Abstract
OBJECTIVE
Closed-loop (CL) insulin delivery effectively maintains glucose overnight but struggles when challenged with meals. Use of single-day, 30-μg/meal pramlintide lowers meal excursions during CL. We sought to further elucidate the potential benefits of adjunctive agents after 3–4 weeks of outpatient dose titration.
RESEARCH DESIGN AND METHODS
Two CL studies were conducted: one evaluating adjunctive pramlintide and the other liraglutide. Ten subjects (age 16–23 years; A1C 7.2 ± 0.6% [55 ± 6.6 mmol/mol]) completed two 24-h sessions: one on CL alone and one on CL plus 60-μg pramlintide (CL + P), after a 3–4-week outpatient dose escalation. Eleven subjects (age 18–27 years; A1C 7.5 ± 0.9% [58 ± 9.8 mmol/mol]) were studied before and after treatment with 1.8 mg liraglutide (CL + L) after a similar 3–4-week dose escalation period. Timing and content of meals during CL were identical within experiments; meals were not announced.
RESULTS
Pramlintide delayed the time to peak plasma glucose (PG) excursion (CL 1.6 ± 0.5 h vs. CL + P 2.6 ± 0.9 h, P < 0.001) with concomitant blunting of peak postprandial increments in PG (P < 0.0001) and reductions in postmeal incremental PG area under the curve (AUC) (P = 0.0002). CL + L also led to reductions in PG excursions (P = 0.05) and incremental PG AUC (P = 0.004), with a 28% reduction in prandial insulin delivery. Outpatient liraglutide therapy led to a weight loss of 3.2 ± 1.8 kg, with a 26% reduction in total daily insulin dose.
CONCLUSIONS
Adjunctive pramlintide and liraglutide treatment mitigated postprandial hyperglycemia during CL control; liraglutide demonstrated the additional benefit of weight loss in an insulin-sparing manner. Further investigations of these and other adjunctive agents in long-term outpatient CL studies are needed.
Introduction
Although the idea of closed-loop (CL) insulin delivery has been around for at least half a century (1,2), the introduction of transcutaneous real-time continuous glucose monitoring devices in combination with computerized control algorithms and insulin pumps has turned this dream into a reality. The past decade has seen a large number of closely supervised inpatient clinical research center studies demonstrating the feasibility of automated insulin delivery systems that allow for more targeted glycemic control while reducing patient burden (3–13). Moreover, as described in a number of articles in this special issue of Diabetes Care (14–18), these systems have now moved out of clinical research centers to experimental, unsupervised use at home in patients with type 1 diabetes.
Despite the remarkable progress that has been made in developing artificial pancreas systems, issues with postprandial hyperglycemia remain. These problems are primarily due to delays in absorption of insulin from the subcutaneous site of insulin infusion and because the algorithm drags out the meal bolus over a period of 2–3 h during fully automated glucose control. We demonstrated the effectiveness of hybrid CL control (4) in which part of the insulin required to cover the carbohydrate content of a meal is provided through a manual bolus dose, but this approach adds to the burden on patients and is subject to human error. Accelerating the rate of absorption of insulin might help to lessen the degree of postprandial hyperglycemia but may require an alternate site of insulin delivery, such as the peritoneum. Use of noninsulin adjunctive agents, including new drugs that have been approved for use in type 2 diabetes, provides another approach to improve postprandial glucose control during open-loop and CL insulin delivery in type 1 diabetes.
We first explored the use of pramlintide as a potential adjunctive therapy to reduce postprandial hyperglycemia during 24 h of CL insulin delivery without manual premeal priming doses of insulin (19). Pramlintide is an analog of the naturally occurring peptide amylin, which has beneficial effects on diabetes control believed to occur in two ways: 1) by slowing carbohydrate appearance by delaying gastric emptying and enabling a better match with insulin absorption and 2) by lowering meal-stimulated increases in plasma glucagon levels. In eight participants, the use of adjunctive treatment with pramlintide 30 μg/meal was associated with a consistent 60-min delay in time to peak postprandial blood glucose levels (19). Despite these delays in carbohydrate absorption, peak postprandial glucose concentrations were reduced by only 25 mg/dL, and 37% of daytime glucose concentrations remained >180 mg/dL (19).
The modest benefit of pramlintide in our first study may have been due to the low dose used in these drug-naive subjects as well as the single-day exposure to the drug. We hypothesized that a longer outpatient treatment period of 3–4 weeks during which pramlintide is titrated up to the full therapeutic dose of 60 μg before each meal would result in an even greater mitigation of prandial glycemic excursions compared with CL alone. Because GLP-1 agonists have been shown to suppress glucagon secretion and to reduce appetite through central action (20), we concurrently explored whether liraglutide might provide another approach to lowering postmeal glucose excursions during CL insulin delivery when titrated to its full therapeutic dose (i.e., 1.8 mg given once daily) over a 3–4-week period. In view of the similarities in the two study designs, including the use of the same fully automated CL system, the results of these two experiments are presented here.
