Abstract
TR3 is an orphan member of the steroid/thyroid/retinoid nuclear receptor superfamily of transcription factors and it plays a pivotal role in regulating cell growth and apoptosis. The expression and function of TR3 in skin have not been well investigated. Using a cDNA expression assay, we discover that TR3 is significantly enriched in human telogen bulge compared with anagen bulb. Immunohistochemical staining confirms that TR3 is highly expressed in the bulge region of human hair follicles and it colocalizes with cytokeratin 15 (K15), an epithelial stem cell marker. To study the function of TR3 in the effect of androgens in keratinocytes, we treat HaCaT keratinocytes and primary human keratinocytes with dihydrotestosterone (DHT) and testosterone (T). The treated keratinocytes show a dose-dependent growth reduction to DHT and T. DHT increases the expression of TR3 in keratinocytes, associated with a concomitant increase of BAD and decrease of Bcl-2 expression. Knockdown TR3 expression by siRNA blocks the inhibitory effect of DHT on keratinocyte proliferation. Our results demonstrate that TR3 is localized to the stem cell compartment in the human hair follicles. Androgen increases TR3 expression in cultured keratinocytes. Our data suggest that TR3 mediates at least part of the inhibitory effect of androgens on keratinocytes.
Hair follicle (HF) is composed of epithelial cells, enclosing in its base the mesenchyme-derived dermal papilla. Hair growth is a highly regulated cyclical process and each HF perpetually goes through three stages: growth (anagen), regression (catagen), and rest (telogen). After telogen, a HF re-enters anagen, a new hair is formed, and the old one shed.1 Hair regrowth requires a distinct population of epithelial stem cells that reside within the bulge area,2 and these stem cells are cytokeratin 15 (K15) positive.3 The bulge consists of a subpopulation of outer root sheath (ORS) cells located in the upper portion of ORS near the insertion of the arrector pili muscle.2,4 The cyclic hair growth requires intricate balance among cell proliferation, differentiation, and apoptosis. Several pathways have been discovered in the regulation of HF growth and regression, such as the apoptotic pathway.5,6 Among them, the Bcl-2 family members are intensely studied. Bcl-2, an apoptosis inhibitor, and Bax, an apoptosis promoter, are strictly hair cycle dependent.7
Androgenetic alopecia (AGA), the most common cause of hair loss, is characterized by a marked decrease in HF size.8 Androgen is thought to be responsible for the gradual miniaturization of genetically susceptible HFs by shortening the duration of the anagen growth phase and reducing the cellular hair matrix volume.9 In susceptible HFs of the scalp, dihydrotestosterone (DHT) binds to the androgen receptor (AR), and then the hormone–receptor complex activates the genes responsible for the transformation of large, terminal follicles to miniaturized follicles.10 The 5α-reductase II inhibitors, which block conversion of testosterone (T) to its more active form, DHT, delay the progression of AGA.10 A few studies show that androgens act through the dermal papilla on follicular epithelial cells by altering the regulatory paracrine factors involved in the differentiation and proliferation of epithelial stem cells.11 However, AR has been demonstrated to be expressed in the HF ORS.12,13 Furthermore, the expression of AR and two 5α-reductase isozymes are significantly higher in the ORS of the HFs in AGA patients’ scalp biopsies, suggesting an important role of ORS in androgen-induced hair miniaturization.9 Nevertheless, the mechanisms underlying the direct effects of androgen on HF epithelial cells have not been well studied.
TR3, a human homolog of mouse Nur77, is an orphan member of the steroid/thyroid/retinoid nuclear receptor superfamily of transcription factors.14 As previously reported, TR3 is involved in diverse cellular activities, including cell proliferation, differentiation, and apoptosis.15 It has been shown to induce apoptosis in a number of cell linages exposed to proapoptotic stimuli by directly targeting the mitochondria and inducing cytochrome c release.15 It plays an important role during cell apoptosis by inducing a Bcl-2 conformational change.16 However, expression and function of TR3 in human skin are poorly understood.
In this study, we show that TR3 is expressed in the bulge region of human HFs and suprabasal layer of epidermis. TR3 expression in the bulge colocalizes with K15. In the cultured keratinocytes, TR3 expression is increased after treatment with DHT. Induction of TR3 expression by DHT is associated with an increased expression of BAD and decreased expression of Bcl-2. Knockdown of TR3 expression blocks DHT-mediated keratinocyte proliferation inhibition. Our results suggest that TR3 mediates at least part of the inhibitory effects of androgens on keratinocytes and it may have a potential role in the development of AGA.
