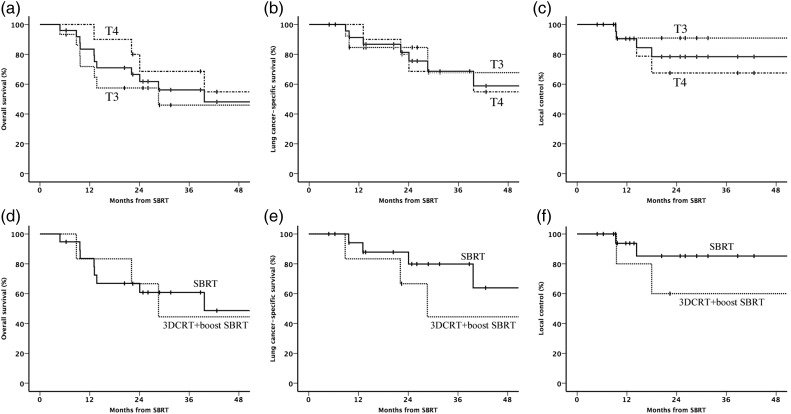
For the 25 patients diagnosed as having cT3 or cT4N0M0 NSCLC and then treated with SBRT, the median follow-up duration was 25 (range: 5–100) months. All patients were followed up except one, who was lost to follow-up after 6 months without relapse. The 2-year overall survival rates, lung cancer–specific survival rates and local control rates for patients with T3 and T4 were 57% (95% confidence interval: 32–83%) and 69% (95% confidence interval: 39–98%) (Fig. 1a), 85% (95% confidence interval: 65–100%) and 69% (95% confidence interval: 39–98%) (Fig. 1b), and 91% (95% confidence interval: 74–100%) and 68% (95% confidence interval: 37–98%) (Fig. 1c), respectively. The 2-year regional control and distant metastasis–free survival rates were 80% (95% confidence interval: 55–100%) and 79% (95% confidence interval: 58–100%) for T3, and 75% (95% confidence interval: 44–100%) and 80% (95% confidence interval: 55–100%) for T4, respectively. None of these outcomes differed significantly between patients with T3 and T4 disease. For 19 patients treated with standard SBRT (40 Gy/5 fr or 50 Gy/5 fr), the 2-year overall survival rates, lung cancer–specific survival rates and local control rates were 61% (95% confidence interval: 38–84%) (Fig. 1d), 80% (95% confidence interval: 59–100%) (Fig. 1e) and 85% (95% confidence interval: 66–100%) (Fig. 1f), respectively. Those for the six patients treated with 3D-CRT following SBRT boost were 67% (95% confidence interval: 29–100%) (Fig. 1d), 67% (95% confidence interval: 29–100%) (Fig. 1e) and 60% (95% confidence interval: 17–100%) (Fig. 1f), respectively. There was no significant difference in outcomes between two groups.
Fig. 1.
Kaplan–Meier plots for all patients (n = 25) (solid line), patients with T3 (n = 15) (dotted line) and patients with T4 (n = 10) (dash-dotted line) disease. (a) Overall survival, (b) lung cancer–specific survival and (c) local control. (d) Overall survival, (e) lung cancer–specific survival and (f) local control for patients (n = 19) treated with standard SBRT (40 Gy/5 fr or 50 Gy/5 fr) (solid line) and patients (n = 6) treated with 3D conformal radiotherapy (3DCRT) following SBRT boost.
SBRT was well tolerated, and all patients completed the treatment course on schedule. Figures 2 and 3 show the data obtained from two patients with cT4 disease. As for toxicities, Grade 0–1, Grade 2 and Grade 3 radiation pneumonitis occurred in 23, 1 and 1 patient, respectively. No other Grade 2 or more acute toxicities (including esophagitis, general fatigue, nausea, fever, chest wall pain, rib fractures and respiratory symptoms) were reported. None of the patients experienced symptomatic late toxicities.
