Abstract
Roughly 1/3rd of immune competent patients will reactivate latent cytomegalovirus (CMV) during critical illness. There are no standard methods to detect reactivation, and some investigators have postulated that presence of DNA in BAL fluid is indicative of viral replication. To test this hypothesis, we used a murine model that allows inclusion of matched healthy controls which is not possible in human studies. BALB/c mice infected with Smith-murine CMV or PBS (mock) had BAL evaluated 7, 14, or 21 days after acute infections, during latency, or during bacterial sepsis. Plaque assay, PCR, and rtPCR were performed on BALs and concomitantly obtained lung tissue. BAL cellular compositions, including tetramer evaluation of CMV-specific T cells were evaluated by flow cytometry. CMV DNA were detected in BAL at all time-points during acute infection, becoming undetectable in all mice during latency, then were detected again during bacterial sepsis, peaking 3 weeks after onset. mCMV specific T-cells were most numerous in BAL after acute viral infections, decreasing to low levels during latency, then fluctuating during bacterial sepsis. Specifically, mCMV-specific T-cells contracted at sepsis onset, expanding 2–4 weeks post-sepsis, presumably in response to increased viral loads at that time point. Altogether, our results support the use of BAL PCR for the diagnosis of CMV replication in immune competent hosts. Additionally, we demonstrate dynamic changes in CMV-specific T cells that occur in BAL during CMV infection and during sepsis induced viral reactivation.
Keywords: cytomegalovirus, virus classification, T cell, immune responses, reactivation, infection, latent infection, infection
INTRODUCTION
Cytomegalovirus (CMV) is a ubiquitous herpes virus that is easily controlled by hosts with intact immunity. Like all herpes viruses, CMV is not eradicated but establishes latency as cellular persistence of viral DNA after resolution of productive infection. In immune competent hosts, latency seems to be actively maintained by host immunity, and viral reactivation likely occurs as a consequence of some combination of transient immune compromise, inflammation, and epigenetic regulation of the major immediate early promoter [Seckert et al., 2013]. Recently, it has been shown that ~35% of immunocompetent latently infected humans have reactivation of CMV during critical illness, and that such reactivation is associated with nearly doubled durations of mechanical ventilation, days in the intensive care unit (ICU), and higher mortality [Kalil and Florescu, 2009; Mansfield et al., 2015].
There are currently no standards for diagnosis of CMV reactivation in immune competent hosts. When reviewing previous studies, various techniques have been used to diagnose CMV reactivation, including antigenemia, PCR of blood, and sputum, lung biopsy, cultures of sputum, blood, bronchoalveolar lavage (BAL), and urine, and more recently PCR (Table I). These studies also vary by population tested (i.e., burn, trauma, medical, and surgical patients). Lungs are widely considered to be an important site of CMV latency, and many investigators have evaluated tracheal aspirates and bronchoalveolar lavage (BAL) for reactivation [Heininger et al., 2001, 2011; Chilet et al., 2010; Smith et al., 2010; Blanquer et al., 2011; Coisel et al., 2012; Doan et al., 2013; Escribano et al., 2013; Friedrichs et al., 2013; Cinel et al., 2014]. To date, it has been assumed that detection of CMV DNA in the BAL of non-immune suppressed patients is consistent with CMV reactivation. Unfortunately studies to date lack appropriate negative controls, with only one study suggesting that CMV is not detectable in BAL of healthy hosts [Fajac et al., 1994]. However, this study was done before widespread availability of highly sensitive PCR-based detection systems. A follow-up study from this same group showed no CMV DNA in BAL from healthy controls, but this study also failed to detect CMV DNA in critically ill patients, a finding that has since been refuted by numerous investigators referenced above. Given recent substantial improvements in detection technologies, and the inherent difficulties associated with such studies in humans, we sought to determine the role of BAL CMV-DNA as an indicator of virus replication in a model that allows deliberate measurements at defined time-points after infection and reactivation stimuli.
TABLE I.
Comparison of Methodologies Used to Diagnosis Cytomegalovirus Reactivation in Immune Competent Hosts
| Study | n | % Positive | Compartment | Testing | Patient population |
|---|---|---|---|---|---|
| Domart et al. [1990] | 29 | 79 | Blood | Cx | SICU |
| Papazian et al. [1996] | 86 | 29 | Lung biopsy | Histology | MICU |
| Stephan et al. [1996] | 23 | 0 | Blood, BAL | Cx, PCR | Mixed |
| Kutza et al. [1998] | 34 | 32 | Blood | PCR, Ag | SICU |
| Cook et al. [1998] | 142 | 14 | Sputum, BAL, blood | Cx | SICU |
| Desachy et al. [2001] | 48 | 2 | Blood | PCR, Ag | Mixed |
| Heininger et al. [2001] | 56 | 36 | Blood, sputum | PCR | SICU |
| Razonable et al. [2002] | 120 | 1 | Blood | PCR | Mixed |
| Cook et al. [2003] | 104 | 15 | Blood, sputum | Cx | SICU |
| Jaber et al. [2005] | 237 | 17 | Blood | Ag | Mixed |
| von Muller et al. [2006] | 25 | 32 | Blood, TA, urine | Ag, Cx | SICU |
| Limaye et al. [2008] | 120 | 33 | Blood | rtPCR | Mixed |
| Ziemann et al. [2008] | 138 | 35 | Blood | PCR | SICU |
| Chiche et al. [2009] | 242 | 16 | Blood, BAL | Ag, Cx | MICU |
| Chilet et al. [2010] | 53 | 40 | Blood, TA | PCR | Mixed |
| Smith et al. [2010] | 61 | 19 | TA | PCR | Mixed |
| Vogel et al. [2008] | 86 | 0 | Blood, TA, urine | PCR, Ag, Cx | SICU |
| Bordes et al. [2011] | 29 | 71 | Blood | PCR | BICU |
| Heininger et al. [2011] | 86 | 41 | Blood, TA | PCR | SICU |
| Coisel et al. [2012] | 93 | 24 | Blood, BAL | Ag, rtPCR | MICU |
| Friedrichs et al. [2013] | 135 | 16 | BAL | PCR | Mixed |
| Escribano et al. [2013] | 42 | 54.5 | Blood, BAL | PCR | Pediatric |
| Ishioka et al. [2014] | 100 | 4 | Blood | Ag | CICU |
| Walton et al. [2014] | 560 | 24 | Blood | PCR | Mixed |
| Frantzeskaki et al. [2015] | 80 | 13.7 | Blood | PCR | Mixed |
| Ong et al. [2015] | 399 | 27 | Blood | PCR | Mixed |
| Lopez Roa et al. [2015] | 150 | 16.5 | Blood | PCR | Cardiac |
TA, tracheal aspirates; BAL, bronchoalveolar lavage; PCR, polymerase chain reaction for CMV DNA; Ag, antigen; rtPCR, real-time PCR; Cx, culture; SICU, surgical intensive care unit; MICU, medical intensive care unit; BICU, burn intensive care unit; CICU, cardiac intensive care unit.