MATERIALS AND METHODS
Tissue Preparation
The human scalps were obtained from the Cooperative Human Tissue Network and were fixed in PBS-buffered 10% formalin. The protocol was approved by the University of Pennsylvania Institutional Review Board. Anagen bulb and telogen bulge were isolated using a dissecting microscope. Total RNA was extracted from the anagen bulb and telogen bulge using Trizol (Life Technologies, USA).
Macroarray
Macroarrays were done as described previously.17 The Atlas Human cDNA Expression Array I (Clontech, USA) was used. This array was spotted in duplicate with 10 ng of PCR-amplified cDNA fragments. Then, 2 μg of total RNA from each sample was first reverse transcribed using the provided reagents and [a-32P]dCTP. The radioactively labeled cDNA probes were then hybridized to the membranes overnight. After washing with 2 × SCC three times, 1% SDS at 68 °C for 30 min, washing with 0.1 × SCC twice, 0.5% SDS at 68 °C for 30 min, the hybridization pattern was analyzed by autoradiography.
Isolation and Culture of Bulb Matrix Cells and Telogen Bulge Cells
Telogen bulges and anagen bulbs were collected and treated with trypsin to produce a single-cell suspension. The keratinocytes were plated with a feeder layer of 3T3-J2 mouse fibroblasts as previously described.18 Multiple colonies of keratinocytes were seen at day 14 and the colonies were allowed to expand for an additional 4 days. The keratinocytes were harvested by first removing the feeder layer with EDTA and then treated with trypsin-EDTA as previously described.18 A fraction of the cells were subcultured onto fresh 3T3-J2 feeder layers.
Quantitative Real-Time PCR
We used TaqMan probes and the Applied biosystems Prism 7700 Sequencing Detection System (Life Technologies) in Figure 1a. The TR3, GAPDH, and ribosomal 18S TaqMan probe and primer mix, as well as the TaqMan Universal PCR Master Mix, were purchased from Applied Biosystems. The TR3 primer sequences were as follows: 5′-ACCTGCTCCAGGAGGTTTGC-3′ and 5′-CGCTATGTGTTAAAAACGGTTTATCT-3′; the TaqMan probe sequence was 5′-TGACCCCA CGATTTGTCTTATCCCCC-3′. GAPDH was used as an internal control for RNA loading. RNA from prostate tissue, known to express TR3, was used to construct the concentration curve for TR3 and GAPDH. Each experiment was performed in triplicate at least three times.
Figure 1.
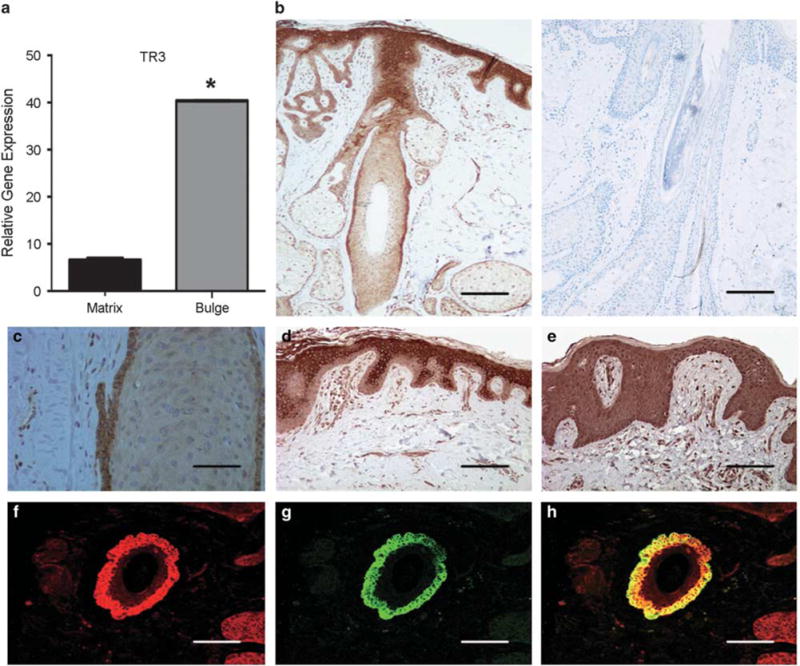
TR3 expression in human hair follicles. (a) qRT-PCR analysis of TR3 expression in anagen bulb matrix and telogen bulge. *P<0.05. Experiments were performed in triplicate at least three times. (b, left) Expression of TR3 protein in human hair follicle from scalp tissue by immunohistochemistry. (b, right) Negative control. Bar indicates 2 mm. (c) Immunostaining analysis of the expression of TR3 in bulge area of an anagen follicle. Bar indicates 50 μm. (d) Immunostaining analysis of the expression of TR3 in the suprabasal layer keratinocytes of the epidermis. Bar indicates 100 μm. (e) Immunostaining analysis of the diffuse expression of Nurr-1 in the epidermis. Bar indicates 100 μm. (f–h) Immunostaining analysis of the expression of TR3 protein in a telogen bulge visualized using red fluorescence (f), expression of K15 protein in the same telogen bulge using green fluorescence (g), and colocalized expression of TR3 and K15 in the telogen bulge (h). Bars indicate 50 μm.
