Abstract
Metformin is and has been considered as first-line therapy for type 2 diabetes for over a quarter of a century. Like other biguanides, metformin can cause a lactic acidosis that is exceptionally rare but fatal. The likelihood of metformin-associated lactic acidosis is substantially higher in patients with kidney impairment and also among those with seemingly normal kidney function who are at risk of acute kidney injury (AKI). Hence, regulatory agencies in many industrialized nations have maintained strict renal restrictions surrounding metformin. However, there have been millions of people exposed to metformin for many years, many of them with serum creatinine values at or close to 1.5 mg/dL with estimated glomerular filtration rates (eGFRs) much below 60 mL/min/1.73 m2 who have not developed lactic acidosis. Thus, there clearly remains controversy in this area, and there has been heightened pressure to remove the renal restrictions of metformin. To provide a discussion on the pros and cons of relaxing the renal restrictions for metformin use, we provide a Point-Counterpoint. In the point narrative below, Drs. Kalantar-Zadeh and Kovesdy provide their argument that although there is little evidence of the potential benefits of metformin in kidney disease, just considering the sheer numbers of metformin users and the high fatality rate of its associated lactic acidosis, the most appropriate practice is to avoid metformin use in people with eGFR <45 mL/min/1.73 m2 or in those who are at high risk of AKI irrespective of underlying eGFR. In the following counterpoint narrative, Drs. Bakris and Molitch argue that the data from a very large analysis demonstrate clearly that serum creatinine should be supplanted with eGFR as the criteria for metformin use and that the incidence of lactic acidosis is only elevated in those with a reduced eGFR who become dehydrated for various reasons or in those exposed to some toxin resulting in AKI. Otherwise the data clearly support the use of metformin under normal circumstances down to eGFR >30 mL/min/1.73 m2.
—William T. Cefalu
Editor in Chief, Diabetes Care
Learning From History and Biochemistry
Metformin, along with buformin and phenformin, belongs to the class of biguanides. Guanidine is a strong base used in plastics and explosives. It is also found in urine as the end product of the metabolism of amino acids including arginine. Guanidino compounds are known uremic toxins, and it has been hypothesized that their increased levels in renal failure may be a potential etiology of the “burnt-out diabetes phenomenon” in advanced uremia (1). In the 1920s, guanidino compounds were discovered in the extracts of Galega officinalis (French lilac) that were used for the treatment of diabetes. In the late 1950s, biguanides were presented as an emerging treatment option for adult-onset diabetes, and by the late 1970s, approximately one-quarter to a half million people were using phenformin as the first commercially widely available biguanide. Given the increasing reports of fatal lactic acidosis cases associated with phenformin use, and the U.S. Food and Drug Administration (FDA) subsequently ordered it withdrawn from the U.S. market and declared it as an “imminent hazard to the public health” (2). As of the late 2000s, phenformin was still available in very few countries, including Italy, Brazil, Poland, and China, where phenformin-associated lactic acidosis continued to be reported. Some herbal products containing phenformin led to cases of lactic acidosis, such that the FDA recalled Chinese “herbal products” containing phenformin. Buformin, which was never available in the U.S., was removed from the market in many but not all countries for the same risk of lactic acidosis (2).
Biguanide-Associated Lactic Acidosis and Other Complications
Although the mechanism of its action is not fully understood, the occurrence of biguanide-associated lactic acidosis is a biologically plausible phenomenon (Fig. 1). Biguanides increase insulin sensitivity in vivo, resulting in reduced plasma glucose concentrations, increased glucose uptake, and decreased gluconeogenesis. They reduce hepatic gluconeogenesis and, as a result, decrease glucose levels in the blood. Whereas the most common side effects of biguanides are diarrhea and dyspepsia (Table 1), which may happen in up to 30% of patients, lactic acidosis is an exceptionally rare but highly feared occurrence given that up to 50% or more of biguanide-associated lactic acidosis cases lead to death. The likelihood of a lactic acidosis event is related to blood concentrations of the biguanide and the severity of kidney dysfunction; therefore, metformin is contraindicated if serum creatinine is ≥1.4 mg/dL in men or ≥1.5 mg/dL in women according to the FDA-mandated package insert. It is believed that phenformin and buformin are more likely to cause lactic acidosis than metformin, although the seemingly lower observed rates of lactic acidosis in metformin may be related to the conservative avoidance of this agent in patients with renal disease. Concomitant use of metformin and H2-receptor antagonists or proton pump inhibitors has the potential to induce vitamin B12 depletion and neuropathy. Other possible side effects of metformin include hypoglycemia, pancreatitis, and vitamin B12 deficiency (independent of H2-receptor antagonists or proton pump inhibitors), as shown in Table 1. Acute pancreatitis may occur secondary to metformin accumulation with or without renal impairment, as reported in the literature (3,4).
