Abstract
The International Diabetes Federation estimates that 415 million adults worldwide now have diabetes and 318 million have impaired glucose tolerance. These numbers are expected to increase to 642 million and 482 million, respectively, by 2040. This burgeoning pandemic places an enormous burden on countries worldwide, particularly resource-poor regions. Numerous landmark trials evaluating both intensive lifestyle modification and pharmacological interventions have persuasively demonstrated that type 2 diabetes can be prevented or its onset can be delayed in high-risk individuals with impaired glucose tolerance. However, key challenges remain, including how to scale up such approaches for widespread translation and implementation, how to select appropriately from various interventions and tailor them for different populations and settings, and how to ensure that preventive interventions yield clinically meaningful, cost-effective outcomes. In June 2015, a Diabetes Care Editors’ Expert Forum convened to discuss these issues. This article, an outgrowth of the forum, begins with a summary of seminal prevention trials, followed by a discussion of considerations for selecting appropriate populations for intervention and the clinical implications of the various diagnostic criteria for prediabetes. The authors outline knowledge gaps in need of elucidation and explore a possible new avenue for securing regulatory approval of a prevention-related indication for metformin, as well as specific considerations for future pharmacological interventions to delay the onset of type 2 diabetes. They conclude with descriptions of some innovative, pragmatic translational initiatives already under way around the world.
Introduction
According to the latest International Diabetes Federation (IDF) calculations, an estimated 415 million adults worldwide (8.8% of the global population) have diabetes—a number that is projected to increase to 642 million (10.4%) by 2040. The vast majority of diabetes cases are attributable to type 2 diabetes. Furthermore, an estimated 318 million adults have impaired glucose tolerance (IGT). That number is expected to climb to 482 million in the next 25 years (1). In the U.S. alone, an estimated 86 million adults have prediabetes (2).
The toll of diabetes and its complications on patients’ health and quality of life is enormous. The burgeoning diabetes pandemic also places a great burden on countries throughout the world, particularly in resource-poor regions. The IDF estimates that global spending to treat diabetes ranged between $673 billion and $1.2 trillion USD in 2015 (1). A recent global analysis found that a substantial portion of this burden falls on patients in low- and middle-income countries as out-of-pocket costs (3). In concert with the predicted increase in new cases of diabetes, global expenditures are projected to rise to between $802 billion and nearly $1.5 trillion USD by 2040 (1).
Diabetes is also a leading cause of death. High blood glucose has been identified as the third largest risk factor for premature mortality worldwide, after high blood pressure and tobacco use. Approximately 5 million deaths were attributable to diabetes in 2015—more than those from HIV/AIDS, tuberculosis, and malaria combined (1,4).
Numerous landmark trials in the past quarter-century have demonstrated conclusively that preventive strategies (i.e., lifestyle modification and various pharmacological interventions) can delay or prevent the development of type 2 diabetes in high-risk individuals with IGT (5–22). Despite the clarity of these findings and large reported effect sizes, translational prevention programs have faced numerous real-world impediments, and none of the tested interventions have been widely adopted as components of routine clinical care.
There are encouraging signs that this may soon change. An independent expert panel recently confirmed that the expansion of the National Diabetes Prevention Program (NDPP) (23), a public-private initiative funded by the Affordable Care Act and launched in 2010 to encourage the provision of evidence-based interventions in communities across the country, would reduce spending and improve the quality of patient care (24). This certification is a crucial step toward expanding NDPP for Medicare beneficiaries with prediabetes.
Despite this encouraging news, key challenges remain, including identifying those interventions most suitable for widespread implementation, selecting appropriately from and tailoring these tools for various populations and settings, and measuring the impact of their implementation on disease progression and on micro- and macrovascular complications (23,25,26). To consider these issues, a Diabetes Care Editors’ Expert Forum was convened in June 2015. Panelists reviewed the prevention evidence to date, discussed areas of controversy, identified unanswered research questions, and explored innovative approaches to translating this research to reduce the incidence of type 2 diabetes. This article summarizes the proceedings of that forum. We use the term “prevention” throughout the article to refer to delaying the progression to type 2 diabetes in a proportion of a population at risk. Ascertaining which individuals will progress from prediabetes to diabetes (and when) is not possible currently. Therefore, it is not feasible to demonstrate that an intervention has prevented the lifelong occurrence of diabetes in an individual or a population of individuals who are otherwise certain to progress. For now, intervention trials can only determine the fraction of the studied population that progresses to diabetes. The number of cases that have been prevented during a trial can be derived from positive results, but this calculation has to be qualified by the limited observation period and the likelihood that treatment must be continued indefinitely to preserve some or all of the prevented cases.
Type 2 Diabetes Prevention Studies: Progress to Date
Charting the future of diabetes prevention and suggesting logical next steps requires a critical review of the key studies. Different interventions and approaches carried out in a variety of settings in diverse populations have yielded nearly uniform evidence in support of both lifestyle modification and pharmacotherapy as viable means for delaying or preventing diabetes in high-risk individuals (5–22) (Table 1).
Table 1.
Major type 2 diabetes prevention trials
| Location | n | Intervention | Reference | |
|---|---|---|---|---|
| Da Qing IGT and Diabetes Study |
China |
577 |
Lifestyle modification |
Pan et al., 1997 (5) |
| Finnish Diabetes Prevention Study (DPS) |
Finland |
522 |
Lifestyle modification |
Tuomilehto et al., 2001 (6) |
| Diabetes Prevention Program (DPP) |
U.S. |
3,234 |
Lifestyle modification, metformin |
Diabetes Prevention Program Research Group, 2002 (7) |
| Indian Diabetes Prevention Programme-1 (IDPP-1) |
India |
531 |
Lifestyle modification, metformin |
Ramachandran et al., 2006 (8) |
| Indian Diabetes Prevention Programme-2 (IDPP-2) |
India |
407 |
Lifestyle modification plus pioglitazone |
Ramachandran et al., 2009 (9) |
| Zensharen Study for Prevention of Lifestyle Diseases |
Japan |
641 |
Lifestyle modification |
Saito et al., 2011 (11) |
| Prevention of type 2 diabetes by lifestyle intervention |
Japan |
458 |
Lifestyle modification |
Kosaka et al., 2005 (12) |
| TRIPOD (Troglitazone in the Prevention of Diabetes) |
U.S. |
266 |
Troglitazone |
Buchanan et al., 2002 (13) |
| DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) |
International |
5,269 |
Rosiglitazone |
DREAM Trial Investigators, 2006 (14) |
| ACT NOW (Actos Now for Prevention of Diabetes) |
U.S. |
602 |
Pioglitazone |
DeFronzo et al., 2011 (10) |
| CANOE (Canadian Normoglycemia Outcomes Evaluation) |
Canada |
207 |
Rosiglitazone plus metformin |
Zinman et al., 2010 (15) |
| ORIGIN (Outcome Reduction With Initial Glargine Intervention) |
International |
12,537 |
Insulin glargine |
ORIGIN Trial Investigators, 2012 (16) |
| STOP-NIDDM (Study to Prevent Non-Insulin-Dependent Diabetes Mellitus) |
International |
1,429 |
Acarbose |
Chiasson et al., 2002 (17) |
| Voglibose for prevention of type 2 diabetes mellitus |
Japan |
1,780 |
Voglibose |
Kawamori et al., 2009 (18) |
| EDIT (Early Diabetes Intervention Trial) |
U.K. |
631 |
Acarbose, metformin |
Holman et al., 2000 (19) |
| NAVIGATOR (Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research) |
International |
9,306 |
Nateglinide, valsartan |
NAVIGATOR Study Group, 2010 (20) |
| XENDOS (Xenical in the Prevention of Diabetes in Obese Subjects) |
Sweden |
3,305 |
Orlistat |
Torgerson et al., 2004 (21) |
| SCALE (Satiety and Clinical Adiposity—Liraglutide Evidence) | International | 3,731 | Liraglutide | Pi-Sunyer et al., 2015 (22) |
The groundbreaking Da Qing IGT and Diabetes Study (5) was the first large-scale prevention trial to test the efficacy of lifestyle intervention and has provided the longest follow-up data. It compared the effects of dietary modification, exercise, or both to a control group given no intervention in high-risk Chinese adults with IGT. After 6 years, the cumulative incidence of diabetes by 1985 World Health Organization (WHO) criteria (fasting plasma glucose [FPG] >140 mg/dL [7.8 mmol/L] or 2-h postload plasma glucose >200 mg/dL [11.1 mmol/L]) was significantly reduced by 31–46% in all intervention groups. Evaluation studies involving 94% of the original cohort at the 20- and 23-year follow-ups (27,28) showed a durable 43% lower diabetes incidence rate, a 47% reduction in severe diabetic retinopathy (29), and, by year 23, significant reductions in cardiovascular (41%) and all-cause (29%) mortality.
The Finnish Diabetes Prevention Study (DPS) (6,30) examined the effects of lifestyle intervention in middle-aged, overweight adults with IGT. Participants were individually randomized to either an intervention group that received ongoing individualized counseling aimed at reducing weight, making healthy dietary modifications, and increasing physical activity or a control group receiving general diet and exercise advice but no individualized counseling. After 4 years, diabetes risk was reduced by 58% in the intervention group, which also had greater improvements in all parameters of the metabolic syndrome. No cases of diabetes developed among people who reached at least four of the study’s five lifestyle intervention targets for diet and physical activity (6). Long-term follow-up found a sustained relative risk reduction in diabetes incidence of 43% after 7 years and 38% after 13 years, while the absolute risk difference between groups continued to increase through 13 years (31,32).
The American Diabetes Prevention Program (DPP) (7) demonstrated that lifestyle modification and, to a lesser extent, metformin therapy can reduce the incidence of diabetes in high-risk individuals. Overweight adults with IGT and an FPG >95 mg/dL (5.3 mmol/L) were randomized to either intensive lifestyle intervention focusing on weight loss and exercise, metformin therapy, or placebo. After a mean 2.8 years, the lifestyle intervention reduced the cumulative incidence of diabetes by 58% (a reduction identical to that noted in the DPS), and the reduction with metformin was 31%, compared with placebo. A fourth DPP arm using the thiazolidinedione (TZD) troglitazone was discontinued early because of the drug’s hepatotoxicity. After a mean 0.9 year of therapy in the troglitazone arm, diabetes incidence was reduced by 75% compared with placebo. However, 3 years after troglitazone withdrawal, the diabetes incidence rate was almost identical to that of the placebo group (33). The DPP lifestyle intervention also yielded improvements in all traditional, as well as many nontraditional, cardiovascular risk factors (34). In the follow-up DPP Outcomes Study, cumulative diabetes incidence rates still differed significantly 10 years (34 and 18% for lifestyle and metformin compared with placebo, respectively) and 15 years (27 and 17%, respectively) after initial randomization into the DPP (34,35). A projection of the DPP interventions’ effects over a lifetime yielded estimates that the lifestyle and metformin interventions delayed diabetes by 11 and 3 years, respectively, but also reduced the absolute incidence of diabetes by 20 and 8%, respectively (36). However, no differences in aggregate microvascular or cardiovascular outcomes by randomized arm have become evident in the DPP, although the expected lower prevalence of microvascular complications in those who did not develop diabetes was reported (35).