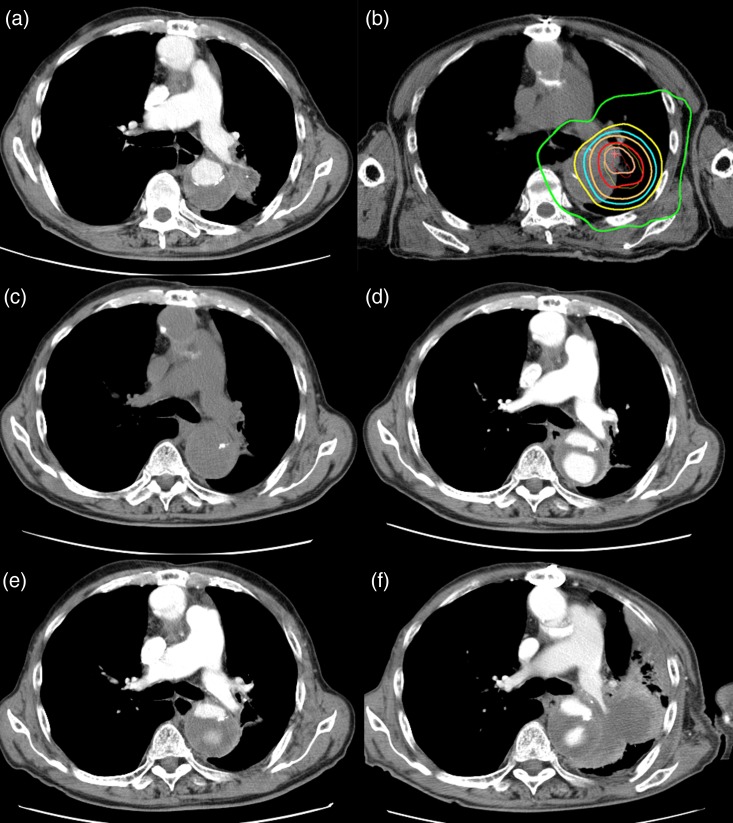
Fig. 2.
Computed tomographic (CT) appearance and dose distribution for a patient with NSCLC adhering to the aorta. (a) CT appearance before SBRT. (b) Dose distribution curves of SBRT. The isodose lines, from outer to inner, represent 10 Gy, 20 Gy, 25 Gy, 30 Gy, 40 Gy and 45 Gy, respectively. (c) (d) (e) CT appearances 4, 8 and 14 months after SBRT, respectively. (f) CT scan obtained 32 months after SBRT shows local recurrence. The patient was a 75-year-old man diagnosed with NSCLC during the follow-up period after aortic dissection. He was judged to have an inoperable tumor because there was widespread adherence to the dissected aorta and aortic invasion was suspected. The patient underwent SBRT (40 Gy in 5 fractions). The tumor was well controlled without toxicity, but at 32 months, local recurrence was observed and he died 39 months after the initial diagnosis.
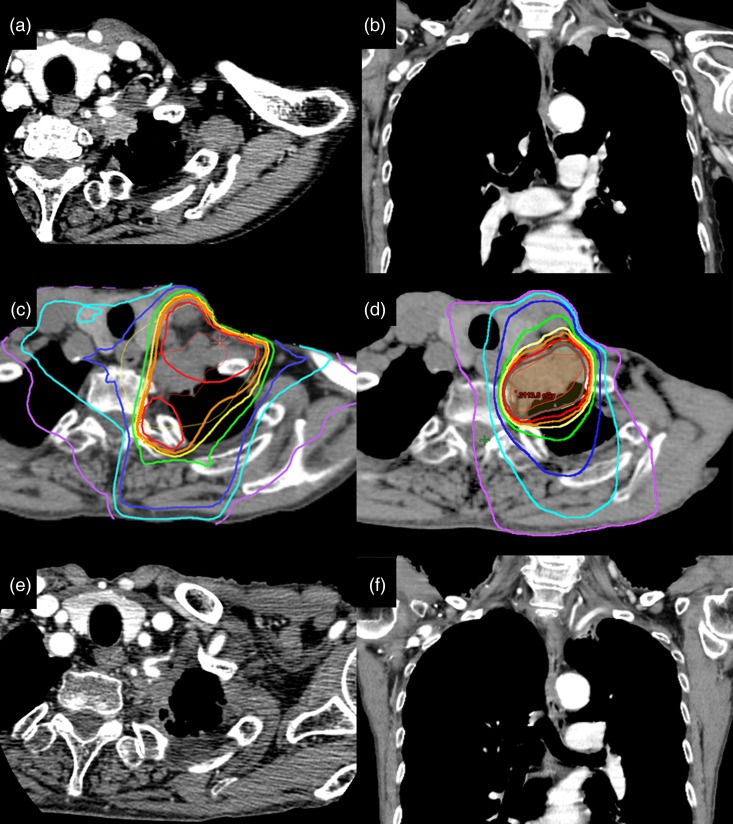
Fig. 3.