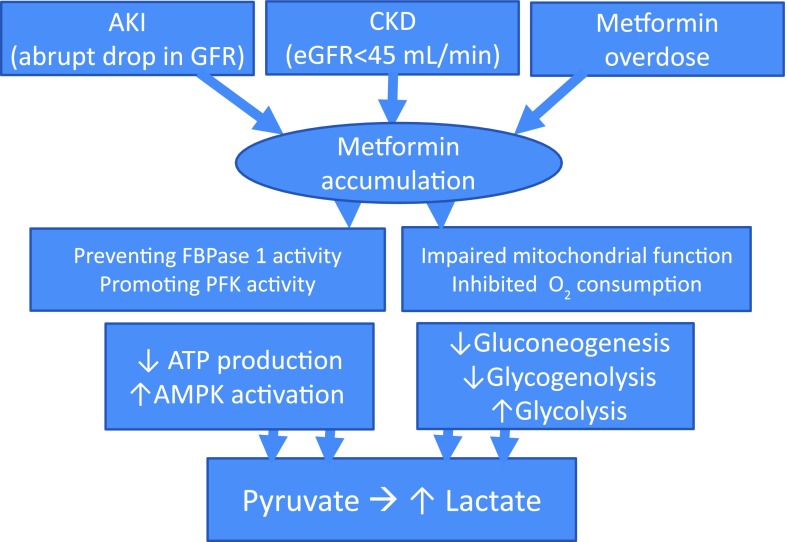
Figure 1.
Putative mechanisms of lactic acidosis with use of metformin under renal impairment. Biguanides, including metformin, may be accumulated with acute worsening of kidney function or upon gradual progression of CKD. The resultant increase in PFK activity along with FBPase 1 activity inhibition suppresses gluconeogenesis and stimulates glycolysis. The subsequent decrease in hepatic energy status activates AMPK, a cellular metabolic sensor, leading to a reduction in hepatic gluconeogenesis and glycogenolysis while glycolysis is promoted and enhanced by impaired mitochondrial function with secondary inhibition of oxygen consumption, resulting in increased lactate generation and accumulation. FBPase 1, fructose-2,6-bisphosphatase 1; PFK, phosphorylated phosphofructokinase 2.
Table 1.