Two Indian Diabetes Prevention Programme studies (IDPP-1 and IDPP-2) (8,9) focused on developing practical, translatable lifestyle interventions and contributed important insights regarding potential ethnicity-based variations in both the pathophysiology of diabetes and responses to lifestyle and pharmacological interventions. In the IDPP-1, Asian Indian adults with IGT—who were younger and leaner than the subjects in the Finnish and American studies (6–8)—were assigned to either a simple lifestyle intervention encouraging healthy dietary changes and increased exercise, low-dose metformin therapy (500 mg daily), a combination of lifestyle modification plus metformin, or no intervention (control). After 3 years, diabetes risk was reduced by 28.5, 26.4, and 28.2% in the lifestyle, metformin, and combination groups, respectively. Both interventions also had positive effects on LDL cholesterol but not on blood pressure (37). The IDPP-2 (9) tested whether adding the TZD pioglitazone would enhance the efficacy of the IDPP-1 lifestyle intervention. In stark contrast to a 72% diabetes risk reduction found with pioglitazone in the U.S. ACT NOW (Actos Now for Prevention of Diabetes) study (10) (discussed below), pioglitazone had no significant effect beyond that of lifestyle intervention in the IDPP-2 population. The potential ethnic differences in responses to pharmacological preventive therapy identified in these studies require further investigation. If substantiated, they represent an important additional issue to be addressed in any global prevention strategy.
The Japanese Zensharen Study for Prevention of Lifestyle Diseases (11) evaluated overweight Japanese adults with FPG levels of 100–125 mg/dL (5.6–6.9 mmol/L) who were randomly assigned to either a frequent or less frequent (control) lifestyle intervention program for 3 years. The frequent intervention resulted in an overall 44% reduction in diabetes incidence, although subgroup analyses revealed that it was effective in participants with combined impaired fasting glucose (IFG) and IGT (59% relative risk reduction) or high baseline A1C (76% relative risk reduction) but had no effect in those with isolated IFG or lower baseline A1C. In another, smaller Japanese lifestyle intervention study, men with IGT were randomly assigned to a standard intervention group (control) or an intensive intervention group (12). Subjects in the control group and in the intensive intervention group were advised to maintain a BMI of <24.0 and <22.0 kg/m2, respectively, through diet and exercise. In the intensive group, detailed instructions on lifestyle modification were repeated every 3–4 months. The cumulative 4-year incidence of diabetes was 9.3% in the control group and 3.0% in the intensive intervention group; reduction in diabetes risk from the intensive intervention was 67.4%.
Because virtually all of the early diabetes prevention research suggested that the success of an intervention lies partly in its ability to improve insulin sensitivity and because TZDs were known to reduce insulin resistance, a number of studies have examined the effect of this class of agents. The TRIPOD (Troglitazone in the Prevention of Diabetes) study (13) demonstrated that troglitazone could reduce the risk of developing diabetes by 55% in Hispanic women with a history of gestational diabetes mellitus (GDM). The DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) trial (14) examined the preventive value of rosiglitazone, and ACT NOW (10) did the same with pioglitazone. In the DREAM trial, adults with IFG (FPG 110 to <126 mg/dL [6.1 to <7.0 mmol/L]), IGT, or both were randomly assigned to either rosiglitazone 8 mg daily or placebo. After 3 years, the composite outcome of incident diabetes or death was reduced by 60% in the rosiglitazone group, most of which was due to a reduction in incident diabetes. Rosiglitazone was also shown to increase the likelihood of reversion to normal glucose tolerance (NGT) by ∼70–80%. In ACT NOW, overweight adults with IGT were randomly assigned to either pioglitazone titrated to 45 mg daily or placebo. During a median follow-up of 2.4 years, the pioglitazone group had a 72% reduction in diabetes risk compared with placebo. In addition, the CANOE (Canadian Normoglycemia Outcomes Evaluation) trial (15) found that low-dose combination therapy with rosiglitazone 2 mg plus metformin 500 mg twice daily in adults with IGT reduced the relative risk of diabetes by 66% during a median 3.9 years of treatment with significantly fewer adverse events than in the DREAM trial. Longer-term passive follow-up of the DREAM cohort showed that at a median 1.6 years after the end of the trial and 4.3 years after randomization the rosiglitazone group retained a 39% lower incidence of the composite outcome but had only 17% more reversion to normoglycemia than the placebo group (38). Thus, limited exposure to a TZD (in this case, rosiglitazone, but also troglitazone in the DPP [33] and pioglitazone in ACT NOW [39]) appears to reduce the long-term incidence of diabetes by delaying, rather than reversing, the underlying disease process.
The ORIGIN (Outcome Reduction With Initial Glargine Intervention) trial (16,40) explored the same question of preventing diabetes by reducing β-cell load, in this case with insulin therapy. Adults with cardiovascular risk factors who either had or were at risk for developing diabetes were randomized to receive either insulin glargine or standard care. Although the study’s primary focus was on cardiovascular outcomes, incident diabetes among at-risk participants was also examined. After a mean follow-up of 6.2 years, glargine reduced the risk of diabetes by 28% compared with standard care based on oral glucose tolerance tests (OGTTs) performed ∼1 month after insulin was stopped. When participants were identified through a second OGTT performed ∼3 months later and those with uncertain new diabetes diagnoses were included, the total risk reduction was 31%. A 2-year passive follow-up study of 4,718 of the original 12,537 ORIGIN participants was consistent with a legacy effect, with new diabetes cases occurring in 41 and 48% of the glargine and standard care groups, respectively, when both confirmed and unconfirmed cases were included (41).
Additional prevention studies have been conducted with other pharmacological agents. The international STOP-NIDDM (Study to Prevent Non-Insulin-Dependent Diabetes Mellitus) trial (17), a 2009 Japanese study (18), and the U.K. Early Diabetes Intervention Trial (EDIT) (19) all investigated the preventive effects of α-glucosidase inhibitors in individuals with IGT. In STOP-NIDDM, participants receiving 100 mg of acarbose three times daily for 3 years had a 25% reduction in relative risk of diabetes compared with placebo. Acarbose therapy also significantly increased the rate of reversion to NGT (17). In the Japanese study, participants receiving 0.2 mg of voglibose three times daily for 4 years had a 40% reduction in the relative risk of diabetes compared with placebo and were significantly more likely to achieve normoglycemia (18). EDIT randomized 631 U.K. participants at risk for diabetes based on two FPG levels of 99–139 mg/dL (5.5–7.7 mmol/L) to acarbose 50 mg, metformin 500 mg, or matched placebo three times daily in a 2 × 2 factorial study. After 3 years, no statistical risk reductions with were observed with acarbose or metformin (8 and 37%, respectively) compared with placebo (19). Although the final EDIT results have not been fully reported, no differences were seen in the relative risk for diabetes by 6 years for acarbose (1.04, P = 0.81), metformin (0.99, P = 0.94), or their combination (1.02, P = 0.91). Interestingly, for those with IGT at baseline, the relative risk of diabetes was reduced by acarbose (0.66, P = 0.046) but not by metformin (1.09, P = 0.70), perhaps because the fasting glucose level and BMI in this study were lower than in the DPP. The investigators concluded that the ability of therapies to reduce the risk of diabetes may differ for those with IGT versus those with IFG (42).
The international NAVIGATOR (Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research) trial (20) tested whether treatment with the meglitinide nateglinide reduces the risk of diabetes and cardiovascular events in adults with IGT and cardiovascular disease or cardiovascular risk factors. The study compared nateglinide up to 60 mg three times daily or valsartan therapy to placebo. During a median follow-up of 5 years, nateglinide did not significantly reduce the cumulative incidence of diabetes compared with placebo (36 vs. 34%, respectively). There was no reduction in the nateglinide group in a core cardiovascular composite outcome that included death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure (7.9 vs. 8.3%, respectively), and there was no reduction in an extended cardiovascular composite outcome that included the individual components of the core composite outcome as well as hospitalization for unstable angina and arterial revascularization (14.2 vs. 15.2%, respectively). Furthermore, nateglinide therapy increased the risk of hypoglycemia.
In the Swedish XENDOS (Xenical in the Prevention of Diabetes in Obese Subjects) trial (21), obese adults with either NGT or IGT were randomly assigned to lifestyle modification plus either orlistat 120 mg or placebo three times daily. After 4 years, the cumulative incidence of diabetes was 9.0% with placebo and 6.2% with orlistat, corresponding to an overall risk reduction of 37.3%. Analyses indicated that the preventive effect of orlistat was explained by its effect on participants with IGT at baseline, in whom orlistat yielded a risk reduction of 52%. Participants receiving orlistat also had significantly greater mean weight loss (5.8 vs. 3.0 kg with placebo), which was not dependent on their glucose tolerance status.
The international SCALE (Satiety and Clinical Adiposity—Liraglutide Evidence) trial (22) investigated the effects of the glucagon-like peptide 1 receptor agonist liraglutide on weight and cardiometabolic risk factors in obese adults without diabetes. Participants were randomly assigned to receive either liraglutide 3.0 mg in once-daily injections or placebo in addition to counseling on lifestyle modification. After 56 weeks, those receiving liraglutide lost significantly more weight (difference –5.6 kg). Weight loss of at least 5% body weight was achieved in 63.2 and 27.1% of the participants in the liraglutide and placebo groups, respectively, and 33.1 and 10.6%, respectively, lost >10% body weight. A1C, fasting glucose, and fasting insulin levels decreased more with liraglutide than with placebo, and the liraglutide group also had lower glucose and higher insulin and C-peptide levels during OGTT. Insulin resistance and β-cell function also improved with liraglutide. The prevalence of prediabetes at week 56 was significantly lower with liraglutide (7.2 vs. 20.7% among participants with baseline normoglycemia and 30.8 vs. 67.3% among those with baseline prediabetes), and type 2 diabetes developed in fewer patients receiving liraglutide than with placebo (4 vs. 14 cases).
Taken together, these studies convincingly support lifestyle modification focusing on healthful eating and increased physical activity and various pharmacological therapies as viable strategies for preventing type 2 diabetes. However, they also raise important new questions, such as 1) how best to identify the most appropriate target populations for intervention, 2) how to disseminate lifestyle interventions in the most cost-effective manner, and 3) how expanding preventive pharmacotherapy options might further the goal of reducing diabetes rates worldwide.