Research Design and Methods
Both studies were reviewed and approved by the Yale University Human Investigation Committee. After a complete explanation of study procedures, written informed consent was obtained for participants ≥18 years of age. For the pramlintide study, written parental permission and participant assent were also obtained for those between age 15 and 18 years. Participants were recruited from the Yale Children’s Diabetes Program and through local advertising.
Eligibility Criteria
Participants for both studies met the following eligibility criteria: clinical diagnosis of type 1 diabetes of at least 1 year’s duration and use of insulin pump therapy for at least 3 months; A1C ≤9% (≤75 mmol/mol); normal hematocrit and serum creatinine level; no history of eating disorders, celiac disease, gastroparesis, or other disorder of intestinal absorption or motility; no history of hypoglycemic seizures in the past 3 months; no other chronic medical condition (except treated hypothyroidism); no current use of medications (other than insulin) known to affect blood glucose level or GI motility, and no prior adverse reactions to the adjunctive agent under study. Female participants could not be pregnant or lactating.
For the pramlintide trial, participants needed to be between 15–30 years old, have a BMI <95% for age and sex, and weight ≥40 kg. Eligible participants in the liraglutide study were aged 18–40 years with a body weight >50 kg; no history of pancreatitis, gallstones, alcoholism, or high triglyceride levels; and no personal or family history of thyroid cancer on multiple endocrine neoplasia type 2.
Study Design
This article describes two separate studies of similar design that used two separate groups of subjects that received two separate adjunctive drug treatments. In both studies, CL system performance was initially assessed before adjunctive drug treatment. Results of the baseline pretreatment studies were compared with CL system performance after 3–4 weeks of outpatient adjunctive drug treatment. Thus, each study involved baseline versus on-treatment paired comparisons for each drug separately, with one group receiving pramlintide and the other liraglutide. The studies were not designed or powered to compare the effects of pramlintide and liraglutide with each other.
In both studies, study staff maintained frequent telephone contact with the participants during the outpatient open-loop treatment phase to titrate doses of pramlintide to 60 μg/meal and liraglutide to 1.8 mg/day and to adjust insulin doses as needed. During these contacts, participants were asked about potential adverse effects from the adjunctive therapy. During the second CL admission, pramlintide was administered 15 min before each meal, whereas liraglutide 1.8 mg was administered once daily at 8:00 a.m. with the start of breakfast. After the second CL admission, study staff aided participants with discontinuation of adjunctive therapy and subsequent readjustment of insulin doses.
Study Outcomes
The primary outcome in both studies was the change in meal-stimulated plasma glucose excursions during CL control as reflected by changes in the peak increment in plasma glucose levels above premeal values as well as the area under the incremental glucose response curve for 5 h after each meal. Other outcomes during CL control were changes in time to peak postprandial glucose levels and the amount of insulin delivered for each meal. Safety outcomes were the number of hypoglycemic events with plasma glucose <60 mg/dL and other adverse events. Outcomes of interest during the open-loop treatment phase were changes in insulin doses and body weight. Safety issues included tolerability of higher drug doses and the occurrence of adverse events.
Participant Preparation for CL Studies
In both studies and on both occasions within each study, participants were admitted to the clinical research center in the midafternoon on study day 1 to allow for initialization of the CL system. Two continuous glucose sensors (the study sensor and a backup sensor) were inserted and calibrated, a new insulin infusion set was placed, and the participant’s usual insulin pump was replaced by the study pump (Paradigm 715; Medtronic). The total daily insulin doses that the participant had received over the past 3, 7, and 14 days were recorded; these data along with the preprogrammed open-loop basal rate were used to adjust the algorithm. An intravenous catheter was placed into an arm vein to facilitate frequent blood sampling.
After dinner on study day 1, a run-in period of CL control was initiated at ∼9:00 p.m. to achieve stable target glucose levels at the start of the CL data collection the next morning (8:00 a.m.). During the 24-h CL control observation period, participants were free to move about their room and hallway.