Adverse events related to use of metformin
| Adverse event | Frequency | Impact by kidney impairment* | Comments |
|---|---|---|---|
| Lactic acidosis | Rare but highly fatal | ++++ | Much more likely with renal impairment; near-fatal to fatal outcomes highly likely |
| Hypoglycemia | Rare to moderate | +++ | More common with worsening renal function |
| Pancreatitis | Rare | ++/+++ | May be concurrent with lactic acidosis |
| Death (independent of lactic acidosis) | Rare, but likely common in CKD | ++++ | Recent cohort study suggests higher death rate in patients with advanced CKD (creatinine >6.0 mg/dL). |
| Cardiovascular toxicity | Rare to moderate | ++/+++ | Guanidine compounds are known uremic toxins causing cardiovascular risk. |
| Hormonal derangement | Rare | −/+ | Studies in fishes show intersex fish and other anomalies. |
| Gastrointestinal | Common (up to 30%): metallic taste, anorexia, soft stool to diarrhea (IR: 12–53%; ER: 10–17%), nausea/vomiting (IR: 7–26%; ER: 7–9%), flatulence (12%) | −/+ | Gastrointestinal symptoms are usually mild, transient, and reversible after dose reduction or discontinuation of the drug. Lactic acidosis per se may also lead to GI symptoms (nausea, vomiting, diarrhea, etc.). |
| Vitamin B12 deficiency and megaloblastic anemia | Rare, dose and therapy duration dependent | −/+ | Metformin reduces intestinal absorption of vitamin B12 in up to 30% of patients and lowers serum vitamin B12 levels in 5–10%, but only rarely causes megaloblastic anemia. |
| Cardiovascular | Rare to common | −/+ | Chest discomfort, flushing, palpitation up to 10%; worsening heart failure may be related to lactic acidosis or via independent mechanisms. |
| Neuromuscular and skeletal | 1–10% | −/+ | Central nervous system: headache (6%), chills, dizziness, lightheadedness, myalgia, weakness, altered mental status including coma |
| Other | Rare | −/+ | Hypotension, arrhythmia, respiratory abnormalities (tachypnea, pulmonary edema), hypothermia-increased diaphoresis, flu-like syndrome, nail disorder |
ER, event rate; IR, incidence rate.
The semiquantitative rating of impact by kidney impairment is from the authors.
Adverse Outcomes of Metformin in Renal Impairment
Metformin is declared as the first-step therapy in the management of type 2 diabetes (T2D) and has also been recommended for the management of prediabetes. Hence, metformin has emerged as the one of the most commonly prescribed medications worldwide and the number one administered drug in T2D. Given the fact that over half of patients with chronic kidney disease (CKD) in the U.S. and many other nations have T2D, and given that one-third of all T2D patients eventually develop CKD, discussion about the use of metformin in CKD is never-ending. Drug regulatory agencies and professional societies in different regions have recommended certain restrictions for metformin in CKD (5). The FDA stipulates a clear-cut contraindication with serum creatinine concentrations of ≥1.5 mg/dL (≥132 µmol/L) and ≥1.4 mg/dL (≥123 µmol/L) in men and women, respectively, irrespective of estimated glomerular filtration rate (eGFR). Other guidelines have recommended similar restrictions (Table 2) (6).
Table 2.
Selected guidelines and recommendations regarding metformin use and kidney function
| Source, year (reference) | Guidelines and recommendation regarding metformin use |
|---|---|
| FDA, 1994 (26) | • Contraindicated if creatinine of ≥1.4 mg/dL (men) or ≥1.5 mg/dL (women) |
| National Kidney Foundation Kidney Disease Outcomes Quality Initiative, 2012 (15) | • Contraindicated if creatinine of ≥1.4 mg/dL (men) or ≥1.5 mg/dL (women) |
| National Institute for Health and Clinical Excellence, 2009 (27) | • To be reviewed if creatinine >1.5 mg/dL or eGFR <45 mL/min/1.73 m2 • Contraindicated if creatinine >1.7 mg/dL or eGFR <30 mL/min/1.73 m2 |
| American Diabetes Association and European Association for Study of Diabetes, 2012 (28) | • Dose reduction if eGFR <45 mL/min/1.73 m2 • Contraindicated if eGFR <30 mL/min/1.73 m2 |
| Kidney Disease: Improving Global Outcomes, 2012 (29) | • To be reviewed if eGFR 30–45 mL/min/1.73 m2 • Contraindicated if eGFR <30 mL/min/1.73 m2 • Temporarily held if eGFR <60 mL/min/1.73 m2 with a concurrent serious illness increasing the risk of AKI |
| Canadian Diabetes Association, 2013 (30) | • Cautioned if eGFR <60 mL/min/1.73 m2 • Contraindicated if eGFR <30 mL/min/1.73 m2 |
| Royal Australian College of General Practitioners, 2014–2015 (31) | • Cautioned and dose reduction advised if eGFR 30–45 mL/min/1.73 m2 • Contraindicated if eGFR <30 mL/min/1.73 m2 |
| Japanese Society of Nephrology, 2012 (32) | • Reevaluated if eGFR <45 mL/min/1.73 m2 • Contraindicated if eGFR <30 mL/min/1.73 m2 |
| Opinion of the authors | • To be reviewed if eGFR 45–60 mL/min/1.73 m2 or any eGFR with prior episodes of AKI or significant proteinuria >0.5 g/day that may predispose to AKI • Contraindicated if eGFR <45 mL/min/1.73 m2, if creatinine ≥1.4 mg/dL (men) or ≥1.5 mg/dL (women), or if cystatin C >1.2 mg/mL • Withhold at any eGFR if high likelihood of AKI, such as IV contrast administration |
Adapted with permission from Rhee and Kalantar-Zadeh (6).