In the current report, we use our murine model to evaluate the diagnostic utility of BAL PCR for detection of CMV replication. Specifically we evaluated kinetics of CMV detection during infection, latency, and following a reactivation trigger. Further, we describe processing techniques of BAL for optimal detection and correlate BAL cellular changes with viral replication. Because natural CMV infections in immune competent hosts do not all appear to be equal, we evaluated the impact of high and low titer infection on BAL detection. We hypothesized that CMV DNA in BAL is representative of viral replication and can be used to diagnose reactivation in immune competent hosts.
METHODS
Animals, Viral Infection, and Confirmation of Latency
Female BALB/c mice (Harlan, Indianapolis, IN) of 6–8 weeks of age were infected with Smith strain mCMV (VR-194/1981, ATCC, Rockville, MD) by intra-peritoneal (i.p.) injection of 1 × 102 PFU (low-titer) or 1 × 106 PFU (high-titer). All mice were housed under specific-pathogen-free conditions adhering to the Guide for the Care and Use of Laboratory Animals prepared by the National Research Council (NIH Publication No. 86–23, revised 1985) following protocol approval by our Institutional Review Board. All virus stocks were stored at −80°C and before use diluted in Dulbecco’s phosphate buffered saline (DPBS) and titrated by plaque assay before in-vivo infections using Primary Mouse Embryonic Fibroblasts (EmbryoMax, Strain C57/BL6, Millipore, Temecula, CA). Mock animals were injected with the same volume of sterile DPBS. As previously published, mice were allowed to become latent over the course of 4 months [Cook et al., 2002].
For acute infection experiments, cohorts of mice (two mock, five low titer, and five high titer per time-point) were evaluated at 7, 14, and 21 days after infection. For latent infection experiments, cohorts of mice (3 mock, 20 low titer, 18 high titer) were evaluated ~4 months after infection. For sepsis/reactivation experiments, latently infected mice underwent an LD40 model of cecal ligation, and puncture (CLP) ~4 months after initial infection. Cohorts of mice (2 mock, 5 low titer, 5 high titer) were evaluated 1, 2, 3, and 4 weeks after CLP.
Bronchoalveolar Lavage
Mice were euthanized with Isoflurane. Using aseptic technique, thoraces were opened widely by removing the anterior rib cage. The incision was carried superiorly and salivary glands were resected to fully expose the trachea. Trachea were cannulated with 22 gauge angiocatheters secured with silk suture passed around the trachea. Care was taken to keep the catheter tip above the level of the carina. Through this cannula lungs were lavaged with 0.5 ml aliquots of cold PBS. Lavage fluids were aspirated into a 2nd sterile syringe. This was repeated for a total of five washings that were stored on ice.
Bronchoalveolar Lavage Processing
BAL were processed into three different components: whole BAL (before processing), supernatants (cell-free component after centrifugation), and remaining cell pellets. Before centrifugation, 200 μl of whole BAL were aliquoted for DNA isolation, and 200 μl were aliquoted in TRIzol (GIBCO BRL, Carlsbad, CA) for RNA isolation. Remaining samples were centrifuged at 800g for 15 min at 4°C. From cell-free supernatants, 200 μl were collected for DNA isolation. One milliliter of remaining cell-free supernatant was used for lytic virus co-culture. Cell pellets were resuspended in 400 μl of enriched Roswell Park Memorial Institute media (ERPMI). From these cell suspensions, 100 μl were used for DNA isolation and 300 μl for flow cytometry. Cells were counted using the Luna FL Dual Fluorescence Cell Counter (Logos Biosystems, Annandale, VA).
Flow Cytometry
Cells were incubated with Fc block (mouse Fcγ III/II Receptor, 2.4G2, BD Pharmingen, San Jose, CA) for 15 min. Flourescent dye-conjugated antibodies specific for CD8 (53–6.7, APC), CD4 (RM4-5, PerCP/Cy5.5), and CD11c (N418, Alexa Fluor 700) were used (BioLegend, San Diego, CA). Alveolar macrophages were defined as CD11C+ and highly auto-fluorescent (Vermaelen 2004). mCMV-specific T-cells were identified using MHC-I tetramers specific for mCMV proteins (m123/pp89, H2Ld-restricted 168YPHFMPTNL176 and m164, H2Dd-restricted 257AGPPRYSRI265) (NIH Tetramer Core Facility, Emory University, Atlanta, GA) as previously described [Holtappels et al., 2002; Sierro et al., 2005]. Briefly, MHC-I peptide tetrameric complexes were produced and assembled with PE-conjugated streptavidin. Cells were incubated with tetramers for 1 hr (37°C), followed by antibody surface staining for 30 min (4°C), fixed, and analyzed by flow cytometry (FACSAria III, Becton Dickinson, San Jose, CA), and results were analyzed using FlowJo software (Tree Star, Ashland, OR).