System Considerations
The CL system used in this study consisted of four components: a Medtronic Paradigm 715 insulin pump, a Medtronic MiniLink REAL-Time transmitter (MMT-7703) adapted for 1-min transmission, a Medtronic continuous glucose sensor (Sof-sensor in the pramlintide study and Enlite sensor in the liraglutide study), and the Medtronic external physiological insulin delivery algorithm. Algorithm calculations were performed on a laptop computer that received the glucose sensor signal each minute from a radiofrequency transmitter and delivered insulin commands to the pump by radiofrequency signaling. The external physiological insulin delivery controller uses a proportional-integral-derivative algorithm modified to include insulin feedback, which has been extensively described (21,22). CL target glucose level was set to 120 mg/dL for the pramlintide protocol and to 100 mg/dL for the liraglutide experiments.
Sensors were calibrated at system initiation and every 12 h thereafter by using a best-fit linear regression calibration scheme and fixed offset with a reference sensor pairing delay of 10 min. Additional calibrations were performed if sensor errors exceeded 20%. Sensor accuracy was similar in both studies: mean absolute relative deviation was 11.5 ± 3.8% in the pramlintide study and 11.1 ± 2.8% in the liraglutide study.
CL Study Procedure
The same breakfast, lunch, and dinner meals were provided at 8:00 a.m., 1:00 p.m., and 6:00 p.m., respectively, during both CL admissions in both studies. Participants self-selected the meals and were not limited by calorie or carbohydrate content. Details regarding macronutrient content of meals are presented in Table 1. No manual boluses were given for meals, and the meals were not announced to the controller.
Table 1.
Macronutrient content of meals during CL experiments
| Macronutrient content of meals (g) |
|||
|---|---|---|---|
| Carbohydrate | Protein | Fat | |
| Pramlintide | |||
| Breakfast | 67.4 ± 23.2 | 21.8 ± 7.7 | 16.4 ± 7.9 |
| Lunch | 78.2 ± 20.8 | 26.5 ± 13.3 | 33.4 ± 8.3 |
| Dinner | 84.0 ± 28.8 | 32.5 ± 13.4 | 27.6 ± 16.0 |
| Liraglutide | |||
| Breakfast | 75.2 ± 49.1 | 24.0 ± 16.3 | 16.9 ± 13.6 |
| Lunch | 78.4 ± 26.9 | 26.4 ± 15.8 | 32.3 ± 13.5 |
| Dinner | 87.8 ± 31.1 | 35.0 ± 15.6 | 35.3 ± 15.4 |
Data are mean ± SD.
Hypoglycemia, defined as a plasma glucose level <60 mg/dL, was treated with 15 g fast-acting carbohydrate. Additional blood glucose measurements were sampled when sensor or plasma glucose levels were <60 mg/dL. Per protocol, urine and blood ketones were measured if plasma glucose was >250 mg/dL for 4 h or >300 mg/dL for 1 h.
Biochemical Analysis
Plasma glucose levels were sampled every 30 min throughout the CL admissions with the YSI 2300 STAT Plus glucose analyzer (YSI Life Sciences, Yellow Springs, OH). These data were used to compare differences in glucose control between the two treatment conditions of CL alone and CL with adjunctive therapy.
Statistical Considerations
Descriptive statistics were calculated for plasma glucose levels and doses of insulin administered. Data are expressed as mean ± SD or SEM as indicated. Meal-stimulated glucose excursions were calculated as the increment in plasma glucose levels above premeal values for the 5 h following each meal. Peak postmeal plasma glucose levels, the incremental plasma glucose area under the curve (AUC), and the time to peak increment in plasma glucose were compared. Statistical comparisons between groups in each protocol (CL alone vs. CL with adjunctive therapy) were accomplished with paired t tests for normally distributed data and Wilcoxon matched pair signed rank tests for nonnormally distributed data. Effects of adjunctive therapy on weight and total daily insulin dose were calculated for each adjunctive therapy. Additionally, a post hoc analysis using an unpaired t test was performed to assess differences in change in weight and insulin dose between the two adjunctive therapies. Calculations were performed with GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA).
Results
Participants
Pramlintide Adjunctive Therapy
Thirteen participants who met the inclusion/exclusion criteria were enrolled in the study; one participant in whom hyperglycemia and ketosis developed before any CL study procedures was discontinued from the study and two other participants withdrew due to scheduling conflicts. The 10 remaining participants (4 males) who completed all study procedures and were included in the analysis ranged in age from 16 to 23 years (mean 19.9 years) and had a diabetes duration of 3.9–15.4 years (mean 9.1 years) and A1C level of 6.7–8.0% (mean 7.2%) (50–64 mmol/mol [mean 55 mmol/mol]).