Type 2 Diabetes Prevention Studies: Challenging Questions
Identifying Appropriate Target Populations
Many longitudinal studies have outlined the trajectories of the metabolic factors (i.e., insulin secretion, insulin sensitivity, and 2-h glucose levels) preceding diagnosis. For example, Tabák et al. (43) showed that insulin secretion and insulin action may be considered to be in the normal range until 2–6 years before diagnosis, when abrupt metabolic changes result in deterioration of fasting and 2-h postload glucose levels. Each of the three clinical categories used to identify the prediabetic state (isolated IFG, isolated IGT, and combined IFG and IGT) may represent a distinctive pathophysiology (44). Individuals in each of these categories have an elevated diabetes risk, and responses to interventions may differ based on the category and severity of the abnormality. Thus, attempts to curb the prevalence of diabetes must focus on effectively screening, identifying, and treating people who are at increased risk by virtue of being in one of these categories.
However, there is not uniform agreement on the specific approach to take. Given the different trajectories of fasting glucose, postprandial glucose, and insulin levels before diagnosis, what specific point would be the most appropriate at which to intervene?
Determining Appropriate Diagnostic Criteria for Prediabetes
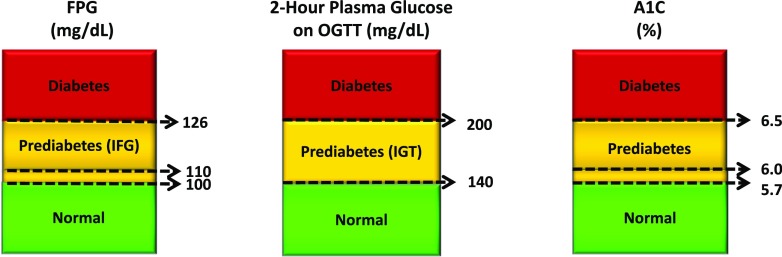
The current diagnostic recommendations for diabetes are an FPG level ≥126 mg/dL (7.0 mmol/L) or a 2-h plasma glucose during an OGTT ≥200 mg/dL (11.1 mmol/L) (Fig. 1). These criteria historically were based on thresholds for the risk of retinopathy (45–48). The term “impaired glucose tolerance,” comprising people at high risk to progress to diabetes, was established in 1979, and its definition (2-h OGTT glucose levels between the normal and diabetic values) essentially has not changed since then (46). In most diabetes prevention studies to date, subjects have had IGT (i.e., a 2-h OGTT glucose level of 140–199 mg/dL [7.8–11.0 mmol/L]).
Figure 1.
Criteria for diagnosing diabetes and prediabetes. Diagnosis of diabetes is made on the basis of an FPG level ≥126 mg/dL (7.0 mmol/L), a 2-h plasma glucose level during an OGTT ≥200 mg/dL (11.1 mmol/L), or an A1C ≥6.5% (48 mmol/mol). When using A1C for diagnosis, it is important to take the patient’s age, race/ethnicity, and anemia/hemoglobinopathy status into consideration. Diabetes can also be diagnosed based on unequivocal symptoms and a random plasma glucose value ≥200 mg/dL (11.1 mmol/L). Any abnormality by any testing method must be repeated and confirmed on a separate day. For the diagnosis of prediabetes, cut points are not as well established. A 2-h plasma glucose during an OGTT of 140–199 mg/dL (7.8–11.0 mmol/L) is known as IGT and considered indicative of prediabetes, but recommended FPG and A1C cut points for prediabetes have varied (FPG ≥100–125 or ≥110–125 mg/dL [5.6–6.9 or 6.1–6.9 mmol/L] and A1C ≥5.7–6.4 or ≥6.0–6.4% [39–46 or 42–46 mmol/mol]). Adapted with permission from American Diabetes Association (45).
The controversy regarding appropriately diagnosing prediabetes and targeting interventions relates to the use of other suggested criteria (FPG and A1C). For example, in 2003, an American Diabetes Association (ADA) Expert Committee suggested that the FPG threshold for IFG be reduced from 110 mg/dL (6.1 mmol/L) to 100 mg/dL (5.6 mmol/L) (48). The committee suggested that this lower cut point may improve the sensitivity of the prediction of diabetes risk, but this change has not been universally accepted because the specificity is markedly reduced by lowering the threshold; thus, many people who may not progress to diabetes are being labeled as having prediabetes (49). In 2009, an A1C cut point of ≥6.5% (48 mmol/mol) was introduced to diagnose diabetes, and this was later endorsed by a WHO consultation committee (50,51). Since 2010, ADA has recommended that an A1C range of 5.7–6.4% (39–46 mmol/mol) be considered indicative of prediabetes (45). However, other organizations have not agreed with these FPG and A1C cut points (51–53). A recent meta-analysis evaluated progression rates by prediabetes definition. It was found that A1C values of 6.0–6.4% (42–46 mmol/mol) might identify individuals at lower risk than other prediabetes definitions, but more research is needed (54).
The success of any broad-based prevention strategy will hinge on attaining consensus regarding the most appropriate diagnostic test and the glycemic cut point at which to begin intervention. The current lack of concordance on this issue is not a trivial matter and has tremendous clinical implications (55). At present, lifestyle intervention has been shown to be unequivocally effective in reducing diabetes incidence only in subjects with isolated IGT or combined IGT and IFG and in subjects with A1C levels of 6.0 to <6.5% (42 to <48 mmol/mol). Without intervention, these individuals have a much higher incidence of diabetes than do individuals who are classified as having prediabetes by other criteria. Existing data are not sufficient to make evidence-based recommendations for those with prediabetes classified by other criteria; targeted studies are needed to provide more specific information.
Clinical Implications of the Prediabetes Diagnosis
More general agreement exists regarding the clinical implications of the prediabetic state. First and foremost, prediabetes is linked to microvascular complications (56–59). For example, using data from the National Health and Nutrition Examination Survey (NHANES) and defining “prediabetes” as an FPG ≥100 mg/dL (5.6 mmol/L) but <126 mg/dL (7.0 mmol/L), Plantinga et al. (56) reported that the prevalence of chronic kidney disease was 17.7% in individuals with prediabetes compared with 10.6% in those with no diabetes and 39.6% and 41.7% in individuals with diagnosed or undiagnosed diabetes, respectively. Second, each category of prediabetes (i.e., IFG, IGT, or a combination of both) increases the relative risk and incident rate of progression to diabetes, although isolated IFG has less predictive value (60). Although previous studies primarily used an IFG cut point of ≥110 mg/dL (6.1 mmol/L), there is evidence that lower (but still elevated) 2-h glucose and A1C values also confer risk (61–63), although data on FPG are less clear.
One major factor to consider is whether the ADA-recommended lower thresholds for A1C and IFG are associated with higher cardiovascular risk. Understanding that risk is a graded continuum, one would expect these values to confer some level of increased risk, and there is evidence to support this (64–66), although some studies have demonstrated less excess risk than others. For example, in the U.S. MESA (Multi-Ethnic Study of Atherosclerosis) trial (64), IFG was defined as no type 2 diabetes and a fasting glucose of 100–125 mg/dL (5.6–6.9 mmol/L) and was associated with an increased incidence of cardiovascular events in univariate, but not multivariate, analysis compared with those with normal fasting glucose. Data from the Emerging Risk Factors Collaboration (ERFC) (65) suggested that “there are generally continuous associations between fasting glucose levels greater than 100 mg per deciliter and risk of death, supporting the view that hyperglycemia (or some factor closely related to it) may be directly relevant.” In addition, Xu et al. (66) performed a meta-analysis on the risk of coronary heart disease (CHD) using different IFG criteria. They found an increased risk of CHD when FPG was as low as 100 mg/dL—the lower ADA cut point for IFG—and concluded that “these results reaffirm the importance of screening for prediabetes using the ADA criteria.”
Collectively, these studies indicate the clinical implications of the continuum of excess risk for microvascular complications, macrovascular complications, mortality, and type 2 diabetes even at the lower values within the glycemic range that defines prediabetes (67).
Disseminating Lifestyle Interventions Cost-Effectively
Numerous efforts have been made to translate lifestyle interventions into real-world practice, although risk reductions generally have not matched the levels achieved in proof-of-concept research studies (68–79). This is not surprising in that clinical practices face different challenges from those in research settings, including personnel training, funding and reimbursement schemes, competing needs in clinics, and compliance and adherence barriers (80).
Clearly, more work is needed to make widespread practical use of the lessons learned thus far, to tailor simple and practical interventions to specific populations and individuals, and to identify patients for whom lifestyle modification may not be enough. A great deal of information is now available about the predictors of incident diabetes and of successful risk reduction through lifestyle intervention (Table 2) (5,7,11,12,27–29,34,35,76,81–97). These findings will help to guide future efforts to determine the most appropriate candidates for widespread translation initiatives among high-risk individuals. Other remaining questions regarding lifestyle intervention include the following:
Table 2.