Computed tomographic (CT) appearance and dose distribution in a patient with a superior sulcus tumor. (a) (b) Axial and coronal CT images obtained before SBRT. (c) Dose distribution curves of 3D conformal radiotherapy (3D-CRT). The isodose lines, from outer to inner, represent 5 Gy, 10 Gy, 15 Gy, 20 Gy, 22.5 Gy, 23.75 Gy, 25 Gy and 26.75 Gy, respectively. (d) Dose distribution curves of SBRT. The isodose lines, from outer to inner, represent10 Gy, 20 Gy, 30 Gy, 40 Gy, 45 Gy, 47.5 Gy and 50 Gy, respectively. (e) (f) Appearance on axial and coronal CT images obtained 15 months after SBRT. The patient was a 70-year-old woman diagnosed with a superior sulcus tumor at another hospital. She initially received chemotherapy (cisplatin and pemetrexed) alone because the hospital did not have a radiation therapy unit. She was then treated at our institution using 3D-CRT with 50 Gy in 25 fractions before SBRT because invasion to the brachial plexus was suspected based on the symptom of left-arm dysesthesia. One month after 3D-CRT, SBRT (25 Gy in 5 fractions) was administered to the residual tumor. Twenty months after SBRT, to date, the tumor remains controlled and there have been no toxicities, such as brachial plexus neuropathy or brachial edema.
Fourteen of the 25 patients (56%) had no local, mediastinal or intrapulmonary progression at 1 year after SBRT. Of those 14 patients, 13 underwent pulmonary function tests. The patient without pulmonary function test data had died at 13 months after SBRT without respiratory symptoms. The median variation in pre-SBRT and post-SBRT forced expiratory volume in 1 s (FEV1) values was –0.1 (‒0.8–0.8). This variation was not statistically significant (P = 0.56). Vital capacity (VC), forced vital capacity (FVC), %VC, and %FEV1 also showed no significant differences between pre-SBRT and post-SBRT values (Table 2).
Table 2.
Pulmonary function test of pre- and post-SBRT
| Pre-SBRT | Post-SBRT | Δ | P value | |
|---|---|---|---|---|
| FVC (L) | 2.1 (0.4–3.7) | 2.1 (1.6–2.7) | −0.1 (−1.1–1.2) | 0.92 |
| VC (L) | 2.3 (1.1–2.9) | 2.2 (1.3–2.7) | −0.1 (−0.3–0.5) | 0.22 |
| %VC (%) | 83 (49–106) | 80 (63–104) | −3.1 (−25–22) | 0.32 |
| FEV1 (L) | 1.3 (0.4–2.1) | 1.3 (0.6–2.0) | −0.1 (−0.8–0.8) | 0.56 |
| %FEV1 (%) | 74 (24–119) | 73 (26–115) | −1.2 (−55–55) | 0.87 |
All data given as mean (range) without P value. SBRT = stereotactic body radiotherapy, Δ = post-treatment value minus pre-treatment value, FVC = forced vital capacity, VC = vital capacity, %VC = percent predicted vital capacity, FEV1 = forced expiratory volume in one second, %FEV1 = percent predicted forced expiratory volume in one second. P value < 0.05 is considered statistically significant.