Liraglutide Adjunctive Therapy
Fifteen participants were enrolled in the study; two withdrew before any inpatient CL admissions, and one withdrew consent due to problems with intravenous access during the first CL admission. Another withdrew after the first CL admission. The 11 remaining participants (4 males) who completed all study procedures and were included in the analysis ranged in age from 18 to 27 years (mean 22 years) and had a diabetes duration of 1–23 years (mean 10.5 years) and A1C level of 5.8–9% (mean 7.5%) (40–75 mmol/mol [mean 58 mmol/mol]).
Effect of Adjunctive Therapy on Prandial Glucose Excursions During CL Control
Pramlintide Adjunctive Therapy
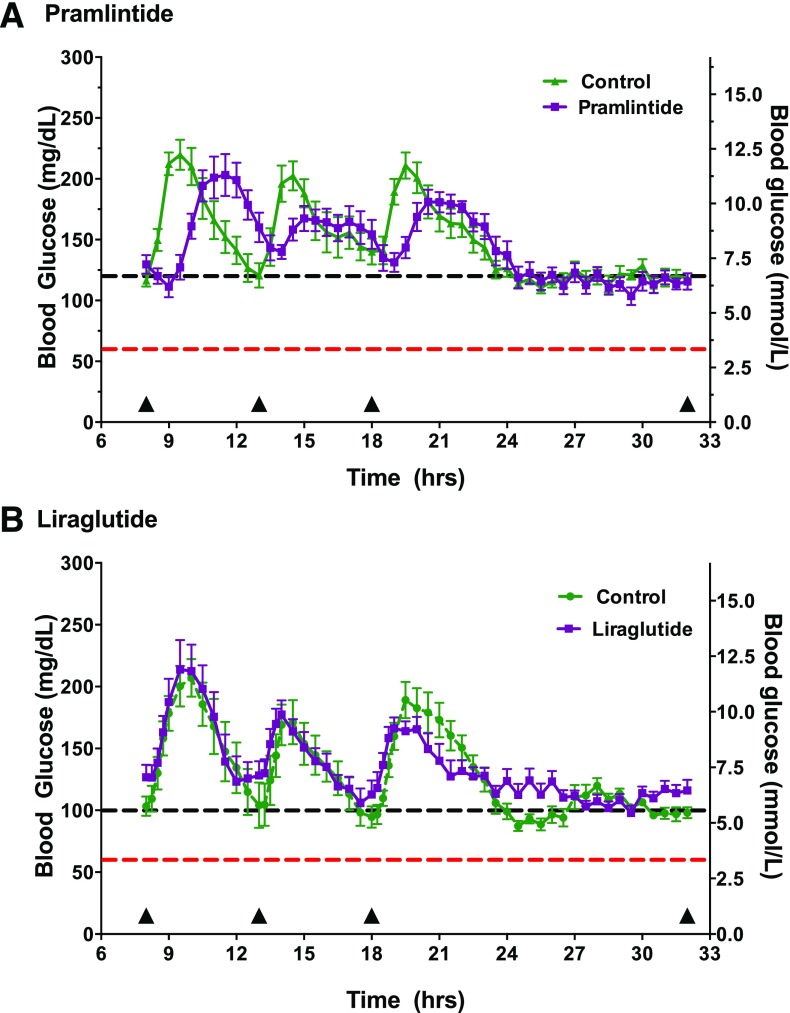
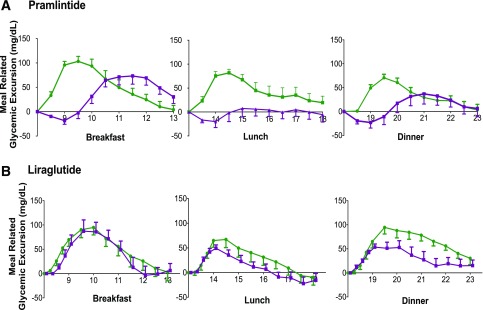
Mean plasma glucose levels during the 24 h of CL control before and after treatment with pramlintide are shown in Fig. 1A, whereas Fig. 2A illustrates the effect of treatment in blunting and delaying meal-stimulated increments in plasma glucose levels. As shown in Table 2, the addition of pramlintide to CL control was associated with a 39% reduction in the peak increment in postprandial plasma glucose levels and incremental plasma glucose AUC as well as with a significant delay in the time to peak plasma glucose level when averaged for all meals and for most individual meals. Meal-related glucose excursions were reduced with pramlintide even in the face of a 13% reduction in prandial insulin delivery (Table 2). Additionally, time in target (70–180 mg/dL) during daytime hours was greater with adjunctive pramlintide (P = 0.004) therapy, as shown in Table 3. One hypoglycemic event occurred during two control admissions and one pramlintide admission. No episodes of nocturnal hypoglycemia were noted during either study.
Figure 1.