Predictors of diabetes risk reduction through lifestyle intervention
| Study | Relevant findings |
|---|---|
| Da Qing IGT and Diabetes Study (5,27–29,81) |
• Diet, exercise, and a combination of diet and exercise intervention for 6 years in Chinese adults with IGT were equally effective in reducing diabetes incidence. |
| • Interventions were effective in people with a BMI higher or lower than 25 kg/m2. | |
| • Benefit could not be wholly ascribed to changes in BMI. | |
| • Interventions were most effective in those with less insulin resistance and greater insulin secretion at baseline. | |
| • Reduced cumulative diabetes incidence persisted for at least 17 years after the termination of the active intervention. | |
| • Lifestyle intervention was associated with a subsequent lower incidence of severe retinopathy and lower mortality. | |
| Finnish Diabetes Prevention Study (DPS) (82–89) |
• Lifestyle intervention was most effective among the oldest participants and those scoring highest on a composite risk assessment at baseline. This scoring instrument or others like it may be useful for identifying individuals most likely to benefit from intensive lifestyle intervention. |
| • Participants who had greater insulin sensitivity and better insulin secretion during the study were less likely to progress to diabetes during a mean follow-up of 6 years. Regression to NGT was more strongly associated with greater insulin secretion than with better insulin sensitivity. | |
| • Participants with greater improvements in weight and BMI during the first year were less likely to develop diabetes. Thus, BMI reduction may be a key goal to improve insulin sensitivity, preserve insulin secretion, and ultimately prevent or delay diabetes. | |
| • Achievement of each of the study’s five lifestyle goals significantly decreased risk. None of the participants who achieved at least four of the five goals developed diabetes by year 4. | |
| • Participants with longer typical sleep durations had a higher risk of developing diabetes in the control group but not in the intervention group; lifestyle intervention was similarly effective regardless of participants’ sleep habits. Thus, lifestyle intervention may reduce the excess risk conferred by longer sleep duration. | |
| • Several genetic variants conferred higher diabetes risk. Post hoc analyses showed that although lifestyle intervention was effective regardless of family history of diabetes its effectiveness varied markedly according to participants’ genetic variant status. This demonstrates the potential role of genotype in diabetes prevention efforts. | |
| Diabetes Prevention Program (DPP) (7,34,35,90–94) |
• Short- and long-term effects of intensive lifestyle intervention were greatest among older participants, those with greater baseline insulin sensitivity and insulin secretion, and those with greater improvements in each during the active study period. |
| • For women with a history of GDM, lifestyle modification and metformin were similarly effective, whereas for women without previous GDM, only lifestyle intervention reduced diabetes risk. | |
| • Lifestyle intervention was similarly effective in those with and without higher genetic risk. | |
| • The presence or absence of diabetes-related antibodies did not affect diabetes risk or predict responses to intervention. | |
| • Individuals from any group who regained NGT at least once during active intervention reduced their diabetes risk by 56% during long-term follow-up compared with those with persistent prediabetes. Thus, even transient reversion to NGT by any means appears to lower future diabetes risk. Reversion was more common in the lifestyle group and was more likely in participants who achieved greater weight loss, were younger, and had lower glucose levels and better β-cell function at baseline. Paradoxically, lifestyle group members who did not revert to NGT during the study were actually at higher risk during follow-up, perhaps because of a particularly strong susceptibility (genetic or environmental) to diabetes. Thus, a combination of interventions may be needed for individuals whose dysglycemia is not reversed through lifestyle modification alone. | |
| Indian Diabetes Prevention Programme-1 (IDPP-1) (95) |
• Baseline A1C was the most significant predictor of diabetes; however, preventive interventions were similarly effective across A1C subgroups. |
| • Lifestyle intervention reduced diabetes risk in this population independent of weight loss. | |
| Indian text-messaging intervention study (76,96,97) |
• Participants who regained NGT by 6 months reduced their risk of progression to diabetes by 75% by year 2 compared with those who did not return to NGT within the first 6 months. Better β-cell function at baseline and its improvement during the study were associated with reversion to NGT by 2 years. |
| • Progression to diabetes was associated with declining β-cell function throughout the study period. | |
| Zensharen Study for Prevention of Lifestyle Diseases (11) |
• Lifestyle intervention was highly effective in participants with combined IGT and IFG and in those with a baseline A1C ≥5.6% (Japan Diabetes Society method). |
| • Lifestyle intervention was ineffective in participants with isolated IFG and in those with a baseline A1C <5.6%. | |
| Japanese study on prevention of type 2 diabetes by lifestyle intervention (12) | • The cumulative 4-year incidence of diabetes, based on confirmed diagnostic FPG levels of 140 mg/dL (7.8 mmol/L) or higher, was 3.0% in the intensive and 9.3% in the conventional group. |
| • Changes in BMI only partially accounted for the lower incidence in the intensive group. |
How much do the dietary and exercise components contribute individually to reducing diabetes risk and delaying or preventing the onset of the disease, or are the two inextricably linked? How much weight loss maintained over how many years is required for sustained prevention of type 2 diabetes?
Are the beneficial effects of exercise or increased leisure-time activity effective independent of weight loss among people who are overweight/obese and those who are of normal weight? Is delay or prevention of diabetes possible in the absence of weight loss in some populations?
Is it appropriate to group individuals with isolated IFG together with those who have IGT or a combination of both as the target for preventive interventions? If not, what strategies might be necessary to reduce diabetes risk in people with each type of metabolic dysregulation (or, for that matter, with higher or lower A1C values)?
How might lifestyle interventions that have proven effective in structured research settings be implemented successfully through large-scale public health initiatives?
Expanding Pharmacotherapeutic Options: Moving Toward a Prevention-Related Indication for Metformin
Relying on diet and physical activity is not enough to delay progression for some at-risk individuals because long-term adherence to healthy lifestyle behaviors can be difficult (26). Furthermore, as previously noted, interventions implemented in research studies can be prohibitively labor intensive and expensive to deliver in real-world settings, where they face different obstacles (80). In addition, the costs of prevention services, such as lifestyle modification programs and health coaching, typically have not been reimbursed by payers (25). The recently announced findings supporting the cost-effectiveness of the NDPP (23,24) are encouraging, as is a recent recommendation from the U.S. Preventive Services Task Force that obese adults aged 40–70 years who do not have symptoms of diabetes should be screened for abnormal blood glucose in the primary care setting (98). Although these and other public health initiatives (99–103) could soon improve this situation, current reimbursement structures and a shortage of qualified lifestyle coaches in primary care and nonmedical settings remain problematic.
For these reasons, preventive pharmacotherapy has been proposed as an adjunct to lifestyle modification (39). As reviewed above, strong evidence from randomized controlled trials has shown the potential of various pharmacological therapies to prevent progression to type 2 diabetes in people with IGT (7–10,13–22,33,104). The preventive effects of these agents, although not fully understood, appear to be related primarily to their ability to lower blood glucose and to preserve or delay the deterioration of β-cell function and thereby modify the disease progression (105), as demonstrated in the DPP (90). TZDs improve insulin sensitivity and glucose utilization and also have direct, positive effects on the β-cells; α-glucosidase inhibitors retard carbohydrate absorption and lower postprandial hyperglycemia, reducing the β-cell load; and metformin, a biguanide, suppresses hepatic glucose production. Each of these mechanisms potentially could modulate factors related to preservation of β-cell function by reducing the demand for insulin secretion.
As preventive monotherapy, only metformin has been studied for longer than ∼3 years, and reductions in diabetes incidence have generally dissipated after discontinuation of glucose-lowering drugs (26). In addition, TZDs have been plagued with concerns regarding serious adverse events, and acarbose is associated with gastrointestinal (GI) symptoms and greater adherence problems (17,104,106). Some newer obesity and diabetes medications (e.g., orlistat and glucagon-like peptide 1 receptor agonists) (21,22,107) have the potential for diabetes prevention based on their positive effects on weight and cardiovascular risk factors and possibly also β-cell function and protection. However, such agents are costly, some are injectables (which may pose a barrier for some people), and all require further study in the population with prediabetes (26,108).
Metformin, with proven effectiveness, long-term safety (109), and cost-effectiveness as the first-line type 2 diabetes treatment (110), is the most likely candidate for widespread use in diabetes prevention (100). Although less effective overall than lifestyle intervention in the DPP, it was as effective as lifestyle modification for younger participants, very obese participants (≥35 kg/m2), and women with a history of GDM. However, it was no more effective than placebo in older participants (>60 years of age) (7,111). A recent risk-based reanalysis of the DPP (112) found that the benefit of metformin was unevenly distributed within the study population such that only subjects in the highest-risk quartile experienced marked risk reduction, whereas the remaining subjects received little or no benefit. Since 2008, ADA has recommended consideration of metformin therapy for individuals who are at very high risk for diabetes (currently including individuals who are <60 years of age, are very obese, have a history of GDM, or have IGT, IFG, or an A1C of 5.7–6.4% [39–46 mmol/mol]) (113).
Despite this recommendation, metformin use for diabetes prevention has been minimal in many countries. Cost may not be a major issue, but GI side effects and the lack of perceived benefit with no measurable targets may play a role in the low rate of metformin use in prediabetes. For example, a recent evaluation of metformin prescription rates in a nationwide sample of >17,000 insured adults aged 19–58 years with prediabetes in the U.S. found that only 3.7% were prescribed metformin in the 2010–2012 period. Even within a subgroup of individuals with a BMI >35 kg/m2 or a history of GDM—characteristics specifically identified in ADA guidelines—only 7.8% received a metformin prescription (114). Although more research is needed to pinpoint the reasons for this lack of uptake for metformin use in prediabetes, the authors suggested that providers’ lack of knowledge about DPP and the natural course of prediabetes, reluctance on the part of patients and providers to “medicalize” prediabetes, and the lack of a metformin prediabetes indication approved by the U.S. Food and Drug Administration (FDA) and similar agencies in other countries all may play a role. That said, a prediabetes indication for acarbose has been approved in several countries (115), but it is uncertain how often that agent is prescribed for this use.
The lack of a prevention-related indication for metformin poses a significant barrier to more widespread use, but perhaps not an insurmountable one. Although conventional wisdom holds that the FDA is reluctant to approve such an indication for any drug, the agency’s 2008 draft guidance for industry on developing diabetes drugs seems to suggest otherwise (116). This document, although never finalized, outlines the FDA’s expectations for “products intended to prevent the development of diabetes” (116). These expectations include that supporting research studies need to 1) be conducted in populations of high-risk individuals (e.g., those with IGT, IFG, or a history of GDM); 2) include a washout period to confirm that the drug truly delays, rather than merely masks, progression of the underlying disease process; 3) be of substantial duration and size; and 4) include as possible end points either delay in type 2 diabetes diagnosis or reduction in the proportion of patients diagnosed with type 2 diabetes relative to placebo.
The guidance notes that, in the absence of a clearly defined “clinically meaningful effect size,” merely delaying a diagnosis of diabetes may not be a sufficiently tangible benefit against which to judge risks. It suggests, however, that certain supporting outcomes, such as demonstration of a durable delay in diabetes onset after therapy ends or evidence of delay or reduction of micro- or macrovascular complications, could strengthen a candidate drug’s claim. In addition, the guidance states that the FDA’s expectations for the safety of prevention-related products are likely to be higher than for drugs aimed at treating type 2 diabetes.
Notably, the FDA guidance does not suggest that a candidate agent must be supported by evidence that it actually “prevents” type 2 diabetes but rather that it durably delays disease onset and thereby can be expected to reduce long-term complications. Some would reserve the term “prevention” for interventions that, at least in a significant proportion of individuals, forestall the onset of diabetes for decades or longer. Others may take a more pragmatic perspective and hold that the terms “reduction,” “prevention,” and “delay” may be considered close enough on the same spectrum to be used interchangeably (117). The distinction may be important to the FDA, which likely would set a higher evidence bar for a “prevention” indication than it would for an indication “for the treatment of prediabetes” or “for reducing the risk of developing type 2 diabetes.” To date, the only evidence in support of strictly defined “prevention” has come from the previously mentioned DPP cost-effectiveness analysis (36) showing that the effects of the metformin intervention, projected over a lifetime, would not only delay diabetes but also reduce its absolute incidence by 8%.
Consistent with the FDA guidance, however, the evidence base for metformin may already be substantial enough to support approval of some form of prevention-related indication. Some work may be necessary to develop expert consensus with regard to what constitutes a clinically meaningful delay in the development of diabetes in the context of metformin’s known benefits and risks; this would be analogous to a previous successful initiative to establish measures of C-peptide as the primary efficacy end point for new type 1 diabetes drugs (118). Notably, the FDA has awarded cardiovascular prevention indications for statins on the basis of results from a single robust trial showing significant reductions in tangible cardiovascular events (119).