As shown in Table 2, even though the renal restrictions of metformin set forth by the FDA and adapted by some professional societies such as the National Kidney Foundation are according to the above-mentioned serum creatinine levels, in some guidelines, they are based on eGFR. It appears that a number of leading professional societies such as the American Diabetes Association recommend an eGFR of <30 mL/min/1.73 m2 as the threshold for absolute contraindication of metformin use. We, too, believe that the use of eGFR may be more reasonable given the wide range of eGFR variations within men and women with the same serum creatinine levels of 1.4 or 1.5 mg/dL, respectively, but across different ages and races (see Supplementary Table 1 showing eGFR values for these serum creatinine levels). However, we suggested a higher eGFR cutoff level of <45 mL/min/1.73 m2 based on the information we have reviewed here and a combination of eGFR and serum creatinine levels, so that renal restrictions not be missed in case eGFR is not readily available or calculated or in case questions about the validity of a given eGFR equation should be raised. It is important to note that for higher eGFR values (such as eGFR in the range of 30–45 mL/min/1.73 m2) some of these guidelines suggest a metformin dose reduction, while others use other statements such as “metformin administration to be reviewed” or “to be more cautious.” To our knowledge, there are no clear data as to what this so-called dose reduction means or in which format, frequency, and intensity it should be implemented; hence, we feel that a blunt suggestion of “dose reduction” is not scientifically sound and may lead to more confusion and practice discrepancies.
Is Metformin-Associated Lactic Acidosis Real?
Biguanide-induced lactic acidosis, also known as type B or nonhypoxemic lactic acidosis, is characterized by increased lactate generation and accumulation from impaired mitochondrial function with secondary inhibition of oxygen consumption and diminished gluconeogenesis and glycogenolysis (Fig. 1) (7). It remains unclear whether metformin is less likely associated with lactic acidosis than other biguanides due to inherent biochemical differences and/or a direct consequence of the aforementioned imposed renal restrictions of metformin (Table 1). This fatal acidosis appears to be a rare occurrence in clinical trials, which may be the result of the selective and conservative nature of these study designs that have historically excluded patients with CKD.
Recently Hung et al. (8) reported that in Taiwan, where until recently metformin was authorized across the entire spectrum of CKD, among 12,350 patients with serum creatinine levels >6 mg/dL (>528 µmol/L), metformin users (n = 1,005) had a 29% higher mortality than nonusers over a median period of 2.1 years. This significantly higher death risk was consistent across all subgroups. There was a dose-dependent relationship between metformin and death, which affirms the biological plausibility of a causal association, notwithstanding that these striking associations may still not be equated as causation (9). In the same study, the number of reported metabolic acidosis events was small, but metformin users had a 23% higher occurrence of metabolic acidosis than nonusers. This study has important strengths including national representation; high mortality rates of 42–53% in metformin nonusers and users, respectively; a long follow-up period (up to 9.8 years), and detailed patient-level data on medication and comorbid conditions (9).
In the U.S., metformin is the sixth most frequently prescribed medication (10). Europe and other regions demonstrate similar practice patterns (7), explaining the realistic likelihood of encountering metformin-associated lactic acidosis given the sheer numbers of its use. Approved by the FDA in 1995 as a safer biguanide (11), metformin is said to bear a lower risk of lactic acidosis compared with phenformin (1–16 vs. 40–64 cases per 100,000 patient-years, respectively [12–14]). However, there has been intense debate with regard to the differential safety and effectiveness of metformin in patients with versus without CKD. Metformin is renally eliminated in unaltered form by glomerular filtration and active tubular secretion, and its clearance may be reduced by 75% in those with eGFR <60 mL/min/1.73 m2 (15).