Glucose profiles during control and adjunctive therapy conditions. A: Pramlintide 120 mg/dL. B: Liraglutide 100 mg/dL. The red line denotes 60 mg/dL, which is the threshold defining hypoglycemia. The black line denotes the system target. Meals are indicated by triangles along the x-axis.
Figure 2.
Meal-related glycemic excursions are presented for the 5-h period after meals. A: Pramlintide. B: Liraglutide. Glucose levels are corrected for baseline glucose at the start of the meal. The control visit is represented in green and the adjunctive therapy in purple.
Table 2.
Data from inpatient CL admissions
| CL alone | CL + adjunctive therapy | P value | |
|---|---|---|---|
| Pramlintide | |||
| Time to peak plasma glucose (h) | |||
| Average of all meals | 1.6 ± 0.5 | 2.9 ± 0.9 | <0.0001 |
| Breakfast | 1.5 ± 0.4 | 3.2 ± 0.6 | <0.0001 |
| Lunch | 1.8 ± 0.8 | 2.7 ± 1.3 | 0.01 |
| Dinner | 1.7 ± 0.3 | 2.8 ± 0.5 | 0.002 |
| Plasma glucose excursion (mg/dL) | |||
| Average all meals | 96 ± 30 | 59 ± 46 | <0.0001 |
| Breakfast | 116 ± 30 | 91 ± 44 | 0.03 |
| Lunch | 99 ± 19 | 42 ± 38 | 0.01 |
| Dinner | 72 ± 23 | 43 ± 42 | 0.11 |
| Plasma glucose AUC meal excursion (mg/dL · h) | |||
| Total all meals | 677 ± 96 | 412 ± 159 | 0.002 |
| Breakfast | 269 ± 117 | 203 ± 113 | 0.06 |
| Lunch | 239 ± 121 | 77 ± 103 | 0.01 |
| Dinner | 168 ± 87 | 110 ± 117 | 0.25 |
| Prandial insulin delivery (units) | |||
| Total all meals | 32.6 ± 6.5 | 28.2 ± 8.8 | 0.047 |
| Breakfast | 10.7 ± 4.4 | 9.8 ± 4.4 | 0.37 |
| Lunch | 10.9 ± 2.0 | 8.4 ± 3.5 | 0.04 |
| Dinner | 12.5 ± 4.2 | 9.1 ± 2.4 | 0.04 |
| Liraglutide | |||
| Time to peak plasma glucose (h) | |||
| Average all meals | 1.8 ± 0.5 | 1.8 ± 0.6 | 0.97 |
| Breakfast | 1.9 ± 0.5 | 2.1 ± 1.1 | 0.75 |
| Lunch | 1.4 ± 0.6 | 1.5 ± 0.9 | 0.99 |
| Dinner | 2.2 ± 0.9 | 1.7 ± 0.8 | 0.27 |
| Plasma glucose excursion (mg/dL) | |||
| Average all meals | 98 ± 24 | 76 ± 17 | 0.05 |
| Breakfast | 114 ± 45 | 103 ± 56 | 0.60 |
| Lunch | 77 ± 53 | 57 ± 21 | 0.17 |
| Dinner | 106 ± 41 | 71 ± 43 | 0.11 |
| Plasma glucose AUC meal excursion (mg/dL · h) | |||
| Total all meals | 789 ± 176 | 500 ± 151 | 0.002 |
| Breakfast | 289 ± 151 | 226 ± 192 | 0.27 |
| Lunch | 178 ± 178 | 106 ± 70 | 0.32 |
| Dinner | 304 ± 143 | 175 ± 153 | 0.05 |
| Prandial insulin delivery (units) | |||
| Total all meals | 22.6 ± 8.4 | 16.3 ± 8.3 | 0.005 |
| Breakfast | 8.8 ± 4.1 | 6.5 ± 4.1 | 0.006 |
| Lunch | 6.4 ± 3.0 | 5.3 ± 2.8 | 0.17 |
| Dinner | 8.2 ± 2.8 | 5.1 ± 2.1 | 0.003 |
Data are mean ± SD. System target glucose was 120 mg/dL for the pramlintide study and 100 mg/dL for the liraglutide study.
Table 3.