DISCUSSION
We herein report our promising treatment outcomes and acceptable toxicity of SBRT for cT3–cT4N0M0 NSCLC in patients who were not candidates for surgery due to advanced age, decreased pulmonary function, and/or comorbidities. Currently, recommended treatment options for such patients are surgery, chemoradiation and chemotherapy, according to the National Comprehensive Cancer Network (NCCN) guideline (Version 5, 2015). Although surgery is preferred if a patient has an operable malignancy, i.e. if the tumor is resectable, there are no reports on treatment outcomes for patients with inoperable T3–4N0M0 NSCLC. In addition, to date, no intensive treatment options that might serve as an alternative to surgery for such patients have been established. Given this situation, SBRT is a potential intensive treatment option. This is the first study, to our knowledge, to examine the use of SBRT for medically inoperable cT3–4N0M0 NSCLC.
Several studies have described the clinical outcomes of surgery for T3 or T4 NSCLC [14–20] (Table 3). In most of these reports, complete resection and N0–1 disease were factors favoring survival. In pathological N0 patients, 5-year survival rates were favorable, ranging between 40 and 70%. The 3-year survival rates in T3N0M0 patients were reported to be ∼60% [4].
Table 3.
Outcomes of surgery for T3 and T4 non–small cell lung cancer
| Author | Case | Age | Invasion | Surgery (%) | R0 (%) | Mortality (%) | 5-y OS (%) | 5-y OS (N0–1) (%) |
|---|---|---|---|---|---|---|---|---|
| (P/L) | ||||||||
| Downey [14], 2009 | 269 | 62 | CW | 20/64 | 52.4 | 6 | – | 49 (N0) |
| Doddoli [15], 2005 | 309 | 60 | CW | 25.6/70.2 | 100 | 7.8 | 30.7 | 40 (N0) |
| Yang [16], 2009 | 146 | 56.1 | T4 | 65.6/34.4 | 72.6 | 3.1 | 22.7 | 40 (N0) |
| Yidizeli [17], 2008 | 271 | 56.3 | T4 | 49.4/39.7 | 92 | 4 | 38.4 | 43 (N0, 1) |
| Ohta [18], 2005 | 16 | 55 | Aorta | 37.5/60.0 | 75 | 12.5 | 48.2 | 70 (N0) |
| Spaggiari [19], 2004 | 109 | 64 | SVC | 50.5/49.5 | 73.4 | 12 | 21 | |
| Stella [20], 2012 | 31 | 65.6 | LA, PV | 83.9/16.1 | 93.5 | 9.7 | 30 | 78 (N0, 1) |
OS = overall survival, P = pneumonectomy, L = lobectomy, CW = chest wall, SVC = superior vena cava, LA = left atrium, PV = pulmonary vein.
The median follow-up time of our study is still short (25 months). However, it is clinically meaningful that the 2-year overall survival rates for patients with T3N0 and T4N0 disease were 57% and 69%, and the 2-year local control rates were 91% and 68%, respectively. Given that our study population consisted of patients for whom surgery was contraindicated, our results may compare favorably with those of surgery.
Significance of SBRT in T4N0M0 disease
Among T4 descriptors, lesions with invasion including the great vessels, left atrium, trachea, or carina, are ‘potentially resectable’ in some cases. In contrast, patients with frank invasion of the heart or esophageal involvement are considered to have ‘definitely unresectable’ tumors. Taking these factors into consideration, in our T4N0 patients (n = 10) treated with SBRT in the current study, there were three with mediastinal invasion and seven with major vessel invasion (Table 1), i.e. with tumors that would be classified as ‘potentially resectable’. As surgery for T4 disease offers promising results, giving these patients intensive local treatment such as SBRT is considered to be clinically meaningful.