There is little doubt that the risk of metformin accumulation is high in patients with any degree of renal impairment. In addition, even CKD patients with minimal renal impairment but the presence of microalbuminuria or proteinuria can easily develop acute kidney injury (AKI) independent of the natural progression and/or severity of their underlying renal insufficiency (16,17). Metformin-associated lactic acidosis is rare but not nonexistent, highlighted by a recent case record in the New England Journal of Medicine (5) as well as other case reports (7,18,19), including a 2010 case series (20). The case series compared metformin-associated acidosis with other types of lactic acidosis, such as postcardiac arrest, septic shock, cardiogenic shock, mesenteric ischemia, and hemorrhagic shock, none of which had a blood pH below 7.0 except for metformin-associated acidosis (20). Despite a more severe acidemia, survival is relatively better with metformin than with other causes of lactic acidosis (20), especially if patients with AKI or CKD who have high metformin levels and profound acidosis receive immediate interventions that remove metformin such as continuous hemodiafiltration therapy, which could also effectively lower metformin notwithstanding its large volume of distribution (7,21). Not many centers can measure metformin levels rapidly; hence, it is usually a late confirmatory test that is available, if at all (21). It is our opinion, based on data reviewed (20,21), that metformin overdose or accumulation is the likely cause of lactic acidosis in any patient who has most or all of the following five criteria in the absence of a high metformin level but with history of metformin administration: 1) hypoglycemia to normoglycemia (random blood glucose <150 mg/dL), 2) a markedly elevated lactate level (e.g., >15 mEq/L) with a large anion gap (>20 mEq/L), 3) severe acidemia with a pH <7.1, 4) a very low serum bicarbonate level (<10 mEq/L), and 5) a history of renal insufficiency (eGFR <45 mL/min/1.73 m2 or serum creatinine >1.5 mg/dL) or high risk of AKI.
Weighing Risks and Benefits of Metformin in CKD
In patients without CKD, metformin appears to improve altered glucose and lipid metabolism, promote weight loss, and delay the onset of full-blown diabetes. It may be argued that excluding metformin in CKD has deprived millions of patients with diabetes from receiving this state-of-the-art standard of care. Does avoiding metformin in kidney impairment really result in poor outcomes, including glycemic control with accelerated diabetic complications? Clinical practice guidelines have proposed various kidney function cutoffs and metrics (serum creatinine vs. eGFR) for defining metformin’s safety threshold in CKD (Table 2). According to the FDA black-box warning label and the National Kidney Foundation Kidney Disease Outcomes Quality Initiative recommendations, metformin is contraindicated even with mild renal insufficiency (15). These cutoffs are conservative estimates of the threshold at which 3 g of metformin can be removed at steady-state levels in 24–48 h (22).
To ensure patients’ safety, even more stringent guidelines are needed, and it is our opinion that metformin use should be reviewed in those patients with eGFR 60–45 mL/min/1.73 m2 (but may be continued if eGFR is ≥60 mL/min/1.73 m2), discontinued in those with eGFR <45 mL/min/1.73 m2, and temporarily held at any eGFR with a concurrent serious illness that increases the risk of AKI (Table 2). Several points should be considered prior to removing restrictions on metformin use in CKD.
The risk of death associated with biguanide-induced lactic acidosis is extremely high (≥50%). As the risk of harm associated with metformin is high, the multitude of alternative options now available for the treatment of diabetes calls for a reevaluation of whether even a small risk of metformin justifies its benefit.
The true incidence of lactic acidosis may be underestimated. Randomized controlled trials that have compared metformin to placebo are not generalizable to real-life scenarios given their selected study populations, close monitoring, and protocol used in the follow-up of participants.