Mean glucose levels and time in target for inpatient CL admissions
| CL alone | CL + adjunctive therapy | P value | |
|---|---|---|---|
| Pramlintide | |||
| Daytime (8:00 a.m.–11:00 p.m.) | |||
| Mean blood glucose (mg/dL) | 166 ± 47 | 160 ± 40 | 0.08 |
| <70 mg/dL | 1.9 | 0.3 | |
| 70–180 mg/dL | 59.7 | 71.1 | 0.004 |
| >180 mg/dL | 38.4 | 28.5 | |
| Nocturnal (11:00 p.m.–6:00 a.m.) | |||
| Mean blood glucose (mg/dL) | 121 ± 20 | 122 ± 28 | 0.87 |
| <70 mg/dL | 0 | 2.7 | |
| 70–180 mg/dL | 98.7 | 93.3 | 0.12 |
| >180 mg/dL | 1.3 | 4 | |
| Liraglutide | |||
| Daytime (8:00 a.m.–11:00 p.m.) | |||
| Mean blood glucose (mg/dL) | 143 ± 55 | 146 ± 47 | 0.13 |
| <70 mg/dL | 5.9 | 3.3 | |
| 70–180 mg/dL | 71.6 | 74.4 | 0.50 |
| >180 mg/dL | 22.4 | 22.3 | |
| Nocturnal (11:00 p.m.–6:00 a.m.) | |||
| Mean blood glucose (mg/dL) | 104 ± 23 | 113 ± 24 | 0.0001 |
| <70 mg/dL | 5.5 | 2.4 | |
| 70–180 mg/dL | 94.5 | 96.4 | 0.13 |
| >180 mg/dL | 0 | 1.2 |
Data are mean ± SD for glucose levels and percent of time in various ranges (<70, 70–180, and >180 mg/dL). System target glucose was 120 mg/dL for the pramlintide study and 100 mg/dL for the liraglutide study.
Liraglutide Adjunctive Therapy
At first glance, adjunctive treatment with liraglutide appeared to have had minimal effects on the mean 24-h plasma glucose levels during CL control (Fig. 1B). Although the time to peak increment in postprandial glucose was not delayed, liraglutide treatment did cause modest, but statistically significant reductions in the peak increment in postmeal plasma glucose levels over all meals combined and a 35% reduction in postmeal plasma glucose AUC. Figure 2B depicts the incremental meal-related glycemic excursions by correcting for baseline glucose values at the start of each meal. These differences were observed even though prandial insulin delivery over the three meals was reduced by 28% during treatment with liraglutide. Eight episodes of hypoglycemia occurred during the liraglutide admissions compared with seven during the control admissions.
Effects of Outpatient Open-Loop Treatment
Pramlintide was well tolerated, and only one participant was unable to increase the pramlintide dose to >30 μg due to loss of appetite and bloating at a higher dose level. During open-loop treatment with liraglutide, 54% of participants experienced GI issues (nausea, decreased appetite, constipation, bloating, and/or diarrhea). However, these issues were transient and did not prevent anyone from reaching the goal dose of 1.8 mg during their second CL admission. In view of the short 3–4-week duration of the outpatient phase of the study, liraglutide-treated participants lost ∼5.0% body weight and lowered total daily insulin doses by ∼26%, changes that were greater than that observed with pramlintide treatment (Table 4). There were no episodes of severe hypoglycemia or diabetic ketoacidosis during the open-loop outpatient dose titration phases.
Table 4.
Comparison of outpatient weight and insulin dose changes
| Pramlintide | Liraglutide | P value | |
|---|---|---|---|
| Weight (kg) | |||
| Baseline | 73.0 ± 13.4 | 66.2 ± 9.3 | |
| Posttreatment | 72.3 ± 13.4 | 62.9 ± 8.1 | |
| Change in weight | 0.7 ± 1.4 | 3.2 ± 1.88 | 0.003 |
| % change in weight | 1 ± 2 | 5 ± 2.4 | 0.002 |
| Insulin dose (units) | |||
| Baseline | 58.7 ± 17.4 | 51.5 ± 14.1 | |
| Posttreatment | 52.1 ± 12.4 | 37.5 ± 12.9 | |
| Change in dose | 6.6 ± 10.9 | 13.9 ± 13.3 | 0.19 |
| % change in dose | 9 ± 14 | 26 ± 21 | 0.05 |
Data are mean ± SD.
Conclusions
An important finding of this study is that treatment with the full recommended dose of 60 μg/meal of pramlintide over 3–4 weeks of outpatient open-loop therapy improved prandial CL glucose control by reducing both peak plasma glucose excursions and incremental plasma glucose AUC by 39%. In contrast, the improvements in both these metrics were only 22% and 26%, respectively, in our prior single-day 30 μg/meal study (19). Because there is no carryover effect of prior premeal doses of insulin before breakfast in the morning, it has been a difficult challenge for most CL systems that do not manually announce meals to effectively control glucose excursions after breakfast. In our earlier study, low-dose pramlintide had little effect on CL control of postbreakfast glucose excursion. Thus, the significant lowering of postbreakfast glucose excursions in the present study is a notable finding.