Total RNAs were isolated using RNeasy Mini Kit (Qiagen, USA) and cDNAs were obtained using SuperScript III First-Stand Synthesis System (Life Technologies) in Figure 4c and d. Real-time PCR analyses were carried out in Bio-Rad machine using iQ SYBR Green Supermix (Bio-Rad, USA) and the following set of specific primers: TR3 (forward: 5′-GGCAGACGGGATAATGTGGT-3′, reverse: 5′-GGCTTG ATGAACTCAGGGGT-3′), Bcl-2 (forward: 5′-GGGGTCATG TGTGTGGAGAG-3′, reverse: 5′-CCCTGCAAATAACACAA GGGC-3′), BAD (forward: 5′-TGAGGTCCTGAGCCGACA-3′, reverse: 5′-CTTCCTCTCCCACCGTAGCG-3′), β-actin (forward: 5′-CACAGAGCCTCGCCTTTGC-3′, reverse: 5′-GCGC GGCGATATCATCATCC-3′).
Figure 4.
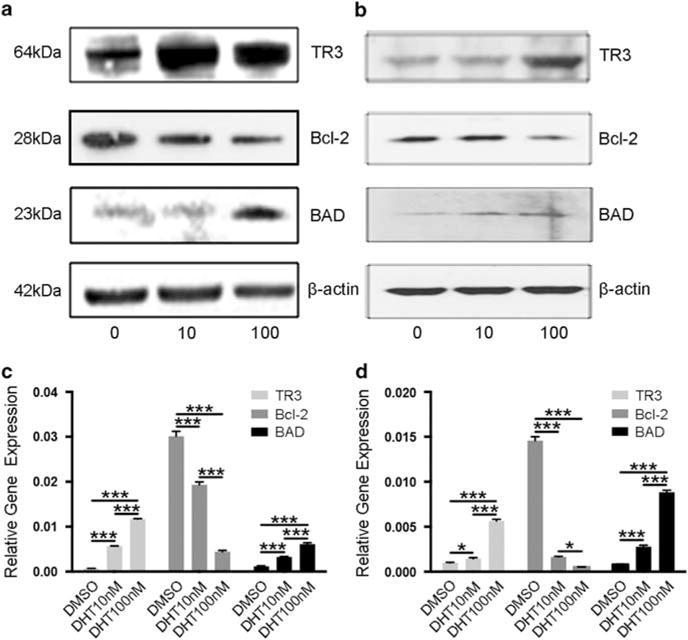
Upregulation of TR3 after DHT treatment. (a) Western blot analyses of TR3 protein expression in DHT-treated HaCaT cells. Cells were treated at 0, 10, and 100 nM, respectively. (b) Western blot analyses of TR3 protein expression in DHT-treated primary keratinocytes. Cells were treated at 0, 10, and 100 nM, respectively. Western blot analyses were performed in triplicate at least three times. (c) Transcriptional analysis of TR3 in DHT-treated HaCaT cells. Cells were treated at 0, 10, and 100 nM, respectively. (d) Transcriptional analysis of TR3 in DHT-treated primary keratinocytes. Cells were treated at 0, 10, and 100 nM, respectively. *P<0.05, ***P<0.001.
Immunohistochemistry and Immunofluorescence
Paraffin sections and cultured cells were stained with specific antibodies that were purchased from Santa Cruz Biotechnology and Dako. DAB or fluorescence was used to visualize antibody binding. The images were taken using a Canon digital camera or a laser scanning confocal microscope in the imaging core facility at the University of Pennsylvania.