Admittedly, the DPP did not evaluate participants’ diabetes status off therapy by including a metformin washout period. In its guidance, the FDA reasonably asks for confirmation that normal glucose regulation persists after washout to exclude the possibility that a glucose-lowering agent is not simply maintaining normoglycemia in those who actually have progressed to diabetes. Evaluating diabetes status after washout must be distinguished from demonstrating the durability of the diabetes-delaying effect. The latter is determined by restarting treatment and determining at subsequent time points (each after treatment washout) what proportion of the population retains normal glucose regulation status. This distinction is less important for trials of long duration with a single washout evaluation at the end and more important for shorter trials with a single end point, for which durability remains an open question, and for long trials with intermediate end points. Measuring durability becomes more important when the safety profile of a candidate drug is not so benign. Here, weighing the durability of benefit against cumulative adversity would be an important aspect of estimating the overall benefit-to-risk relationship of what for some could be a lifelong therapy.
In the case of metformin, the long-term safety profile is well established, making the drug suitable for lifelong use once it is started for prevention, although there have been associated GI tolerability issues and concerns about vitamin B12 deficiency and anemia, as well as neuropathy in those with low vitamin B12 levels (120). Similar to current ADA recommendations, the indication for metformin should limit its use to subpopulations found in the DPP to be at high risk (i.e., those with a history of GDM, who are very obese, or who have more severe or progressive hyperglycemia) until additional evidence supports expansion of the indicated population. Of interest in this regard, metformin reduced IGT progression to diabetes in the IDPP’s lean Indian population about as well as it did in the DPP’s obese population (7,8).
Key practical questions are: how could approval of a new metformin indication be achieved, and who will lead this effort? The conventional mechanism would be for one or more of the companies marketing metformin in the U.S. to compile and submit a supplementary new drug application containing the data and other necessary information to allow the FDA to approve the indication. However, these companies may not wish to spend the significant resources required for such an application given that metformin is already available in generic form. Realizing the long-term societal benefits of type 2 diabetes prevention may require other avenues for FDA approval (25). One approach could be for the FDA to receive a comprehensive package of evidence and other relevant material and call an advisory committee for a full public discussion. With a positive vote of its advisory committee, the FDA could conclude that the indication should be granted and allow companies with approved metformin new drug applications to add the new indication to their labels. An organization such as ADA could very appropriately lead the effort to compile the briefing package for the FDA and facilitate other supportive actions.
Unmet Research Needs
We need to learn more about racial and ethnic differences in responses to lifestyle and, particularly, pharmacological interventions. Are there meaningful differences, or are they more perceived than real because different populations were not compared in the same study? The similar effectiveness of the DPP interventions in five ethnic groups suggests that this may be more theoretical than real (7).
Much remains to be learned about the genetics of diabetes risk, the effects of genetics as a modifier of prevention interventions, and the potential role of genetic testing to identify appropriate subjects for future research. In this regard, genetics has been somewhat disappointing to date. Compilation of genetic risk scores has demonstrated that greater genetic risk is associated with a greater likelihood of progression to diabetes. However, this work has also demonstrated that a high genetic risk score in adults is typically also associated with elevated glucose levels, meaning that glucose itself is as good a predictor and certainly less expensive. Thus, the true utility of genetic risk scores may be in identifying young individuals who are at the greatest risk and initiating preventive therapy in them well before their glucose levels start to increase substantially.
More detailed evidence is needed to inform efforts to tailor preventive measures based on patients’ phenotype, genotype, composite diabetes risk scores, personal characteristics, socioeconomic factors, and other relevant parameters.
The Future Is Now: Translation Through National and Community Campaigns
Carefully conducted landmark prevention trials have provided more than enough evidence that prevention or delay of type 2 diabetes is possible in high-risk individuals. Clearly, the next step is to implement these strategies, and new technologies and innovations offer promising ways to reduce the labor and costs associated with scaling up prevention initiatives on a population level. Individuals can be screened for prediabetes in numerous low-cost ways for enrollment in an intervention. Community or workplace health screenings, provider referrals, and reviews of electronic medical records all can be leveraged to screen for diabetes risk. Individuals can also self-screen using one of the many available risk scoring instruments. Such risk questionnaires, originating from many sources in various populations, are available on the Internet and can be publicized through traditional advertising and social media (82,121–125).
Throughout the world, countries are now developing practical, affordable population-based programs that can be tailored for individual and cultural differences and implemented relatively inexpensively. Some of these programs are described below, and their reported results, where available, are summarized in Table 3 (24,68,76–78,126–129).
Table 3.
Sample translational prevention initiatives and their reported outcomes
| Study or initiative | Findings/results |
|---|---|
| Indian text-messaging intervention study (76) |
After 2 years, participants receiving twice-weekly motivational text messages had a 36% relative reduction in diabetes risk compared with those receiving standard advice but no text messages. |
| Finnish National Diabetes Prevention Program (FIN-D2D) (77) |
After 1 year, average weight loss in this high-risk population was 1.3 kg in men and 1.1 kg in women, with a 1.3-cm reduction in waist circumference. Decreases in blood pressure were 0.8 mmHg systolic and 1.5 diastolic in men, and 1.9 and 1.6 mmHg, respectively, in women. Total cholesterol, LDL cholesterol, and triglyceride levels decreased by 5–8% in men and 2–5% in women. Overall, 17.5% of subjects lost ≥5% of their body weight, 16.8% lost 2.5–4.9% weight, 46.1% maintained their baseline weight, and 19.6% gained ≥2.5% weight. The relative risk of diabetes was 0.31 in the group who lost ≥5% weight, 0.72 in the group who lost 2.5– 4.9% weight, and 1.10 in the group who gained ≥2.5% compared with the group who maintained weight. |
| Australian Life! Taking Action on Diabetes program (78) |
Between 2007 and mid-2011, there were >14,800 referrals to the program, and >8,400 individuals started the program. Participants who attended the first 5 sessions (offered every 2 weeks) lost a mean 1.4 kg in weight and 2.5 cm in waist circumference; those who also attended the sixth session (offered 8 months after the first) lost a mean 2.4 kg in weight (2.7% weight) and 3.8 cm in waist circumference, for an imputed potential diabetes risk reduction of 21–39%. |
| VA MOVE! Weight Management Program (68) |
Retrospective, observational analysis found a significant, dose-dependent, inverse association between incident diabetes and participation. Compared with nonparticipation, intense and sustained participation (at least eight sessions within 6 months over at least a 4-month span) was associated with a 33% reduction in diabetes incidence, and lower-intensity participation yielded a 20% reduction in diabetes incidence. Those who participated intensively also lost more weight than low-intensity participants (–2.2 vs. –0.64% over 3 years). However, the program has not reached a substantial proportion of the eligible population; only 13% participated in at least one session between 2005 and 2012. |
| Special Diabetes Program for Indians–Diabetes Prevention (SPDI-DP) (126) |
More than 2,500 participants started the 16-session program by 31 July 2008, with clinical assessments performed at baseline, soon after completing the program, and annually for up to 3 years. Crude incidence of diabetes was ∼3.5% per year among those who finished all 16 sessions, whereas it more than doubled (7.5% per year) among those who did not finish the program. Participants on average lost 9.6 lb immediately after completing the program, representing a 4.4% weight loss. This attenuated over the three annual visits but still differed significantly from no weight loss. By the end of the program sessions, 22.5% of participants had achieved the 7% weight loss goal; at the 3-year follow-up, 17.5% had achieved this goal. The percentage of participants achieving the 150 min/week exercise goal increased from 22% at baseline to 56% after the program and was ≥38% at each of the annual assessments. Participants also had significant improvements in blood glucose, blood pressure, and lipid parameters throughout the follow-up period. |
| DEPLOY (Diabetes Education & Prevention with a Lifestyle Intervention Offered at the YMCA) pilot study (127,128) and YMCA Diabetes Prevention Program (129) |
In the DEPLOY pilot study, body weight at 6 months decreased by a mean 5.7 kg or 6.0% in intervention participants and 1.8 kg or 2.0% in control subjects; this difference persisted through 12 months, with no racial or sex differences. Also, significant between-group differences in total cholesterol levels at both follow-up points were observed. At 4 and 12 months, the intervention group had significant decreases in 10-year coronary heart disease risk of 3.28 and 2.23%, respectively, compared with control subjects, who had a decrease in 10-year risk of 0.78% at 4 months and an increase in risk of 1.88% at 12 months. |
| As of December 2015, 39,435 individuals had attended at least one program session at one of 202 YMCA centers in 43 states. The average weight loss among participants was 4.7% at the end of the 16 sessions and 5.4% by 1 year. On average, participants undertook 157.5 min/week of physical activity. | |
| NDPP/Medicare/YMCA demonstration program (24) | Through this program, funded by CMS under the Affordable Care Act, eligible Medicare beneficiaries at high risk for diabetes attended initial weekly meetings and monthly follow-up sessions with a lifestyle coach to address long-term dietary and lifestyle modification to reduce their risk for diabetes. Mean weight loss was 4.7% for those who attended at least four weekly sessions and 5.2% for those who attended at least nine sessions. More than 80% of recruited participants attended at least four sessions. When compared with similar Medicare beneficiaries not in the program, CMS estimated a savings of $2,650 for each enrollee over a 15-month period, which was more than enough to cover the cost of the program. |
Taking advantage of new technologies can aid in the translational effort by reducing costs and expanding reach to a wide audience. For example, an Indian study assessed the effectiveness of a text-messaging lifestyle intervention and convincingly demonstrated that a low-cost, low-labor program offering lifestyle modification support through a widely available medium can produce clinically meaningful outcomes (76). Similar approaches are being implemented in other countries, some under the auspices of the WHO/International Telecommunication Union Be He@lthy, Be Mobile program (130).
Elsewhere, numerous public health initiatives have begun translating prevention research into effective community-based, or even nationwide, interventions. The Finnish National Diabetes Prevention Program (FIN-D2D) (77,131,132) was the first of these efforts. Implemented in 2003 in five districts covering a population of 1.5 million, it encompasses three concurrent strategies: a high-risk strategy aimed at incorporating diabetes prevention and cardiovascular risk reduction into routine primary care using existing resources, a population strategy focused on raising awareness, and an early treatment strategy for individuals identified through screening as having diabetes. The ultimate goal of FIN-D2D is to refine and expand this multistrategy approach to serve all of Finland. The Finnish experience also has been used in the large European effort DE-PLAN (Diabetes in Europe: Prevention Using Lifestyle, Physical Activity and Nutritional Intervention) to develop screening for type 2 diabetes risk followed by lifestyle programs in several local settings, mainly in primary care (133). This has been further elaborated within the IMAGE (Development and Implementation of a European Guideline and Training Standards for Diabetes Prevention) network, which has developed tools for the implementation of prevention in practice (134).