CKD patients have a lower threshold for kidney function decline, including greater susceptibility to AKI and accelerated rate of kidney function decline. Current clinical practice guidelines do not consider the dynamic fluctuations in kidney function (i.e., unpredicted AKI or anticipated CKD progression over time) that may substantially increase the risk of metformin accumulation and fatal lactic acidosis.
CKD patients have multiple concomitant risk factors for lactic acidosis. CKD patients are typically of advanced age and have a high burden of underlying illness (e.g., infection/sepsis, congestive heart failure, myocardial infarction, liver failure, etc.) further increasing their risk of lactic acidosis.
Conclusions: Let’s Stand by Renal Restrictions for Metformin
Metformin-associated lactic acidosis is a real and highly fatal complication. It is likely that the infrequent reports of this complication are due to the fact that we have not liberally prescribed this medication to CKD patients thanks to the well-justified FDA-mandated renal restrictions. Given recent observational study by Hung et al. (8) in a setting where metformin was previously widely prescribed to patients with renal impairment, there remains little doubt that metformin could lead to excess death in T2D patients with advanced CKD. In addition to the safety concerns, it remains uncertain as to whether metformin may yield the same multiorgan benefits in CKD patients as in other populations without kidney disease. To date, no randomized trial has specifically evaluated the potential benefits and risks of metformin in patients with CKD (22). As the natural progression of CKD is often associated with prolonged insulin half-life and decline in lipids, glucose, and hemoglobin A1c levels along with seemingly counterintuitive improvement in metabolic and glycemic burden, it is not clear what role metformin may have in CKD. Worsening uremia also leads to the accumulation of lactate and uremic toxins, including guanidino compounds that are closely related in structure to biguanides, which may negate the need for metformin given the potential harms of the expanding pool of guanidino compounds in the system and the natural progression toward “burnt-out diabetes” (23).
Although there is an ongoing need to examine real-world contexts where metformin may be frequently administered to those with advanced CKD, particularly as recent publications have advocated the expansion of metformin use in this population (24), it is highly premature and unwise to liberalize the use of metformin. Given the data by Hung et al. (8) and other case reports describing the gravity of metformin-related adverse events (5), along with historical data on the toxicities of other biguanides, the aforementioned restrictive regulations have likely protected tens of thousands of people from lactic acidosis, hypoglycemia, and pancreatitis and have saved literally thousands of lives each year. The renal restrictions of metformin should be maintained given the utmost priority of practicing safe and conservative medicine and the lack of evidence of benefits in CKD. The story of Frances Oldham Kelsey reinforces our “never again” motto (25).
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge Dr. Connie M. Rhee for her review of the manuscript and her suggestions.
Funding. K.K.-Z. is supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIH NIDDK) grant K24-DK091419 as well as philanthropist grants from Harold Simmons, Louis Chang, Dr. Joseph Lee, the National Kidney Foundation, and the American Society of Nephrology. K.K.-Z. and C.P.K. are also supported by NIH NIDDK grant R01-DK096920.
Duality of Interest. K.K.-Z. is supported by a grant from AVEO Pharmaceuticals. He has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, AstraZeneca, AVEO Pharmaceuticals, Chugai Pharmaceutical Co., DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, Relypsa, Resverlogix, Sanofi, Shire, Vifor Pharma, and ZS Pharma. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-2327/-/DC1.
See accompanying article, p. 1287.