PCR and RT-PCR
DNA were isolated from BAL fluid and lung tissue using Qiagen DNeasy Blood & Tissue Kit (Hilden, Germany) following manufacturer’s protocol. DNA were amplified in a total volume of 25 μl in iQ SYBR Green Supermix (BioRad, Hercules, CA) using manufacturer’s instructions. Total RNA were extracted from BAL fluid and tissues using TRIzol reagent. RNA were normalized to a fixed quantity. Reverse transcription (RT) reactions were carried out using QuantiTect Reverse Transcription Kit (QIAgen) per manufacturer’s instructions. Following RT reactions, 2 μl of the resulting cDNA was amplified using the same conditions as outlined above for PCRs. DNA and RNA samples were evaluated with nanodrop (Thermo Scientific, Wilmington, DE) for quantification and purity via 260/280 and 260/230 ratios.
Primers for mCMV-glycoprotein B (gB) (5′-GAG AAC TGC GAC ACG AAC AG -3′ and 5′-AGC ACC TTG AAG TCG GTG TT-3′) were used. Parathyroid-related protein (PTHrP) (5′-CAA GGG CAA GTC CAT CCA AG-3′ and 5′-GGG ACA CCT CCG AGG TAG CT-3′) was used as a housekeeping gene in PCR and RT-PCR reactions. DNA and RNA were quantified by PCR and rtPCR respectively with BioRad’s iCycler IQ using the following program: initial denaturation for 10 min at 95°C, 35 cycles of denaturation for 30 sec at 95°C, annealing for 30 sec at 54°C, and elongation for 30 sec at 72°C, followed by a final elongation for 7 min at 72°C and then holding at 4°C. Concomitant “no-RT” reactions were performed for each sample for each run to confirm lack of DNA contamination.
Plaque Assay
For plaque assays, Primary Mouse Embryonic Fibroblasts (EmbryoMax, Strain C57/BL6, Millipore, Temecula) were grown to confluence in six-well plates in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL). One milliliter of cell-free BAL supernatant was placed in each well. Cells were incubated for 30 min at 37°C in 5% CO2. To maximize sensitivity centrifugal enhancement was performed at 300g at 37°C for 15 min and specimens incubated for 1 hr longer. Plates were washed with phosphate-buffered saline (PBS) and then covered with 1 ml of 1% agar in DMEM. To enumerate lytic virus, following 6 days of incubation (37°C in 5% CO2), plates were fixed in 10% formalin, stained with 1% crystal violet, overlays removed, and analyzed for plaque formation by low-power phase-contrast microscopy.
Sepsis and Reactivation
For sepsis experiments, latently infected mice were anesthetized using inhaled isoflurane. The abdomen was shaved and prepped with betadine. An upper midline incision was made and the peritoneum incised. The cecum was identified and ligated using 3.0 silk suture at approximately 1 cm from the distal tip. Two enterotomies (through-and-through) were made using a 23G needle. The cecum was gently squeezed to ensure leakage of stool. The cecum was returned to the abdomen. The abdomen was closed with skin staples. After emergence from anesthesia mice received injections of buprenex for post-operative analgesia twice daily. The mice were housed in our animal facility with ample access to food and water. Surviving mice were then euthanized 7, 14, 21, or 28 days after sepsis.
RESULTS
Acute Infections
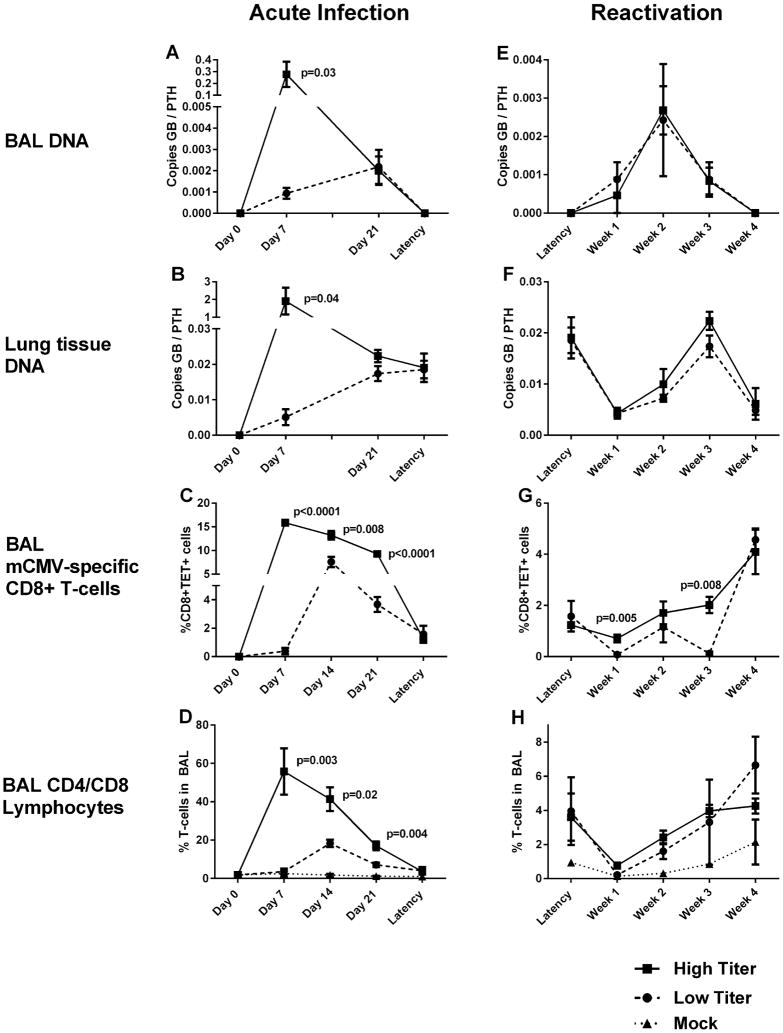
To determine the most sensitive method of mCMV-gB DNA detection in BAL specimens, we used our acutely infected mice as positive controls. Mean BAL recovery volume was 2.30 ml (SEM 0.023, range 1.0–2.75 ml). Whole BAL (BAL fluid + cells) analyses showed mCMV-gB DNA detectable by quantitative PCR only 1 week after high-titer infection (mean 0.35, +/− 0.10 copies mCMV-gB relative to PTH. mCMV-gB was not detectable in whole BAL at any other time-point. Supernatants (cell-free BAL) contained very low concentrations of DNA via nanodrop, with PTH controls inconsistently detectable, and mCMV-gB undetectable in all samples. In contrast, mCMV-gB DNA were detectable in BAL cell pellets (BAL-cells) during acute infection (both high and low titer) at both time points studied. Mean viral DNA loads were roughly two logs higher 1 week after high versus low-titer infection in BAL-cells (P = 0.016), but by 3 weeks after infection BAL DNA loads in high and low titer mice were not significantly different (Table II) (Fig. 1A). Based upon these results, we conclude that cell pellets from BAL are more sensitive for detecting CMV DNA, thus all subsequent BAL analyses were performed with BAL-cell pellets.