The second large-scale prevention program reported was Victoria, Australia’s Life! Taking Action on Diabetes program (78). Established in 2007 and built in part on the Finnish experience, this statewide program has been implemented in a systematic approach featuring a structured group intervention, standardized facilitator training and accreditation, a facilitator payment process linked to return of data, a participant manual, a continuous quality improvement process, and ongoing evaluation. The program uses an Australian risk tool to screen potential participants and a multifaceted social marketing and communications plan to raise awareness and facilitate recruitment.
In Singapore, several initiatives have been launched as part of the government’s Healthy Living Master Plan to encourage the population in general, and individuals with prediabetes specifically, to adopt lifestyle changes to reduce their diabetes risk (135–138). A low-cost, nurse educator–led prediabetes intervention program offers participants three individual counseling sessions at community centers or primary care clinics and telephone follow-up after 6, 9, and 12 months. OGTTs are performed after 1 year to determine whether participants’ dysglycemia has improved. In addition, the Healthier Dining Programme encourages food and beverage companies to improve the nutritional quality of their offerings, and the Workplace Health Programme encourages and provides resources to Singaporean employers to provide an integrated approach to workplace health.
Israel recently launched an innovative approach to prevention funded through a social impact bond. Under this arrangement, private donors finance a 1-year intensive intervention program in high-risk individuals. Health maintenance organizations and the Israeli National Insurance Institute will repay donors their original investment plus a dividend based on cost reductions realized through health improvements resulting from the program (139).
In the U.S., numerous small DPP translational interventions have been implemented in diverse settings using a variety of innovative formats (69–75). On a larger scale, the most promising programs to date have been implemented in the veteran population (68) and American Indian/Alaska Native (AI/AN) communities (126) and through YMCA centers throughout the country (129).
The U.S. Department of Veterans Affairs (VA) MOVE! Weight Management Program (68) offers interactive education sessions on nutrition, physical activity, self-management, and goal setting for veterans who are overweight or obese and have a weight-related disorder. Since 2005, >500,000 veterans have participated, making MOVE! the largest such program in the U.S.
The Special Diabetes Program for Indians–Diabetes Prevention (SPDI-DP) (126) targets the AI/AN population, which has the highest diabetes prevalence rate of any segment of the U.S. population (2). Since 2006, the SPDI-DP has tested the feasibility and impact of an adapted DPP lifestyle intervention offered in native communities that lack essential resources and have diverse health care settings and mobile populations, all of which pose challenges to recruitment, retention, and effectiveness. The program is being offered through 36 health care programs serving 80 AI/AN tribes in 18 states.
The YMCA Diabetes Prevention Program is based on DEPLOY (Diabetes Education & Prevention with a Lifestyle Intervention Offered at the YMCA), a 2005–2010 pilot study of a DPP-type intervention adapted for community implementation through YMCAs (127). The year-long course is open to overweight adults who meet glucose criteria for prediabetes, have had GDM, or have a high score on a qualifying risk test. It provides group education and counseling with goals of 5–7% weight loss and ≥150 min/week of physical activity.
The YMCA program operates as part of NDPP (23), which offers training for lifestyle coaches and a formal recognition process for high-quality intervention programs. NDPP has facilitated a partnership between the YMCA and United Health Group through which the YMCA receives pay-for-performance reimbursement for the program from insurers and private employers. NDPP is now working to expand that partnership to include more programs and payers and to refine a marketing strategy to increase referrals to and participation in lifestyle intervention programs nationwide.
In 2011, the U.S. Centers for Medicare & Medicaid Services (CMS) awarded the National Council of YMCAs of the USA $11.8 million to enroll eligible Medicare beneficiaries at high risk for diabetes in a prevention program targeting weight loss of ≥5%. The results of this initiative led to the recent independent findings in support of the expansion of the NDPP model throughout the Medicare program (24).
Next Steps
The programs described above have developed innovative, pragmatic methods of delivering lifestyle change interventions; many have made use of the latest information and communication technologies; and each has involved the active cooperation of key stakeholders in the medical community, nongovernmental organizations, and public and private sector agencies. We are eagerly awaiting reports of their outcomes and hope that the components deployed will play an important role in reducing the prevalence of a disease with such wide-ranging personal, societal, and economic consequences.
A logical early step in advancing preventive strategies may be for the diabetes medical community to reach consensus on how to approach widespread translational programs that potentially can be implemented on a global level. Although lifestyle interventions are effective, our sole reliance on people’s adherence to diet and physical activity recommendations will not be enough to delay progression for a large portion of the at-risk population. Thus, attention also must be given to recommendations for pharmacological therapy to yield long-term societal benefits in the area of type 2 diabetes prevention and reduction in diabetes complications, including cardiovascular disease. Hence, in this Expert Forum, we also discussed a possible alternative avenue for FDA approval of a new prevention indication for metformin. Specifically, we developed a compelling argument that the evidence base for metformin already may be substantial enough to support approval of such an indication. We readily admit that additional work will be needed, and many hurdles remain. Still, this is an exciting time for diabetes prevention, and although real-world translation is the next greatest hurdle, it also represents the biggest opportunity to stem the tide of the global diabetes pandemic.
Article Information
Acknowledgments. Writing and editing support services for this article were provided by Debbie Kendall of Kendall Editorial in Richmond, VA. The Editorial Committee recognizes that the work of the journal and contributions such as this Expert Forum would not be possible without the dedicated work and continued support of many individuals. Specifically, the planning, logistics, and funding of the meeting and the incredible editorial support would not have been possible without the tireless efforts of Chris Kohler and his staff at the ADA Publishing office. In addition, the Editorial Committee thanks Lyn Reynolds and her staff in the ADA editorial office for support and Anne Gooch at the Pennington Biomedical Research Center for her valuable assistance in helping to organize the Expert Forum. W.T.C. is supported in part by National Institutes of Health (NIH) grant 1U54-GM-104940, which funds the Louisiana Clinical and Translational Science Center, and NIH grant P50-AT-002776.
Duality of Interest. W.T.C. has served as principal investigator on clinical research grants received by his institutions from AstraZeneca, Janssen, MannKind Corp., and Sanofi and has served as a consultant for Intarcia Therapeutics, Adocia, and Sanofi. J.B.B. has been an investigator and/or consultant under contracts between his employer and the following companies: Adocia, Andromeda, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Dance Biopharm, Elcelyx, Eli Lilly and Co., F. Hoffman-La Roche, GI Dynamics, GlaxoSmithKline, Halozyme, Intarcia Therapeutics, Johnson & Johnson, Lexicon, MacroGenics, Medtronic Minimed, Merck Sharp & Dohme, Metavention, Novo Nordisk, Orexigen, Osiris, Quest, Sanofi, Scion NeuroStim, Takeda, Tolerex, and vTv Therapeutics. He is a consultant to and has stock options from PhaseBio. J.T. has received grant support from AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Merck Serono, Regeneron, and Sanofi. He has received honoraria for speaking engagements from AstraZeneca, Eli Lilly and Co., Novo Nordisk, and Sanofi and consulting fees from AstraZeneca, Bayer Pharma, and Novo Nordisk. He is a stock shareholder of Orion Pharma. G.A.F. is a panel member/consultant for Acosti, Adocia, Arisaph, Artemis, Bio-Cancer Treatment International Ltd., Becton Dickinson, BioCon, Diasome, Dompé, Emperra, Exsulin, Thermo Fisher Scientific, Gilead, Hyperion, IMTherapeutics, Innoneo, Intarcia Therapeutics, Islet Sciences, Lexicon, Locemia, Johnson & Johnson, MannKind Corp., Mars, MediWound, Melior, N-Gene, NuSirt, Royalty Pharma, Pfizer, Rhythm, Sanofi, SkyePharma, Strongbridge Biopharma, SynAgile, Takeda, Teva, Thermalin, Thetis, ThromboGenics, Tolerion, VeroScience, and Versartis. He is a stock/shareholder of Ammonett Pharma, Exsulin Corporation, Locemia, Innoneo, SynAgile, and Thetis. E.F. is an advisory board member for Boehringer Ingelheim/Eli Lilly and Co. and Merck Sharp & Dohme. He is a consultant for AstraZeneca, GlaxoSmithKline, and Janssen and a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Co., Merck Sharp & Dohme, Mitsubishi, Novo Nordisk, Sanofi, and Takeda. He receives research grant support from Eli Lilly and Co. H.C.G. has received grant support from AstraZeneca, Eli Lilly and Co., Merck Sharp & Dohme, and Sanofi; honoraria for speaking engagements from AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Novo Nordisk, and Sanofi; and consulting fees from Abbott Pharmaceuticals, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Co., Kaneq Bioscience, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. A.R. is an advisory board member for AstraZeneca and Merck Sharp & Dohme. He has received honoraria for speaking engagements from Bayer, Eli Lilly and Co., Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi. The India Diabetes Research Foundation, of which he is president, has received research funding support from AstraZeneca and Novartis. I.R. has served on advisory boards for AstraZeneca/Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly and Co., LabStyle Innovations, Merck Sharp & Dohme, Novo Nordisk, Orgenesis, Sanofi, and SmartZyme Innovation; has been a consultant for AstraZeneca/Bristol-Myers Squibb, FuturRx, Gili Medical, Insuline Medical, Kamada, and NephroGenex; has been a speaker for AstraZeneca/Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly and Co., Johnson & Johnson, Merck Sharp & Dohme, Novartis Pharma AG, Novo Nordisk, Sanofi, and Teva; and is a stock/shareholder in Glucome, Insuline Medical, LabStyle Innovations, Orgenesis, and SmartZyme Innovation. J.R. has served on advisory boards and received honoraria or consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly and Co., Intarcia Therapeutics, Janssen, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. He has received grants/research support from Asahi, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly and Co., GlaxoSmithKline, Hanmi, Intarcia Therapeutics, Janssen, Lexicon, MannKind Corp., Merck Sharp & Dohme, Novo Nordisk, Pfizer, Sanofi, and Takeda. S.E.K. is a consultant/advisory board member for Boehringer Ingelheim, Elcelyx, Eli Lilly and Co., GlaxoSmithKline, Intarcia Therapeutics, Janssen, Merck Sharp & Dohme, Novo Nordisk, and Receptos and has received grant support from Eli Lilly and Co. No other potential conflicts of interest relevant to this article were reported.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Available from www.diabetesatlas.org. Accessed 31 December 2015