References
- 1.Park J, Lertdumrongluk P, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr Diab Rep 2012;12:432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fantus IG. Metformin's contraindications: needed for now. CMAJ 2005;173:505–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fimognari FL, Corsonello A, Pastorell R, Antonelli-Incalzi R. Metformin-induced pancreatitis: A possible adverse drug effect during acute renal failure. Diabetes Care 2006;29:1183. [DOI] [PubMed] [Google Scholar]
- 4.Mallick S. Metformin induced acute pancreatitis precipitated by renal failure. Postgrad Med J 2004;80:239–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Uppot RN, Lewandrowski KB. Case records of the Massachusetts General Hospital. Case 23-2013. A 54-year-old woman with abdominal pain, vomiting, and confusion. N Engl J Med 2013;369:374–382 [DOI] [PubMed] [Google Scholar]
- 6.Rhee CM, Kalantar-Zadeh K. Restricting metformin in CKD: continued caution warranted. Am J Kidney Dis 2015;66:1101–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vecchio S, Protti A. Metformin-induced lactic acidosis: no one left behind. Crit Care 2011;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung SC, Chang YK, Liu JS, et al. Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. Lancet Diabetes Endocrinol 2015;3:605–614 [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Rhee CM. Metformin in chronic kidney disease: more harm than help? Lancet Diabetes Endocrinol 2015;3:579–581 [DOI] [PubMed] [Google Scholar]
- 10.Held C, Hjemdahl P, Håkan Wallén N, et al.; The Angina Prognosis Study in Stockholm . Inflammatory and hemostatic markers in relation to cardiovascular prognosis in patients with stable angina pectoris. Results from the APSIS study. Atherosclerosis 2000;148:179–188 [DOI] [PubMed] [Google Scholar]
- 11.Kumano K, Yokota S, Go M, et al. Quantitative and qualitative changes of serum albumin in CAPD patients. Adv Perit Dial 1992;8:127–130 [PubMed] [Google Scholar]
- 12.Brown JB, Pedula K, Barzilay J, Herson MK, Latare P. Lactic acidosis rates in type 2 diabetes. Diabetes Care 1998;21:1659–1663 [DOI] [PubMed] [Google Scholar]
- 13.Stang M, Wysowski DK, Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care 1999;22:925–927 [DOI] [PubMed] [Google Scholar]
- 14.Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 2007;335:508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012;60:850–886 [DOI] [PubMed] [Google Scholar]
- 16.Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial 2010;23:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: A1C remains the gold standard outcome predictor in diabetic dialysis patients. Diabetes Care 2012;35:1625–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisberg LS. Lactic acidosis in a patient with type 2 diabetes mellitus. Clin J Am Soc Nephrol 2015;10:1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Protti A, Russo R, Tagliabue P, et al. Oxygen consumption is depressed in patients with lactic acidosis due to biguanide intoxication. Crit Care 2010;14:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friesecke S, Abel P, Roser M, Felix SB, Runge S. Outcome of severe lactic acidosis associated with metformin accumulation. Crit Care 2010;14:R226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen HL, Concepcion L. Metformin intoxication requiring dialysis. Hemodial Int 2011;15(Suppl. 1):S68–S71 [DOI] [PubMed] [Google Scholar]
- 22.Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care 2011;34:1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Deyn PP, Vanholder R, Eloot S, Glorieux G. Guanidino compounds as uremic (neuro)toxins. Semin Dial 2009;22:340–345 [DOI] [PubMed] [Google Scholar]
- 24.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bren L. Frances Oldham Kelsey. FDA medical reviewer leaves her mark on history. FDA Consum 2001;35:24–29 [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration Metformin hydrochloride tablets. Available from http://www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf. Accessed 14 May 2016
- 27.National Institute for Health and Care Excellence (NICE) NICE guidelines: the management of type 2 diabetes. Available from https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations. Accessed 14 May 2016
- 28.Inzucchi SE, Bergenstal RM, Buse JB, et al.; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidney Disease: Improving Global Outcomes KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013;3:1–163 [DOI] [PubMed] [Google Scholar]
- 30.Booth G, Cheng AY; Canadian Diabetes Association Clinical Practice Guidelines Expert Committee . Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Methods. Can J Diabetes 2013;37(Suppl. 1):S4–S7 [DOI] [PubMed] [Google Scholar]
- 31.The Royal Australian College of General Practitioners General practice management of type 2 diabetes. 2014-2015. Available from http://www.racgp.org.au/download/Documents/Guidelines/Diabetes/2014diabetesmanagement.pdf. Accessed 14 May 2016
- 32.Japanese Society of Nephrology Clinical Practice Guidebook for Diagnosis and Treatment of Chronic Kidney Disease. Tokyo, Japan, Tokyo Igakusha, 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.