TABLE II.
Relative Expression of mCMV-Glycoprotein B DNA to Control Gene in BAL-Cell Pellets
| Group | Low titer (mean ± SEM) | High titer (mean ± SEM) | P-value |
|---|---|---|---|
| Acute infection–1 week | 0.00094 ± 0.00026 | 0.278 ± 0.107 | 0.016 |
| Acute infection–3 weeks | 0.0022 ± 0.00080 | 0.0020 ± 0.00067 | 0.436 |
| Latency | Not detectable | Not detectable | N/A |
| Sepsis–1 week | 0.0009 ± 0.0005 | 0.00046 ± 0.0005 | 0.269 |
| Sepsis–2 weeks | 0.00058 ± 0.00026 | 0.00268 ± 0.00063 | 0.008 |
| Sepsis–3 weeks | 0.00088 ± 0.00045 | 0.0010 ± 0.00037 | 0.421 |
| Sepsis–4 weeks | Not detectable | Not detectable | N/A |
p-values that are italicized are significantly different.
Fig. 1.
Bronchoalveolar lavage (BAL) fluid and lung tissues were collected from euthanized mice infected with murine cytomegalovirus (mCMV) at low titer (1 × 102 plaque forming units (PFU)) and high titer (1 × 106 PFU) initial infections. Time points studied include: 7, 14, and 21 days following acute infection, during latency (4 months after infection), and 1, 2, 3, and 4 weeks following bacterial sepsis from cecal ligation and puncture. Day 0 time-points are prior to infection and mock mice (without infection) are included where applicable. BAL specimens were analyzed by polymerase chain reaction for mCMV-glycoprotein B (GB) relative to control gene parathyroid-related protein (PTHrP) during acute infection (A) and sepsis-induced viral reactivation (E). Similar PCR analyses of lung tissues obtained after BAL collection (B and F). mCMV-specific CD8+ T cells were evaluated via flow cytometry from BAL fluid using tetramers specific for viral peptides m123 or m164 (TET+) during acute infection (C) and sepsis-induced reactivation (G). Overall CD4+ and CD8+ lymphocytes were also assessed via flow cytometry of BAL fluid in mock, low-titer, and high-titer mice during acute infection (D) and sepsis-induced reactivation (H). Each point represents results mean values from at least n = 5 mice, and bars represent standard error of mean. P-values indicate statistically significant differences between high titer and low titer mice (Student’s t-test, two tailed).
mCMV DNA from lung tissues (after lavage) showed an almost identical pattern to BAL during acute infection (Fig. 1B). High titer infection produced roughly two log higher viral loads 1 week after infection than low titer infections. Similar to BAL DNA, high titer and low titer tissue DNA concentrations converged by day 21 after infections. mCMV DNA concentrations in tissues were also significantly higher at each time point after infection in tissues than in BAL (analysis not shown).
RNA were not detectable in BAL due to extremely low RNA concentrations (RNA of control genes were not detectable). In lavaged lung tissues, mCMV-gB RNA were quantifiable only after high titer infection at 1 week (mean relative expression 3.36 ± 1.17 to control gene). All lavaged lungs were negative for mCMV-gB RNA via quantitative rtPCR 3 weeks after infection. Lytic virus was recovered from BAL of high-titer mice at 1 week (average 7.5 PFU/ml BALF) post-infection. No lytic virus was recovered from low-titer or mock infected mice at any time-point. Additionally, no lytic virus was recovered at any other time-point during latency or sepsis.
CMV-specific T-cells were significantly more numerous in BAL at all time points during acute infection after high titer compared to low titer infections (Fig. 1C). These cells peaked at day 7 after high titer infection, and day 14 after low titer infections. In high titer mice, lytic virus correlated with developing CMV-specific T-cells 1 week after infection (R2=0.86, P = 0.0003). As shown in Fig. 1D, mCMV infection induces substantial CD4 and CD8 lymphocyte alveolar migration during high titer infection. There was also notable alveolar CD4/CD8 migration after low titer infection, but this was significantly less than high titer infection (P < 0.01 at all time-points).
Latency
During latency, regardless of the fraction tested (BAL cell pellets, whole BAL, or supernatants), mCMV gB DNA were not detectable by quantitative PCR (n = 20 low titer, n = 18 high titer). rtPCR and plaque assays were also negative for RNA or virus during latency. Previous infection was confirmed in all mice via identification of CMV gB DNA in lung tissue via PCR (Fig. 1B). Interestingly, low titer and high titer infections resulted in comparable lung tissue viral DNA loads during latency (Fig. 1B, P = 0.91). It has been previously shown that mCMV infection causes accumulation of large populations of mCMV-specific T-cells in lungs of latently infected mice [Reddehase et al., 1984; Holtappels et al., 2000, 2002; Karrer et al., 2003; Pahl-Seibert et al., 2005; Sierro et al., 2005; Munks et al., 2006; Snyder et al., 2008; Snyder et al., 2011]. Recent work has suggested that these populations reside in the vascular compartment [Smith et al., 2014], and to confirm this cell pellets from BAL samples in latent mice were analyzed. As shown in Fig. 1C, there are very few CMV-specific T-cells in alveoli during latency, especially when compared to acute infection. Likewise overall CD4/CD8 lymphocyte accumulation has mostly resolved in BAL by onset of latency, with no significant differences in alveolar CD4/CD8 counts between naïve and latently infected mice (Fig. 1D, high vs. mock P = 0.17, low vs. mock P = 0.22).