- 2.Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA, U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 3.Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics 2015;33:811–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland, World Health Organization, 2009 [Google Scholar]
- 5.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 6.Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 7.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme . The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevention type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran A, Snehalatha C, Mary S, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2). Diabetologia 2009;52:1019–1026 [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Tripathy D, Schwenke DC, et al.; ACT NOW Study . Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 11.Saito T, Watanabe M, Nishida J, et al.; Zensharen Study for Prevention of Lifestyle Diseases Group . Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–1360 [DOI] [PubMed] [Google Scholar]
- 12.Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152–162 [DOI] [PubMed] [Google Scholar]
- 13.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 14.Gerstein HC, Yusuf S, Bosch J, et al.; DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators . Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 15.Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 2010;376:103–111 [DOI] [PubMed] [Google Scholar]
- 16.Gerstein HC, Bosch J, Dagenais GR, et al.; ORIGIN Trial Investigators . Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 17.Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group . Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–2077 [DOI] [PubMed] [Google Scholar]
- 18.Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K; Voglibose Ph-3 Study Group . Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009;373:1607–1614 [DOI] [PubMed] [Google Scholar]
- 19.Holman RR, North BV, Tunbridge FK. Possible prevention of type 2 diabetes with acarbose or metformin. Diabetes 2000;49(Suppl. 1):A111 [Google Scholar]
- 20.Holman RR, Haffner SM, McMurray JJ, et al.; NAVIGATOR Study Group . Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1463–1476 [DOI] [PubMed] [Google Scholar]
- 21.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–161 [DOI] [PubMed] [Google Scholar]
- 22.Pi-Sunyer X, Astrup A, Fujioka K, et al.; SCALE Obesity and Prediabetes NN8022-1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22 [DOI] [PubMed] [Google Scholar]
- 23.Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med 2013;44(Suppl. 4):S346–S351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Service. Independent experts confirm that diabetes prevention model supported by the Affordable Care Act saves money and improves health [Internet], 2016. Available from http://www.hhs.gov/about/news/2016/03/23/independent-experts-confirm-diabetes-prevention-model-supported-affordable-care-act-saves-money.html. Accessed 4 April 2016
- 25.Fradkin JE, Roberts BT, Rodgers GP. What’s preventing us from preventing type 2 diabetes? N Engl J Med 2012;367:1177–1179 [DOI] [PubMed] [Google Scholar]
- 26.Kahn R, Davidson MB. The reality of type 2 diabetes prevention. Diabetes Care 2014;37:943–949 [DOI] [PubMed] [Google Scholar]
- 27.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 28.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–480 [DOI] [PubMed] [Google Scholar]
- 29.Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–307 [DOI] [PubMed] [Google Scholar]
- 30.Lindström J, Louheranta A, Mannelin M, et al.; Finnish Diabetes Prevention Study Group . The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 31.Lindström J, Ilanne-Parikka P, Peltonen M, et al.; Finnish Diabetes Prevention Study Group . Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 32.Lindström J, Peltonen M, Eriksson JG, et al.; Finnish Diabetes Prevention Study (DPS) . Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013;56:284–293 [DOI] [PubMed] [Google Scholar]
- 33.Knowler WC, Hamman RF, Edelstein SL, et al.; Diabetes Prevention Program Research Group . Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005;54:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman WH, Hoerger TJ, Brandle M, et al.; Diabetes Prevention Program Research Group . The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snehalatha C, Mary S, Joshi VV, Ramachandran A. Beneficial effects of strategies for primary prevention of diabetes on cardiovascular risk factors: results of the Indian Diabetes Prevention Programme. Diab Vasc Dis Res 2008;5:25–29 [DOI] [PubMed] [Google Scholar]
- 38.Gerstein HC, Mohan V, Avezum A, et al.; DREAM On (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication Ongoing Follow-up) Investigators . Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes. Diabetologia 2011;54:487–49521116607 [Google Scholar]
- 39.DeFronzo RA, Abdul-Ghani M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care 2011;34(Suppl. 2):S202–S209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J; ORIGIN Trial Investigators . Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J 2008;155:26–32, 32.e1–32.e6 [DOI] [PubMed] [Google Scholar]
- 41.ORIGIN Trial Investigators Cardiovascular and other outcomes postintervention with insulin glargine and omega-3 fatty acids (ORIGINALE). Diabetes Care 2016;39:709–716 [DOI] [PubMed] [Google Scholar]
- 42.Holman RR, Blackwell L, Stratton IM, Manley SE, Tucker L, Frighi V. Six-year results from the Early Diabetes Intervention Trial. Diabet Med 2003;20(Suppl. 2):15 [Google Scholar]
- 43.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 45.American Diabetes Association Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S13–S22 [DOI] [PubMed] [Google Scholar]
- 46.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 47.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 48.Genuth S, Alberti KG, Bennett P, et al.; Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva, Switzerland, World Health Organization, 2006 [Google Scholar]
- 50.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva, Switzerland, World Health Organization, 2011 [PubMed] [Google Scholar]
- 52.World Health Organization Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland, World Health Organization, 1999 [Google Scholar]
- 53.National Institute for Health and Clinical Excellence Preventing Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk. NICE Public Health Guidance 38. Manchester, U.K., National Institute for Health and Clinical Excellence, 2012 [Google Scholar]
- 54.Morris DH, Khunti K, Achana F, et al. Progression rates from HbA1c 6.0-6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 2013;56:1489–1493 [DOI] [PubMed] [Google Scholar]
- 55.James C, Bullard KM, Rolka DB, et al. Implications of alternative definitions of prediabetes for prevalence in U.S. adults. Diabetes Care 2011;34:387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plantinga LC, Crews DC, Coresh J, et al.; CDC CKD Surveillance Team . Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol 2010;5:673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tesfaye S, Boulton AJ, Dyck PJ, et al.; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu JS, Yang YC, Lin TS, et al. Epidemiological evidence of altered cardiac autonomic function in subjects with impaired glucose tolerance but not isolated impaired fasting glucose. J Clin Endocrinol Metab 2007;92:3885–3889 [DOI] [PubMed] [Google Scholar]
- 59.Schroeder EB, Chambless LE, Liao D, et al.; Atherosclerosis Risk in Communities (ARIC) study . Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2005;28:668–674 [DOI] [PubMed] [Google Scholar]
- 60.Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305–312 [DOI] [PubMed] [Google Scholar]
- 61.Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990-2000. Diabet Med 2007;24:200–207 [DOI] [PubMed] [Google Scholar]
- 62.Anjana RM, Shanthi Rani CS, Deepa M, et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the Chennai Urban Rural Epidemiology Study (CURES). Diabetes Care 2015;38:1441–1448 [DOI] [PubMed] [Google Scholar]
- 63.Tirosh A, Shai I, Tekes-Manova D, et al.; Israeli Diabetes Research Group . Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 2005;353:1454–1462 [DOI] [PubMed] [Google Scholar]
- 64.Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2011;58:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seshasai SR, Kaptoge S, Thompson A, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu T, Liu W, Cai X, et al. Risk of coronary heart disease in different criterion of impaired fasting glucose: a meta-analysis. Medicine 2015;94:e1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ning F, Zhang L, Dekker JM, et al.; DECODE Finnish and Swedish Study Investigators . Development of coronary heart disease and ischemic stroke in relation to fasting and 2-hour plasma glucose levels in the normal range. Cardiovasc Diabetol 2012;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jackson SL, Long Q, Rhee MK, et al. Weight loss and incidence of diabetes with the Veterans Health Administration MOVE! lifestyle change programme: an observational study. Lancet Diabetes Endocrinol 2015;3:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mau MK, Keawe’aimoku Kaholokula J, West MR, et al. Translating diabetes prevention into native Hawaiian and Pacific Islander communities: the PILI 'Ohana Pilot project. Prog Community Health Partnersh 2010;4:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pagoto SL, Kantor L, Bodenlos JS, Gitkind M, Ma Y. Translating the diabetes prevention program into a hospital-based weight loss program. Health Psychol 2008;27(Suppl.):S91–S98 [DOI] [PubMed] [Google Scholar]
- 71.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care 2008;31:684–689 [DOI] [PubMed] [Google Scholar]
- 72.Boltri JM, Davis-Smith YM, Seale JP, Shellenberger S, Okosun IS, Cornelius ME. Diabetes prevention in a faith-based setting: results of translational research. J Public Health Manag Pract 2008;14:29–32 [DOI] [PubMed] [Google Scholar]
- 73.Aldana SG, Barlow M, Smith R, et al. The Diabetes Prevention Program: a worksite experience. AAOHN J 2005;53:499–505; quiz 506–507 [PubMed] [Google Scholar]
- 74.Whittemore R, Melkus G, Wagner J, Dziura J, Northrup V, Grey M. Translating the Diabetes Prevention Program to primary care: a pilot study. Nurs Res 2009;58:2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res 2015;17:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramachandran A, Snehalatha C, Ram J, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2013;1:191–198 [DOI] [PubMed] [Google Scholar]
- 77.Saaristo T, Peltonen M, Keinänen-Kiukaanniemi S, et al.; FIN-D2D Study Group . National type 2 diabetes prevention programme in Finland: FIN-D2D. Int J Circumpolar Health 2007;66:101–112 [DOI] [PubMed] [Google Scholar]
- 78.Dunbar JA, Jayawardena A, Johnson G, et al. Scaling up diabetes prevention in Victoria, Australia: policy development, implementation, and evaluation. Diabetes Care 2014;37:934–942 [DOI] [PubMed] [Google Scholar]
- 79.Sakane N, Sato J, Tsushita K, et al.; Japan Diabetes Prevention Program (JDPP) Research Group . Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health 2011;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wareham NJ. Mind the gap: efficacy versus effectiveness of lifestyle interventions to prevent diabetes. Lancet Diabetes Endocrinol 2015;3:160–161 [DOI] [PubMed] [Google Scholar]
- 81.Li G, Hu Y, Yang W, et al.; DA Qing IGT and Diabetes Study . Effects of insulin resistance and insulin secretion on the efficacy of interventions to retard development of type 2 diabetes mellitus: the DA Qing IGT and Diabetes Study. Diabetes Res Clin Pract 2002;58:193–200 [DOI] [PubMed] [Google Scholar]
- 82.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–731 [DOI] [PubMed] [Google Scholar]
- 83.Lindström J, Peltonen M, Eriksson JG, et al.; Finnish Diabetes Prevention Study (DPS) Group . Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care 2008;31:857–862 [DOI] [PubMed] [Google Scholar]