Culture of Primary Human Keratinocytes and HaCaT Cells
Primary human keratinocytes were derived from neonatal foreskin19 and cultured as previously described.20 Human skin was incubated with Dispase (Life Technologies) at 4 °C overnight, and epidermal sheets were separated from the dermis. Single-cell suspensions were obtained by incubating in trypsin (Life Technologies) at 37 °C for 5 min. After neutralizing by fetal calf serum (Life Technologies), stratum corneum debris was removed. Isolated epidermal keratinocytes were maintained in keratinocyte serum-free medium (Life Technologies).
The spontaneously immortalized human keratinocytes line HaCaT cells (ATCC, USA) were grown in a modified DMEM/Ham’s F12 1:1 with 10% fetal calf serum, 100 U penicillin, and 100 μg streptomycin per ml.
Cell Viability Assay
Cell viability was assessed by Trypan blue exclusion test. Cells were plated at 5 × 104 cells/ml in triplicate. Cells were serum starved for 24 h before the addition of DHT, T, and DMSO (control) as indicated for additional 24 h, and then the cells were resuspended and 100 μl was removed for counting (mixed with 100 μl of Trypan blue solution (Sigma, USA)). Cells were counted on a hemocytometer and both live and dead (Trypan blue-positive) cells were scored. Each experiment was performed in triplicate at least three times.
MTT Assay
The number of viable cells was assessed using MTT assay (Roche, USA) according to the manufacturer’s instructions. Briefly, cells were seeded in 96-well plates at 5000 cells per well in a total volume of 100 μl culture medium and then incubated for 24 h. Cells were changed to serum free medium for 16 h and then exposed to DHT for 24 h at 37 °C. After the incubation period, 10 μl of the MTT labeling reagent was added to each well and plates were incubated at 37 °C for 4 h. At the end of the incubation period with dye, 100 μl of the Solubilization solution was added to each well. Then, the plates were incubated overnight in 37 °C. Spectrophotometrical absorbance of the samples was measured at 570 nM wavelength using a microplate reader (Bio-TEK, Winooski, VT, USA). Each experiment was performed in triplicate at least three times.
Western Blot Analysis
Specific primary antibodies were purchased from Santa Cruz Biotechnology, Cell Signaling Technology, and Dako. Protein concentration assay kit was obtained from Bio-Rad (USA). Proteins were separated on a Nu-PAGE (4–12% Bis-Tris Gel, Life Technologies) and subsequently transferred to a PVDF membrane (HybondTM-P, Amersham Biosciences, USA). Membranes were incubated with the primary antibodies specific for TR3 (sc-5569, Santa Cruz, USA, 1:200), BAD (9292, CST, USA, 1:1000), Bcl-2 (2876, CST, USA, 1:1000), and β-actin (A1978, Sigma, 1:5000) overnight at 4 °C, followed by incubation with corresponding HRP-conjugated secondary antibodies. Immunoreactive bands were visualized using chemiluminescence. Bands were scanned and intensities were quantified using a ChemiDoc XRS system (Bio-Rad).
Knockdown of TR3 Expression by RNA Interference The following Silencer siRNA Expression Cassettes were synthesized: TR3 siRNA cassette 1, 5′-AGACTACACAAATCT GTGCACTCCCCCAAGTCGGTGTTTCGTCCTTTCCACAA G-3′; and TR3 siRNA cassette 2, 5′-CTCCTACACAAAGAG GAGCATGGCTCCACTGCCGGTGTTTCGTCCTTTCCACAA G-3′. The cassettes were cloned into a Silencer express (human H1) vector according to manufacturer’s instructions (Life Technologies). A negative control was a scrambled siRNA that was supplied by the manufacture. The plasmids were transfected into cells using Oligofectamine reagent (Life Technologies). Stable transfection was achieved by selecting transfected HaCaT cells under G418 (50 μg/ml), and well-formed colonies were selected and expanded in vitro.
Statistical Analysis
All averages listed are mean ± s.e.m. Statistical analysis was performed using a SPSS 13.0 software package. Two-tailed Student’s t-test was used for comparisons between two independent groups. Analysis of comparisons among three or more independent groups was done by one-way ANOVA. For all statistical tests, a P-value of <0.05 was defined to be statistically significant.