Sepsis
Bacterial sepsis was induced using an LD40 model of cecal ligation and puncture in mCMV latent mice, and cohorts were evaluated weekly for evidence of virus in BAL samples. mCMV-gB DNA became detectable in BAL in 36% of latent mice 1 week after bacterial sepsis induction and 82% by week 1. All mice (100%) were BAL positive for mCMV-gB DNA by week 3 post-sepsis, but by week 4 mCMV-DNA were no longer detectable in BAL. Relative mCMV-DNA copy numbers were comparable in high and low-titer mice during sepsis (Fig. 1E). All mock mice were negative for CMV DNA at all time-points (not shown).
Curiously, in lung tissue of low and high titer mice mCMV-gB DNA decreased from latent levels during early sepsis (1 week) (P = 0.02 high titer, P = 0.01 low titer) (Fig. 1F). Lung tissue DNA loads recovered later during sepsis (3 weeks) to latency levels in both groups (P = 0.42 low titer, P = 0.30 high titer), but dropped again 4 weeks after sepsis onset during the maximal alveolar lymphocyte response (P = 0.02 high titer, P = 0.06 low titer).
Similar to the contraction previously observed in lung tissue during a septic stimulus [Campbell et al., 2012], alveolar CMV-specific T-cells decreased during early sepsis (1 week) from already low latent levels (P = 0.036 high titer, P = 0.025 low titer). During the ensuing 3 weeks, alveolar CMV-specific T-cells recovered, becoming higher than pre-sepsis levels by week 4 (P = 0.003) (Fig. 1G). Overall alveolar lymphocytes (CD4/CD8) showed a similar pattern, first contracting then rebounding to latency levels by week 4 (Fig. 1H). Notably, contractions of mCMV specific and general CD4/CD8 alveolar T-cells precede detection of mCMV DNA in septic mice, and rebounds in mCMV-specific and overall CD4/CD8 alveolar T-cells are coincident with BAL specimens becoming mCMV-DNA negative.
DISCUSSION
This study shows that bronchoalveolar lavage can be used to diagnose CMV replication in immune competent hosts. Specifically, we have found that CMV DNA are quantifiable in BAL cells during acute infection and after reactivation stimuli. Perhaps more importantly, CMV DNA are not quantifiable in BAL from healthy hosts during latent infection. It is notable that there are few alveolar CMV-specific T-cells present during latency, but these become numerous during acute infection and after a reactivation trigger. Together these data show dynamic changes in the alveolar compartment during primary infection, latency, and bacterial sepsis that can be monitored as a surrogate for viral replication.
As previously mentioned, a number of recent studies in immune competent hosts have assumed that detectable CMV-DNA in BAL or tracheal aspirates is evidence of CMV replication/reactivation [Heininger et al., 2001; Chilet et al., 2010; Smith et al., 2010; Heininger et al., 2011; Coisel et al., 2012; Doan et al., 2013; Escribano et al., 2013; Friedrichs et al., 2013; Cinel et al., 2014]. Unfortunately, there are few data evaluating healthy immune competent seropositive hosts, and none during the era of quantitative PCR. It is well known that alveolar macrophages are the predominant cell type in BAL during health, a finding that we confirmed in latently infected mice (not shown). Because monocyte/macrophages are known to harbor latent virus [Taylor-Wiedeman et al., 1994; Reeves and Sinclair, 2013; Poole et al., 2015], we were concerned that BAL for CMV-DNA might be persistently positive, even during latency. The primary purpose of the current study was therefore to determine the presence of CMV DNA in BAL during acute infection, latency, and after a reactivation stimulus.
Using our murine model, we found that CMV DNA in BAL may indeed be a good clinical indicator of CMV replication. mCMV DNA are easily detected in BAL-cells during acute infection and after a reactivation challenge. In BAL specimens, mCMV DNA are detectable 1 week after primary infection, and remain detectable for at least 3 weeks after infection onset. In contrast, large cohorts of healthy mice show that CMV DNA are not detectable in any BAL specimens during latency. After using the previously described reactivation trigger (bacterial sepsis) [Cook et al., 2002] mCMV DNA once again become detectable in BAL for 7–21 days. This finding is consistent with results in critically ill humans [Chilet et al., 2010; Heininger et al., 2001, 2011]. In the current report, 4 weeks after sepsis onset mCMV DNA become undetectable in BAL consistent with resumption of latency.
Accompanying these BAL DNA changes are significant changes in BAL cellular composition that also support the hypothesis that BAL-DNA are indicative of viral replication. Dramatic increases in CD8+ cells were seen during acute infection that mirror DNA levels. The overall CD4 and CD8 responses were significantly larger than CMV-specific responses, however only immunodominant CD8 epitopes, m164, and m123, were evaluated. We suspect that many of the tetramer-negative CD8 and CD4 cells recruited to alveoli during acute infection are specific to other CMV epitopes, but this will require future confirmation. It is interesting that alveolar CD8 responses to acute infection seem dependent on the infecting titer of mCMV, but that by onset of latency the infecting titer is not distinguishable by the number of CMV-specific T-cells harbored in the alveolar compartment.
In the current report contraction of alveolar CMV-specific T-cells preceded detection of CMV-DNA in the alveolar space, and conversely recovery of these same cells was associated with disappearance of CMV-DNA from the alveolar space. Such memory contraction and recovery has been previously associated with viral reactivation [Campbell et al., 2012]. These same associations have been suggested by studies in immune competent patients, where low CMV-specific T-cell counts have been associated with reactivation [Clari et al., 2013], and expansion of these cells with viral control [von Muller et al., 2007; Chilet et al., 2010; Blanquer et al., 2011; Clari et al., 2013]. Similarly, alveolar CMV-specific T-cells have been recently shown to play a significant role in antiviral responses to replicating virus in transplant patients [Pipeling et al., 2008; Akulian et al., 2013], so it seems logical that these mCMV specific T-cells are dynamically responding to virus replication. Although, previous work has suggested that sepsis can activate CMV specific T-cells [Forster et al., 2010], it is possible that transient contraction of these cells during sepsis might also be accompanied by some functional compromise, a hypothesis that will require future testing.