- 84.de Mello VDF, Lindström J, Eriksson J, et al. Insulin secretion and its determinants in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals: the Finnish Diabetes Prevention Study. Diabetes Care 2012;35:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tuomilehto H, Peltonen M, Partinen M, et al.; Finnish Diabetes Prevention Study Group . Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care 2009;32:1965–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J, Kuusisto J, Vänttinen M, et al. Variants of transcription factor 7-like 2 (TCF7L2) gene predict conversion to type 2 diabetes in the Finnish Diabetes Prevention Study and are associated with impaired glucose regulation and impaired insulin secretion. Diabetologia 2007;50:1192–1200 [DOI] [PubMed] [Google Scholar]
- 87.Siitonen N, Lindström J, Eriksson J, et al. Association between a deletion/insertion polymorphism in the α2B-adrenergic receptor gene and insulin secretion and type 2 diabetes. The Finnish Diabetes Prevention Study. Diabetologia 2004;47:1416–1424 [DOI] [PubMed] [Google Scholar]
- 88.Laaksonen DE, Siitonen N, Lindström J, et al.; Finnish Diabetes Prevention Study Group . Physical activity, diet, and incident diabetes in relation to an ADRA2B polymorphism. Med Sci Sports Exerc 2007;39:227–232 [DOI] [PubMed] [Google Scholar]
- 89.Uusitupa MI, Stancáková A, Peltonen M, et al. Impact of positive family history and genetic risk variants on the incidence of diabetes: the Finnish Diabetes Prevention Study. Diabetes Care 2011;34:418–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kitabchi AE, Temprosa M, Knowler WC, et al.; Diabetes Prevention Program Research Group . Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aroda VR, Christophi CA, Edelstein SL, et al.; Diabetes Prevention Program Research Group . The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab 2015;100:1646–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Florez JC, Jablonski KA, Bayley N, et al.; Diabetes Prevention Program Research Group . TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 2006;355:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dabelea D, Ma Y, Knowler WC, et al.; Diabetes Prevention Program Research Group . Diabetes autoantibodies do not predict progression to diabetes in adults: the Diabetes Prevention Program. Diabet Med 2014;31:1064–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE; Diabetes Prevention Program Research Group . Regression from pre-diabetes to normal glucose regulation is associated with long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramachandran A, Snehalatha C, Samith Shetty A, Nanditha A. Predictive value of HbA1c for incident diabetes among subjects with impaired glucose tolerance--analysis of the Indian Diabetes Prevention Programmes. Diabet Med 2012;29:94–98 [DOI] [PubMed] [Google Scholar]
- 96.Nanditha A, Ram J, Snehalatha C, et al. Early improvement predicts reduced risk of incident diabetes and improved cardiovascular risk in prediabetic Asian Indian men participating in a 2-year lifestyle intervention program. Diabetes Care 2014;37:3009–3015 [DOI] [PubMed] [Google Scholar]
- 97.Ram J, Snehalatha C, Selvam S, et al. The oral disposition index is a strong predictor of incident diabetes in Asian Indian prediabetic men. Acta Diabetol 2015;52:733–741 [DOI] [PubMed] [Google Scholar]
- 98.Siu AL; U S Preventive Services Task Force . Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015;163:861–868 [DOI] [PubMed] [Google Scholar]
- 99.Congress.gov. H.R.2102: Medicare Diabetes Prevention Act of 2015 [Internet]. Available from https://www.congress.gov/bill/114th-congress/house-bill/2102. Accessed 6 November 2015
- 100.Patient-Centered Outcomes Research Institute. Future Research Prioritization: Comparative Effectiveness of Strategies for Diabetes Prevention and Prediabetes [Internet], July 2015. Available from http://www.pcori.org/sites/default/files/PCORI-Assessment-Options-AP-Meeting-Topic-1-Brief-CER-Prevention-Pre-Diabetes-07-09-10-2015.pdf. Accessed 6 November 2015
- 101.Ackermann RT, Kenrik Duru O, Albu JB, et al.; NEXT-D Study Group . Evaluating diabetes health policies using natural experiments: the natural experiments for translation in diabetes study. Am J Prev Med 2015;48:747–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thorpe KE. Analysis & commentary: the Affordable Care Act lays the groundwork for a national diabetes prevention and treatment strategy. Health Aff (Millwood) 2012;31:61–66 [DOI] [PubMed] [Google Scholar]
- 103.Community Preventive Services Task Force. Diabetes prevention and control: combined diet and physical activity promotion programs to prevent type 2 diabetes among people at increased risk: task force finding and rationale statement [Internet]. Available from http://www.thecommunityguide.org/diabetes/supportingmaterials/RRcombineddietandpa.html. Accessed 6 November 2015
- 104.Phung OJ, Sood NA, Sill BE, Coleman CI. Oral anti-diabetic drugs for the prevention of Type 2 diabetes. Diabet Med 2011;28:948–964 [DOI] [PubMed] [Google Scholar]
- 105.DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab 2011;96:2354–2366 [DOI] [PubMed] [Google Scholar]
- 106.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med 2010;170:1191–1201 [DOI] [PubMed] [Google Scholar]
- 107.Astrup A, Carraro R, Finer N, et al. NN8022-1807 Investigators . . Safety, tolerability, and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes 2012;36:843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.RISE Consortium Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diabetes Prevention Program Research Group Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diabetes Prevention Program Research Group The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012;35:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ratner RE, Christophi CA, Metzger BE, et al.; Diabetes Prevention Program Research Group . Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sussman JB, Kent DM, Nelson JP, Hayward RA. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ 2015;350:h454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.American Diabetes Association Prevention or delay of type 2 diabetes. Sec. 4. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S36–S38 [DOI] [PubMed] [Google Scholar]
- 114.Moin T, Li J, Duru OK, et al. Metformin prescription for insured adults with prediabetes from 2010 to 2012: a retrospective cohort study. Ann Intern Med 2015;162:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hanefeld M, Schaper F. Acarbose: oral anti-diabetes drug with additional cardiovascular benefits. Expert Rev Cardiovasc Ther 2008;6:153–163 [DOI] [PubMed] [Google Scholar]
- 116.U.S. Food and Drug Administration. Guidance for Industry: Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention [Draft Guidance] [Internet], February 2008. Available from http://www.fda.gov/downloads/Drugs/GuidanceCompliance RegulatoryInformation/Guidances/UCM071624.pdf. Accessed 12 November 2015
- 117.Gerstein HC. Point: If it is important to prevent type 2 diabetes, it is important to consider all proven therapies within a comprehensive approach. Diabetes Care 2007;30:432–434 [DOI] [PubMed] [Google Scholar]
- 118.Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve β-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes 2004;53:250–264 [DOI] [PubMed] [Google Scholar]
- 119.U.S. Food and Drug Administration. FDA approves new indication for Crestor [Internet]. Available from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm200128.htm. Accessed 17 February 2016
- 120.Aroda VR, Edelstein SL, Goldberg RB, et al.; Diabetes Prevention Program Research Group . Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2016;101:1754–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alssema M, Vistisen D, Heymans MW, et al.; DETECT-2 collaboration . The Evaluation of Screening and Early Detection Strategies for Type 2 Diabetes and Impaired Glucose Tolerance (DETECT-2) update of the Finnish diabetes risk score for prediction of incident type 2 diabetes. Diabetologia 2011;54:1004–1012 [DOI] [PubMed] [Google Scholar]
- 122.American Diabetes Association. Type 2 diabetes risk test [Internet]. Available from http://www.diabetes.org/are-you-at-risk/diabetes-risk-test/?loc=atrisk-slabnav. Accessed 17 January 2016
- 123.Diabetes UK. Diabetes risk score assessment tool [Internet]. Available from https://www.diabetes.org.uk/professionals/diabetes-risk-score-assessment-tool. Accessed 17 January 2016
- 124.Chen L, Magliano DJ, Balkau B, et al. AUSDRISK: an Australian type 2 diabetes risk assessment tool based on demographic, lifestyle and simple anthropometric measures. Med J Aust 2010;192:197–202 [DOI] [PubMed] [Google Scholar]
- 125.Mohan V, Deepa R, Deepa M, Somannavar S, Datta M. A simplified Indian diabetes risk score for screening for undiagnosed diabetic subjects. J Assoc Physicians India 2005;53:759–763 [PubMed] [Google Scholar]
- 126.Jiang L, Manson SM, Beals J, et al.; Special Diabetes Program for Indians Diabetes Prevention Demonstration Project . Translating the Diabetes Prevention Program into American Indian and Alaska Native communities: results from the Special Diabetes Program for Indians Diabetes Prevention demonstration project. Diabetes Care 2013;36:2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community: the DEPLOY pilot study. Am J Prev Med 2008;35:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lipscomb ER, Finch EA, Brizendine E, Saha CK, Hays LM, Ackermann RT. Reduced 10-year risk of coronary heart disease in patients who participated in a community-based diabetes prevention program: the DEPLOY pilot study. Diabetes Care 2009;32:394–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.YMCA. Measureable progress, unlimited support: Diabetes Prevention Program fact sheet [Internet], December 2015. Available from http://www.ymca.net/diabetes-prevention/statistics.html. Accessed 21 December 2015
- 130.Prasad VM. Using mobile technology for health [Internet]. Available from http://www.searo.who.int/entity/noncommunicable_diseases/events/ncd-bengaluru-mobile-technology-for-health.pdf. Accessed 17 December 2015
- 131.Finnish Diabetes Association. Implementation of type 2 diabetes prevention plan: project plan 2003–2007, FIN-D2D project [Internet]. Available from http://www.diabetes.fi/files/1107/Implementation_of_Type_2_Diabetes_Prevention._Project_Plan_2003-2007.pdf. Accessed 18 December 2015
- 132.Saaristo T, Moilanen L, Korpi-Hyövälti E, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schwarz PE, Lindström J, Kissimova-Scarbeck K, et al.; DE-PLAN project . The European perspective of type 2 diabetes prevention: diabetes in Europe--prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. Exp Clin Endocrinol Diabetes 2008;116:167–172 [DOI] [PubMed] [Google Scholar]
- 134.Lindström J, Neumann A, Sheppard KE, et al. Take action to prevent diabetes--the IMAGE toolkit for the prevention of type 2 diabetes in Europe. Horm Metab Res 2010;42(Suppl. 1):S37–S55 [DOI] [PubMed] [Google Scholar]
- 135.Nanditha A, Ma RCW, Ramachandran A, et al. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care 2016;39:472–485 [DOI] [PubMed] [Google Scholar]
- 136.Singapore Health Promotion Board. Pre-diabetes intervention programme [Internet]. Available from http://www.hpb.gov.sg/HOPPortal/programmes-article/5844. Accessed 18 December 2015
- 137.Singapore Health Promotion Board. Healthier dining programme [Internet]. Available from http://www.hpb.gov.sg/HOPPortal/programmes-article/HPB063209. Accessed 3 March 2016
- 138.Singapore Health Promotion Board. Workplace health promotion [Internet]. Available from http://www.hpb.gov.sg/HOPPortal/programmes-article/5568. Accessed 3 March 2016
- 139.Neudorfer Y. Impact investing in Israel. Presentation to the G8 Impact Investment Taskforce. 9 July 2015 [Internet]. Available from http://www.socialimpactinvestment.org/reports/Impact-Investment-in-Israel-Yaron-Neudorfer.pptx. Accessed 3 March 2016