GLP-1 agonists, like liraglutide, have been suggested as alternatives to pramlintide as an adjunctive treatment of type1 diabetes because their mechanisms of action (i.e., suppression of plasma glucagon levels and delays in gastric emptying) appear to be similar to pramlintide and their longer duration of action allows once-daily to once-weekly injections. Thus, that we were able to show only a modest improvement in CL control of meal-stimulated glucose excursions in the present study is disappointing. Moreover, unlike pramlintide, there was no evidence of lasting effects of liraglutide in delaying rates of gastric emptying. Single-dose experiments have shown that GLP-1 agonists suppress meal-stimulated increases in plasma glucagon levels (23). However, more-recent studies have indicated that treatment with GLP-1 agonists over weeks and months may actually increase circulating plasma glucagon levels; such increases in glucagon may help to explain the limited benefit of liraglutide during CL control in the present study (24).
Nevertheless, the present results indicate that liraglutide may play an adjunctive role in open-loop treatment of type 1 diabetes. A surprising finding of this study was the degree of weight loss (and the corresponding reduction in insulin requirements) observed over a remarkably short period of treatment with liraglutide. Although the central action of liraglutide to reduce appetite and promote weight loss in obese individuals with or without type 2 diabetes is well recognized, these actions may also be beneficial for patients with type 1 diabetes. In the current intensive treatment era, an increasingly large proportion of pediatric and adult patients with type 1 diabetes are overweight or obese, which in turn contributes to problems in achieving optimal metabolic control and increases the risk of future cardiovascular disease (25). The recent failure of metformin to improve metabolic control of obese adolescents with type 1 diabetes (26) illustrates a continuing unmet need for an adjunctive therapy like liraglutide that could promote weight loss and reduce insulin requirements in type 1 diabetes.
The CL system used in the present experiments relied on total daily insulin dose as the primary variable to tune algorithm parameters; subjects requiring lower doses have less aggressive insulin delivery during meal-related glucose excursions. Thus, the 26% reduction in total daily doses achieved during outpatient, open-loop treatment with liraglutide may have offset our ability to demonstrate differences in prandial increments in plasma glucose during the second CL experiments.
Of note, the present studies were not designed or powered to provide a direct comparison of the effects of pramlintide with liraglutide as adjunctive agents to CL insulin delivery. As noted in Table 3, both the control and the liraglutide experiments had lower mean glucose levels than that achieved during the pramlintide experiments, which is likely due to the lower system set point (target 120 mg/dL for the pramlintide study vs. 100 mg/dL for the liraglutide study). The lower set point used in the liraglutide studies may also explain the higher frequency of hypoglycemia with liraglutide than with pramlintide.
The first generations of ambulatory CL systems approaching commercial development must be shown to be safe before gaining regulatory approval, trading some controller aggressiveness in insulin delivery for avoidance of hypoglycemia. Consequently, all these systems require manual boluses of premeal insulin to handle meal-related challenges. The use of adjunctive therapy may ease the burden placed on these CL systems to mitigate postprandial glycemic excursions in an insulin-sparing manner, thereby achieving lower glycemic excursions with a lower risk of hypoglycemia. Although pramlintide appears to provide robust and durable reductions and delays in prandial glucose excursions, its attractiveness as an adjunctive treatment is reduced by the need for premeal injections. GLP-1 agonists require only once-daily or once-weekly injections, but their ability to mitigate postmeal excursions with long-term treatment remains to be established. The present data suggest that the major benefit of these agents as adjunctive treatment may be for weight loss and concomitant increases in insulin sensitivity in overweight and obese patients with type 1 diabetes. Outpatient studies of CL insulin delivery with these adjunctive agents are needed to determine their full efficacy and safety profiles.
Article Information
Acknowledgments. The authors thank the participants and their families, the health care professionals and staff of Yale Children’s Diabetes Clinic, the Yale Center for Clinical Investigation, and the dedicated nursing staff of the hospital research unit for the support and participation that made this project possible.
Funding. This study was made possible through the support of grants from JDRF (22-2009-799, 17-2013-5, and 5-ECR-2014-112-A-N), the National Institutes of Health (R01-DK-085618, K12-DK-094714, UL1-TR-000142, and P30-DK-45735), and the Michael D. Ryan and Rosemary McNicholas Ryan Pediatric Diabetes Research Fund. Medtronic Diabetes provided the pumps, sensors, infusion sets, reservoirs, and laptop computers for the CL experiments.
Duality of Interest. W.V.T. is a consultant for Novo Nordisk and Sanofi. E.C. is a speaker for Novo Nordisk. S.A.W. is a consultant for Medtronic Diabetes and Tandem and serves on a medical advisory board for Insulet. No other potential conflicts of interest relevant to this article were reported.