The source of CMV DNA in alveoli during acute infection and sepsis is currently not known. It is possible that CMV is simply “shed” into the alveolar secretions as a consequence of cell lysis, similar to viral shedding in saliva, and urine. Surprisingly, viral loads in lung tissues drop significantly during early sepsis as compared to latency. It is interesting to speculate that cells harboring latent virus, such as monocytes/macrophages, might migrate into alveoli during sepsis, and thence be retrieved during BAL. Such migration during sepsis is associated with monocyte activation/differentiation, which is known to allow permissive infection [Taylor-Wiedeman et al., 1994; Soderberg-Naucler et al., 1997, 2001; Poole et al., 2015]. It is also very intriguing that the same inflammatory stimuli that accompany sepsis have also recently been shown to enhance CMV reactivation in these cells [Reeves and Sinclair, 2013]. More conclusive proof of the BAL-DNA hypothesis however would require demonstration of lytic virus or at the very least RNA in alveoli or lung tissue, which for now may escape our sensitivity of detection.
We have previously shown that bacterial sepsis causes pulmonary transcriptional reactivation 2–3 weeks after onset [Cook et al., 2002] and therefore attempted in the current study to correlate mCMV RNA with DNA from BAL specimens. Although, we were able to detect CMV RNA in BAL samples very early after acute high titer infection (not shown), we were unable to quantitate RNA in lung tissue during acute infection, nor could we detect RNA in BAL or lung tissue during sepsis. We suspect that this is consequent to lower sensitivity of quantitative methods used in the current report, because our previous reactivation studies have required nested RT-PCR for mCMV RNA detection during sepsis [Cook et al., 2002, 2006; Campbell et al., 2012]. In addition, our previous studies evaluated whole lung, and the current study divided lungs into BAL and BAL free tissue, which might simply reduce already very low RNA concentrations into separate fractions. Nevertheless, given our previously shown association between sepsis and transcriptional reactivation, and the cellular changes observed during acute infection and reactivation in the current study, it seems likely that appearance of mCMV-DNA in alveoli of latently infected hosts is most consistent with pulmonary reactivation. By extension regularly monitoring BAL viral DNA during critical illness should give a good indication of virus quiescence or reactivation.
Finally, the current study suggests that the most sensitive compartment to detect CMV in BAL specimens is the cellular fraction. Evaluation of whole BAL without centrifugation during acute infections allowed poor and inconsistent DNA detection. Only BAL cell pellets allowed DNA detection after low titer or high titer infections and during sepsis. It is important to note that only small volumes of BALF are recoverable in mice (average 2.3 ml of 3 ml initially instilled), and consequently small numbers of cells (average 1.31E +05 cells per mouse) are recovered. In contrast, human lavage typically allows recovery of more cells and volume, which could possibly increase sensitivity in humans. Recent work with human alveolar macrophages (AM), however, has shown that similar to our model, hCMV DNA can only be detected from purified AM cells using nested qualitative PCR in healthy subjects [Poole et al., 2015]. We therefore expect that similar to mice, quantitative PCR for hCMV-DNA would be negative during latency in humans, but validation in healthy seropositive patients will be required.
In conclusion, we have shown that the alveolar compartment undergoes dynamic changes between primary infection, latency, and during transcriptional reactivation of cytomegalovirus. The detection of DNA in alveolar cells in our model correlates with suspected virus replication and importantly CMV-DNA are not quantifiable during latency. Moreover, expansion, contraction, and subsequent re-expansion of alveolar mCMV-specific T cells during acute infection, latency, and sepsis correlate with viral DNA detection, further supporting this hypothesis. Taken together, these data suggest that presence of CMV-DNA in bronchoalveolar lavage specimens is a useful indicator of viral replication in immunocompetent hosts.
Acknowledgments
We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC I tetramers.
Grant sponsor: National Institutes of Health Awards; Grant numbers: T32-CA090223; RO1- GM 066115
Footnotes
Disclosure statement: The authors have no conflicts of interest to report.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Akulian JA, Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. High-quality CMV-specific CD4+ memory is enriched in the lung allograft and is associated with mucosal viral control. Am J Transplant. 2013;13:146–156. doi: 10.1111/j.1600-6143.2012.04282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquer J, Chilet M, Benet I, Aguilar G, Munoz-Cobo B, Tellez A, Costa E, Bravo D, Navarro D. Immunological insights into the pathogenesis of active CMV infection in non-immunosup-pressed critically ill patients. J Med Virol. 2011;83:1966–1971. doi: 10.1002/jmv.22202. [DOI] [PubMed] [Google Scholar]
- Bordes J, Maslin J, Prunet B, d’Aranda E, Lacroix G, Goutorbe P, Dantzer E, Meaudre E. Cytomegalovirus infection in severe burn patients monitoring by real-time polymerase chain reaction: A prospective study. Burns. 2011;37:434–439. doi: 10.1016/j.burns.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Campbell J, Trgovcich J, Kincaid M, Zimmerman PD, Klenerman P, Sims S, Cook CH. Transient CD8-memory contraction: A potential contributor to latent cytomegalovirus reactivation. J Leukoc Biol. 2012;92:933–937. doi: 10.1189/jlb.1211635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiche L, Forel JM, Roch A, Guervilly C, Pauly V, Allardet-Servent J, Gainnier M, Zandotti C, Papazian L. Active cytomegalo-virus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. 2009;37:1850–1857. doi: 10.1097/CCM.0b013e31819ffea6. [DOI] [PubMed] [Google Scholar]