The potential of SBRT as a new treatment option for cT3–4N0M0 patients not indicated for surgery
The advantage of SBRT is its feasibility for patients with inoperable malignancies. In a Japanese survey of SBRT, peri-treatment morbidities and mortalities were very rare, and the rate of Grade 5 toxicity was very low (0.6%) [21]. According to a report that compared overall survival after SBRT with surgery for elderly (median age of 79) patients with Stage I NSCLC, the overall survival rates at 1 and 3 years were 75% and 60% after surgery, and 87% and 42% after SBRT, respectively, while the 30-day mortality rates were 8.3% and 1.7%, respectively [22]. Good tolerance of SBRT has been reported for octogenarians [22, 23] and patients with GOLD III–IV COPD [24]. SBRT offers patients with inoperable Stage I NSCLC the advantages of a high local control rate and low treatment-related mortality. Opting for either no treatment or conventional radiotherapy alone would lead to poor survival and decreased quality of life (QOL). Therefore, SBRT may be better suited than resection for elderly patients with comorbidities.
Although the safety of SBRT for central tumors is controversial in T1–2N0M0 NSCLC, favorable outcomes are often reported for patients with centrally located tumors and those who are frail, such as the elderly. Nuyttens et al. [25] reported the actuarial 2-year local tumor control rate to be 85% for central tumors treated with a BED10 >100 Gy. Haasbeek et al. [26] reported the 3-year overall survival rates for central and peripheral early-stage NSCLC to not differ statistically, at 64% and 51%, respectively. Since tumors in patients with T3–4N0M0 disease are often located in the central area of the lung, the promising SBRT results obtained for centrally located tumors are encouraging, although it must be kept in mind that they were for T1–2N0M0 NSCLC. However, it should be noted that fatal toxicities can occur when central tumors are treated with SBRT [27]. We reported [28] the limited toxicity of SBRT (40–60 Gy in 5 fractions [BED3 = 147–300 Gy]) in 133 centrally located lung tumors that received >25 Gy to the organ at risk in the mediastinum and pulmonary hilum. Five cases (3.8%) had Grade 3, and 2 (1.5%) had Grade 5 hemoptysis among the 133 patients. Therefore, central lung tumors should be treated with careful consideration of the irradiation dose delivered to organs at risk. Furthermore, fully informed consent must be obtained after carefully explaining all possible risks to patients.
Post-treatment pulmonary function and QOL
Respiratory function decline is among the most critical problems encountered in managing patients after surgery. For T4N0M0 NSCLC patients, pneumonectomy is frequently selected (Table 3). Menna et al. [29] reported a FEV1 decline of 0.8 l after pneumonectomy for lung cancer. Moreover, curative resection was not indicated for many NSCLC patients because of underlying COPD with poor pulmonary function.
On the other hand, the decline in respiratory function caused by SBRT is minimal. Studies investigating respiratory function after SBRT for lung cancer [30, 31] found that this treatment had little or no effect on FEV1 and FVC. In fact, we observed no respiratory function decline in this study. Preservation of respiratory function contributes to better QOL for the remainder of a patient's life, an advantage of SBRT.
Limitations
This study has several limitations, including short follow-up periods, small sample sizes, and its retrospective nature with possible selection bias. Due to retrospective nature, toxicities were possibly understated, and dose fractionation was not uniform in SBRT with increased fractionation and conventional radiation therapy. In addition, there was a potential bias due to the lack of pathological staging that could have been provided by surgery. According to a report [32] that compared outcomes of clinical and surgical–pathological staging in IIIA NSCLC, overstaging was present in 40.8% of cases in terms of T status and in 38.7% in terms of N status. Therefore, our study subjects treated with SBRT might have included pathological T2 or node-positive patients, which would have influenced the treatment results. Another possible disadvantage of SBRT is that treatment of mediastinal lymphatics, which is routinely performed in surgery or conventional irradiation, is omitted. However, recently, a prophylactic effect on ipsilateral hilar relapse by SBRT was reported [33].