No sponsor had any role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Author Contributions. J.L.S. researched data and wrote the manuscript. N.S.P., C.I.M., M.M.P.-C., M.A.V.N., E.C., L.R.C., and E.M.T. researched data and contributed to the discussion. W.V.T. and S.A.W. researched data, contributed to the discussion, and reviewed and edited the manuscript. J.L.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, PA, 8–12 June 2012; 12th Annual Diabetes Technology Meeting, Bethesda, MD, 8–10 November 2012; the 6th International Conference on Advanced Technologies & Treatments for Diabetes, Paris, France, 27 February–2 March 2013; 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013; 8th International Conference on Advanced Technologies & Treatments for Diabetes, Paris, France, 18–21 February 2015; and 15th Annual Diabetes Technology Meeting, Bethesda, MD, 22–24 October 2015.
Footnotes
References
- 1.Albisser AM, Leibel BS, Ewart TG, Davidovac Z, Botz CK, Zingg W. An artificial endocrine pancreas. Diabetes 1974;23:389–396 [DOI] [PubMed] [Google Scholar]
- 2.Albisser AM, Leibel BS, Ewart TG, et al. . Clinical control of diabetes by the artificial pancreas. Diabetes 1974;23:397–404 [DOI] [PubMed] [Google Scholar]
- 3.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006;55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 4.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 5.Hovorka R, Allen JM, Elleri D, et al. . Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 6.Hovorka R, Kumareswaran K, Harris J, et al. . Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovatchev B, Cobelli C, Renard E, et al. . Multinational study of subcutaneous model-predictive closed-loop control in type 1 diabetes mellitus: summary of the results. J Diabetes Sci Technol 2010;4:1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimri R, Atlas E, Ajzensztejn M, Miller S, Oron T, Phillip M. Feasibility study of automated overnight closed-loop glucose control under MD-logic artificial pancreas in patients with type 1 diabetes: the DREAM Project. Diabetes Technol Ther 2012;14:728–735 [DOI] [PubMed] [Google Scholar]
- 9.Nimri R, Danne T, Kordonouri O, et al. . The “Glucositter” overnight automated closed loop system for type 1 diabetes: a randomized crossover trial. Pediatr Diabetes 2013;14:159–167 [DOI] [PubMed] [Google Scholar]
- 10.Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care 2012;35:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castle JR, Engle JM, El Youssef J, et al. . Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haidar A, Legault L, Dallaire M, et al. . Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 2013;185:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Bon AC, Jonker LD, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a bihormonal closed-loop system to control postexercise and postprandial glucose excursions. J Diabetes Sci Technol 2012;6:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinsker JE, Lee JB, Dassau E, et al. . Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care 2016;39:1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson SM, Raghinaru D, Pinsker JE, et al. . Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016;39:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renard E, Farret A, Kropff J, et al. ; AP@home ConsortiumDay-and-night closed-loop glucose control in patients with type I diabetes under free-living conditions: results of a single-arm 1-month experience compared with a previously reported feasibility study of evening and night at home. Diabetes Care 2016;39:1151–1160 [DOI] [PubMed] [Google Scholar]
- 17.Tauschmann M, Allen JM, Wilinska ME, et al. . Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2016;39:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Favero S, Boscari F, Messori M, et al. . Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care 2016;39:1180–1185 [DOI] [PubMed] [Google Scholar]
- 19.Weinzimer SA, Sherr JL, Cengiz E, et al. . Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:1994–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nauck MA. Is glucagon-like peptide 1 an incretin hormone? Diabetologia 1999;42:373–379 [DOI] [PubMed] [Google Scholar]
- 21.Steil GM, Palerm CC, Kurtz N, et al. . The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab 2011;96:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palerm CC. Physiologic insulin delivery with insulin feedback: a control systems perspective. Comput Methods Programs Biomed 2011;102:130–137 [DOI] [PubMed] [Google Scholar]
- 23.Hare KJ, Knop FK, Asmar M, et al. . Preserved inhibitory potency of GLP-1 on glucagon secretion in type 2 diabetes mellitus. J Clin Endocrinol Metab 2009;94:4679–4687 [DOI] [PubMed] [Google Scholar]
- 24.Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: insight from the LIBRA trial. J Clin Endocrinol Metab 2015;100:3702–3709 [DOI] [PubMed] [Google Scholar]
- 25.Chillarón JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism 2014;63:181–187 [DOI] [PubMed] [Google Scholar]
- 26.Libman IM, Miller KM, DiMeglio LA, et al. . Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. JAMA 2015;314:2241–2250 [DOI] [PubMed] [Google Scholar]