- Chilet M, Aguilar G, Benet I, Belda J, Tormo N, Carbonell JA, Clari MA, Costa E, Navarro D. Virological and immunological features of active cytomegalovirus infection in nonimmunosup-pressed patients in a surgical and trauma intensive care unit. J Med Virol. 2010;82:1384–1391. doi: 10.1002/jmv.21825. [DOI] [PubMed] [Google Scholar]
- Cinel G, Pekcan S, Özçelik U, Alp A, Yalçin E, Doğru Ersöz D, Kiper N. Cytomegalovirus infection in immunocompetent wheezy infants: The diagnostic value of CMV PCR in bronchoalveolar lavage fluid. J Clin Pharm Ther. 2014;39:399–403. doi: 10.1111/jcpt.12169. [DOI] [PubMed] [Google Scholar]
- Clari MA, Aguilar G, Benet I, Belda J, Giménez E, Bravo D, Carbonell JA, Henao L, Navarro D. Evaluation of cytomegalovirus (CMV)-specific t-cell immunity for the assessment of the risk of active CMV infection in non-immunosuppressed surgical and trauma intensive care unit patients. J Med Virol. 2013;85:1802–1810. doi: 10.1002/jmv.23621. [DOI] [PubMed] [Google Scholar]
- Coisel Y, Bousbia S, Forel J-M, Hraiech S, Lascola B, Roch A, Zandotti C, Million M, Jaber S, Raoult D, Papazian L. Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS ONE. 2012;7:e51340. doi: 10.1371/journal.pone.0051340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cook C, Zhang X, McGuinness B, Lahm M, Sedmak D, Ferguson R. Intra-abdominal bacterial infection reactivates latent pulmonary cytomegalovirus in immunocompetent mice. J Infect Dis. 2002;185:1395–1400. doi: 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- Cook CH, Martin LC, Yenchar JK, Lahm MC, McGuinness B, Davies EA, Ferguson RM. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. 2003;31:1923–1929. doi: 10.1097/01.CCM.0000070222.11325.C4. [DOI] [PubMed] [Google Scholar]
- Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, tumor necrosis factor alpha, or inter-leukin-1{beta} triggers reactivation of latent cytomegalovirus in immunocompetent mice. J Virol. 2006;80:9151–9158. doi: 10.1128/JVI.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CH, Yenchar JK, Kraner TO, Davies EA, Ferguson RM. Occult herpes family viruses may increase mortality in critically ill surgical patients. Am J Surg. 1998;176:357–360. doi: 10.1016/s0002-9610(98)00205-0. [DOI] [PubMed] [Google Scholar]
- Desachy A, Ranger-Rogez S, Francois B, Venot C, Traccard I, Gastinne H, Denis F, Vignon P. Reactivation of human herpesvirus type 6 in multiple organ failure syndrome. Clin Infect Dis. 2001;32:197–203. doi: 10.1086/318474. [DOI] [PubMed] [Google Scholar]
- Doan TT, Phung TT, Pham HV, Pham SH, Nguyen LT. Effect of ganciclovir for the treatment of severe cytomegalovirus-associated pneumonia in children without a specific immuno-compromised state. BMC Infect Dis. 2013;13:424. doi: 10.1186/1471-2334-13-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domart Y, Trouillet JL, Fagon JY, Chastre J, Brun-Vezinet F, Gibert C. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery [see comments] Chest. 1990;97:18–22. doi: 10.1378/chest.97.1.18. [DOI] [PubMed] [Google Scholar]
- Escribano A, Chilet M, Clari MA, Lucas R, Costa E, Bravo D, Munoz-Cobo B, Borras R, Navarro D. Frequent detection of cytomegalovirus (CMV) DNA in the lower respiratory tract in CMV-seropositive pediatric patients with underlying chronic bronchopulmonary diseases lacking canonical immunosuppression. J Med Virol. 2013;85:888–892. doi: 10.1002/jmv.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajac A, Vidaud M, Lebargy F, Stephan F, Ricci S, Geslin P, Goossens M, Bernaudin JF. Evaluation of human cytomegalovirus latency in alveolar macrophages. Am J Respir Crit Care Med. 1994;149:495–499. doi: 10.1164/ajrccm.149.2.8306052. [DOI] [PubMed] [Google Scholar]
- Forster MR, Trgovcich J, Zimmerman P, Chang A, Miller C, Klenerman P, Cook CH. Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice. Antiviral Res. 2010;85:496–503. doi: 10.1016/j.antiviral.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantzeskaki FG, Karampi ES, Kottaridi C, Alepaki M, Routsi C, Tzanela M, Vassiliadi DA, Douka E, Tsaousi S, Gennimata V, Ilias I, Nikitas N, Armaganidis A, Karakitsos P, Papaevangelou V, Dimopoulou I. Cytomegalovirus reactivation in a general, nonimmunosuppressed intensive care unit population: Incidence, risk factors, associations with organ dysfunction, and inflammatory biomarkers. J Crit Care. 2015;30:276–281. doi: 10.1016/j.jcrc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Friedrichs I, Bingold T, Keppler OT, Pullmann B, Reinheimer C, Berger A. Detection of herpesvirus EBV DNA in the lower respiratory tract of ICU patients: A marker of infection of the lower respiratory tract? Med Microbiol Immunol. 2013;202:431–436. doi: 10.1007/s00430-013-0306-1. [DOI] [PubMed] [Google Scholar]
- Heininger A, Haeberle H, Fischer I, Beck R, Riessen R, Rohde F, Meisner C, Jahn G, Koenigsrainer A, Unertl K, Hamprecht K. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care. 2011;15:R77. doi: 10.1186/cc10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heininger A, Jahn G, Engel C, Notheisen T, Unertl K, Hamprecht K. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. 2001;29:541–547. doi: 10.1097/00003246-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Holtappels A, Pahl-Seigert M, Thomas D, Reddehase M. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L10 memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtappels R, Thomas D, Podlech J, Reddehase MJ. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J Virol. 2002;76:151–164. doi: 10.1128/JVI.76.1.151-164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishioka H, Sanui M, Tsutsumi Y, Yanase F, Shiotsuka J. Low prevalence of active cytomegalovirus infection in a cardiovascular intensive care unit. J Inten Care. 2014;2:12. doi: 10.1186/2052-0492-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber S, Chanques G, Borry J, Souche B, Verdier R, Perrigault P-F, Eledjam J-J. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005;127:233–241. doi: 10.1378/chest.127.1.233. [DOI] [PubMed] [Google Scholar]
- Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infections in non-immunosuppressed ICU patients. Crit Care Med. 2009;37:2350–2358. doi: 10.1097/CCM.0b013e3181a3aa43. [DOI] [PubMed] [Google Scholar]
- Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: Continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- Kutza AS, Muhl E, Hackstein H, Kirchner H, Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998;26:1076–1082. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang M-L, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Roa P, Perez-Granda MJ, Munoz P, Catalan P, Alonso R, Sanchez-Perez E, Novoa E, Bouza E. A prospective monitoring study of cytomegalovirus infection in non-immunosuppressed critical heart surgery patients. PLoS ONE. 2015;10:e0129447. doi: 10.1371/journal.pone.0129447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield S, Grieβl M, Gutknecht M, Cook C. Sepsis and cytomegalovirus: Foes or conspirators? Medical Microbiology and Immunology. 2015:1–7. doi: 10.1007/s00430-015-0407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- Ong DY, Spitoni C, Klein Klouwenberg PC, Verduyn Lunel F, Frencken J, Schultz M, van der Poll T, Kesecioglu J, Bonten MM, Cremer O. Cytomegalovirus reactivation and mortality in patients with acute respiratory distress syndrome. Intensive Care Medicine. 2015:1–9. doi: 10.1007/s00134-015-4071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl-Seibert M-F, Juelch M, Podlech J, Thomas D, Deegen P, Reddehase MJ, Holtappels R. Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. J Virol. 2005;79:5400–5413. doi: 10.1128/JVI.79.9.5400-5413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian L, Fraisse A, Garbe L, Zandotti C, Thomas P, Saux P, Pierrin G, Gouin F. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996;84:280–287. doi: 10.1097/00000542-199602000-00005. [DOI] [PubMed] [Google Scholar]
- Pipeling MR, West EE, Osborne CM, Whitlock AB, Dropulic LK, Willett MH, Forman M, Valsamakis A, Orens JB, Moller DR, Lechtzin N, Migueles SA, Connors M, McDyer JF. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol. 2008;181:546–556. doi: 10.4049/jimmunol.181.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole E, Juss JK, Krishna B, Herre J, Chilvers ER, Sinclair J. Alveolar macrophages isolated directly from human cytomegalovirus (HCMV)-seropositive individuals are sites of HCMV reactivation in vivo. J Infect Dis. 2015;211:1936–1942. doi: 10.1093/infdis/jiu837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razonable RR, Fanning C, Brown RA, Espy MJ, Rivero A, Wilson J, Kremers W, Smith TF, Paya CV. Selective reactivation of human herpesvirus 6 variant a occurs in critically ill immunocompetent hosts.[see comment] J Infect Dis. 2002;185:110–113. doi: 10.1086/324772. [DOI] [PubMed] [Google Scholar]
- Reddehase MJ, Keil GM, Koszinowski UH. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984;14:56–61. doi: 10.1002/eji.1830140111. [DOI] [PubMed] [Google Scholar]
- Reeves MB, Sinclair JH. Circulating dendritic cells isolated from healthy seropositive donors are sites of human cytomegalo-virus reactivation in vivo. J Virol. 2013;87:10660–10667. doi: 10.1128/JVI.01539-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckert CK, Griessl M, Buttner JK, Freitag K, Lemmermann N, Hummel M, Liu XF, Abecassis M, Angulo A, Messerle M, Cook CH, Reddehase M. Immune surveillance of cytomegalovirus latency and reactivation in murine models: link to memory inflation. In: Reddehase MJ, editor. Cytomegaloviruses. Norfolk, UK: Caister Academic Press; 2013. pp. 374–416. [Google Scholar]
- Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- Smith CA, Conroy LT, Pollock M, Ruddy J, Binning A, McCruden EA. Detection of herpes viruses in respiratory secretions of patients undergoing artificial ventilation. J Med Virol. 2010;82:1406–1409. doi: 10.1002/jmv.21794. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Turula H, Snyder CM. Systemic hematogenous maintenance of memory inflation by MCMV infection. PLoS Pathog. 2014;10:e1004233. doi: 10.1371/journal.ppat.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB. Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS Pathog. 2011;7:e1002295. doi: 10.1371/journal.ppat.1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest. 1997;100:3154–3163. doi: 10.1172/JCI119871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Streblow DN, Fish KN, Allan-Yorke J, Smith PP, Nelson JA. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J Virol. 2001;75:7543–7554. doi: 10.1128/JVI.75.16.7543-7554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan F, Meharzi D, Ricci S, Fajac A, Clergue F, Bernaudin JF. Evaluation by polymerase chain reaction of cytomegalovirus reactivation in intensive care patients under mechanical ventilation. Intensive Care Medicine. 1996;22:1244–1249. doi: 10.1007/BF01709343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons P, Sinclair J. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol. 1994;68:1597–1604. doi: 10.1128/jvi.68.3.1597-1604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T, Vadonis R, Kuehn J, Eing BR, Senninger N, Haier J. Viral reactivation is not related to septic complications after major surgical resections. APMIS. 2008;116:292–301. doi: 10.1111/j.1600-0463.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- von Muller L, Klemm A, Durmus N, Weiss M, Suger-Wiedeck H, Schneider M, Hampl W, Mertens T. Cellular immunity and active human cytomegalovirus infection in patients with septic shock. J Infect Dis. 2007;196:1288–1295. doi: 10.1086/522429. [DOI] [PubMed] [Google Scholar]
- von Muller L, Klemm A, Weiss M, Schneider M, Suger-Wiedeck H, Durmus N, Hampl W, Mertens T. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. 2006;12:1517–1522. doi: 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, Pachot A, Brooks TL, Deych E, Shannon WD, Green JM, Storch GA, Hotchkiss RS. Reactivation of multiple viruses in patients with sepsis. PLoS ONE. 2014;9:e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann M, Sedemund-Adib B, Reiland P, Schmucker P, Hennig H. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Crit Care Med. 2008;36:3145–3150. doi: 10.1097/CCM.0b013e31818f3fc4. [DOI] [PubMed] [Google Scholar]