CONCLUSION
SBRT for T3 and T4N0M0 NSCLC was found to be both effective and feasible. Considering the favorable survival and low morbidity rates obtained, SBRT is a potential treatment option for patients with T3 and T4N0M0 NSCLC. Further research with longer follow-up is warranted.
FUNDING
There was no funding support.
ACKNOWLEDGEMENTS
Part of the content of this manuscript was presented at the 27th Annual Meeting of the Japan 3-D Conformal External Beam Radiotherapy Group (22 February 2014).
REFERENCES
- 1. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. http://seer.cancer.gov/statfacts/html/lungb.html (8 August 2015, date last accessed). [Google Scholar]
- 2.De Pas T, Raimondi S, Pelosi G et al. A critical appraisal of the adjuvant chemotherapy guidelines for patients with completely resected T3N0 non-small-cell lung cancer. Acta Oncol 2010;49:480–4. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Guidelines®. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (8 August 2015, date last accessed). [Google Scholar]
- 4.Kawaguchi K, Miyaoka E, Asamura H et al. Modern surgical results of lung cancer involving neighboring structures: a retrospective analysis of 531 pT3 cases in a Japanese Lung Cancer Registry Study. J Thorac Cardiovasc Surg 2012;144:431–7. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg RJ, Vokes EE, Raben A. Non-small cell lung cancer. In: Devita VT, Hellman S, Rosenberg SA (eds). Cancer Principles and Practice of Oncology. Philadelphia: Lippincott-Raven, 1997. [Google Scholar]
- 6.Martini N, Yellin A, Ginsberg RJ et al. Management of non-small cell lung cancer with direct mediastinal involvement. Ann Thorac Surg 1994;58:1447–51. [DOI] [PubMed] [Google Scholar]
- 7.Van Raemdonck DE, Schneider A, Ginsberg RJ. Surgical treatment for higher stage non-small cell lung cancer. Ann Thorac Surg 1992;54:999–1013. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya R, Asamura H, Kondo H et al. Extended resection of the left atrium, great vessels, or both for lung cancer. Ann Thorac Surg 1994;57:960–5. [DOI] [PubMed] [Google Scholar]
- 9.Pitz CC, Brutel de la Riviere A, van Swieten HA et al. Results of surgical treatment of T4 non-small cell lung cancer. Eur J Cardiothorac Surg 2003;24:1013–8. [DOI] [PubMed] [Google Scholar]
- 10.Onishi H, Araki T. Stereotactic body radiation therapy for stage I non-small-cell lung cancer: a historical overview of clinical studies. Jpn J Clin Oncol 2013;43:345–50. [DOI] [PubMed] [Google Scholar]
- 11.Casamassima F, Masi L, Bonucci I et al. Relevance of biologically equivalent dose values in outcome evaluation of stereotactic radiotherapy for lung nodules. Int J Radiat Oncol Biol Phys 2008;71:145–51. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Guo W, Lu Y et al. Dose-individualized stereotactic body radiotherapy for T1–3N0 non-small cell lung cancer: long-term results and efficacy of adjuvant chemotherapy. Radiother Oncol 2008;88:351–8. [DOI] [PubMed] [Google Scholar]
- 13.Takeda A, Kunieda E, Sanuki N et al. Dose distribution analysis in stereotactic body radiotherapy using dynamic conformal multiple arc therapy. Int J Radiat Oncol Biol Phys 2009;74:363–9. [DOI] [PubMed] [Google Scholar]
- 14.Downey RJ, Martini N, Rusch VW et al. Extent of chest wall invasion and survival in patients with lung cancer. Ann Thorac Surg 1999;68:188–93. [DOI] [PubMed] [Google Scholar]
- 15.Doddoli C, D'Journo B, Le Pimpec-Barthes F et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg 2005;80:2032–40. [DOI] [PubMed] [Google Scholar]
- 16.Yang HX, Hou X, Lin P et al. Peripheral direct adjacent lobe invasion non-small cell lung cancer has a similar survival to that of parietal pleural invasion T3 disease. J Thorac Oncol 2009;4:1342–6. [DOI] [PubMed] [Google Scholar]
- 17.Yildizeli B, Dartevelle PG, Fadel E et al. Results of primary surgery with T4 non-small cell lung cancer during a 25-year period in a single center: the benefit is worth the risk. Ann Thorac Surg 2008;86:1065–75; discussion 74–5. [DOI] [PubMed] [Google Scholar]
- 18.Ohta M, Hirabayasi H, Shiono H et al. Surgical resection for lung cancer with infiltration of the thoracic aorta. J Thorac Cardiovasc Surg 2005;129:804–8. [DOI] [PubMed] [Google Scholar]
- 19.Spaggiari L, Magdeleinat P, Kondo H et al. Results of superior vena cava resection for lung cancer. Analysis of prognostic factors. Lung Cancer 2004;44:339–46. [DOI] [PubMed] [Google Scholar]
- 20.Stella F, Dell'Amore A, Caroli G et al. Surgical results and long-term follow-up of T(4)-non-small cell lung cancer invading the left atrium or the intrapericardial base of the pulmonary veins. Interact Cardiovasc Thorac Surg 2012;14:415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagata Y, Hiraoka M, Mizowaki T et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-D Conformal External Beam Radiotherapy Group. Int J Radiat Oncol Biol Phys 2009;75:343–7. [DOI] [PubMed] [Google Scholar]
- 22.Palma D, Visser O, Lagerwaard FJ et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240–4. [DOI] [PubMed] [Google Scholar]
- 23.Takeda A, Sanuki N, Eriguchi T et al. Stereotactic ablative body radiation therapy for octogenarians with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;86:257–63. [DOI] [PubMed] [Google Scholar]
- 24.Palma D, Lagerwaard F, Rodrigues G et al. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys 2012;82:1149–56. [DOI] [PubMed] [Google Scholar]
- 25.Nuyttens JJ, van der Voort van Zyp NC, Praag J et al. Outcome of four-dimensional stereotactic radiotherapy for centrally located lung tumors. Radiother Oncol 2012;102:383–7. [DOI] [PubMed] [Google Scholar]
- 26.Haasbeek CJ, Lagerwaard FJ, Slotman BJ et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036–43. [DOI] [PubMed] [Google Scholar]
- 27.Timmerman R, McGarry R, Yiannoutsos C et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833–9. [DOI] [PubMed] [Google Scholar]
- 28.Takeda A, Enomoto T, Sanuki N et al. Reassessment of declines in pulmonary function ≥1 year after stereotactic body radiotherapy. Chest 2013;143:130–7. [DOI] [PubMed] [Google Scholar]
- 29.Menna C, Ciccone AM, Ibrahim M et al. Pneumonectomy: quality of life and long-term results. Minerva Chir 2012;67:219–26. [PubMed] [Google Scholar]
- 30.Bishawi M, Kim B, Moore WH et al. Pulmonary function testing after stereotactic body radiotherapy to the lung. Int J Radiat Oncol Biol Phys 2012;82:e107–10. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura S, Takeda A, Sanuki N et al. Toxicities of organs at risk in the mediastinal and hilar regions following stereotactic body radiotherapy for centrally located lung tumors. J Thorac Oncol 2014;9:1370–6. [DOI] [PubMed] [Google Scholar]
- 32.Muehling B, Wehrmann C, Oberhuber A et al. Comparison of clinical and surgical–pathological staging in IIIA non-small cell lung cancer patients. Ann Surg Oncol 2012;19:89–93. [DOI] [PubMed] [Google Scholar]
- 33.Lao L, Hope AJ, Maganti M et al. Incidental prophylactic nodal irradiation and patterns of nodal relapse in inoperable early stage NSCLC patients treated with SBRT: a case-matched analysis. Int J Radiat Oncol Biol Phys 2014;90:209–15. [DOI] [PubMed] [Google Scholar]