RESULTS
TR3 Expression in Human HFs
To identify differentially expressed genes in bulge stem cells, we performed a cDNA macroarray study using RNAs isolated from human telogen bulge and anagen bulb and found that TR3 was one of the genes highly expressed in bulge area as compared with the bulb region. Quantitative real-time PCR was performed and confirmed that TR3 mRNA level in telogen bulge cells was sixfold higher than that in anagen matrix cells (Figure 1a). We performed immunostaining using an anti-TR3 antibody on paraffin-embedded human scalp tissue. We found that TR3 was highly expressed in the bulge region of HF (Figure 1b, c, and f). TR3 was also expressed in the suprabasal layer of epidermis (Figure 1d). On the contrary, Nurr1, another member of the NR4A subfamily of the nuclear receptors, was diffusely positive in the epidermis (Figure 1e) but negative in the bulge (data not shown). We then performed double immunohistochemical staining using antibodies against TR3 and K15, a well-known marker for bulge epithelial stem cells,3 and found that TR3 colocalized with K15 in the bulge (Figure 1g and h).
TR3 Expression in Cultured Bulge Keratinocytes
To study the expression of TR3 in the cultured bulge epithelial stem cells, we dissected out hair bulge region or anagen bulb region, disassociated cells, and cultured them as previously described.21 These cells formed discrete colonies in the culture (Figure 2a and b). Then, we performed immunocytochemistry and found that early passage bulge keratinocytes expressed both K15 and TR3, whereas the bulb keratinocytes expressed lower level of TR3 but not K15 (Figure 2c–f). However, after only several passages, bulge keratinocytes rapidly lost K15 expression with increased expression of TR3 (data not shown).
Figure 2.
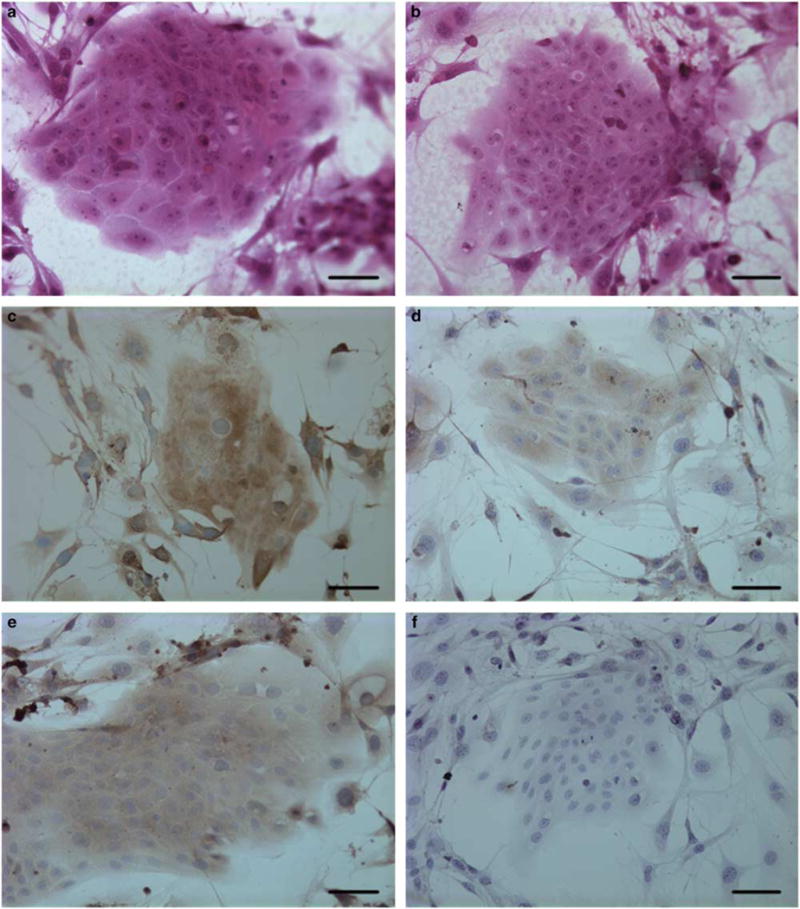
TR3 and K15 expression in cultured bulge keratinocytes. (a) Morphology of a keratinocyte colony derived from cell suspension of trypsinized telogen bulge follicles, stained with H&E. (b) Morphology of a keratinocyte colony derived from cell suspension of trypsinized hair matrix cells, stained with H&E. (c) Telogen bulge keratinocyte colonies stained with anti-TR3 antibody. (d) Hair matrix cell colonies stained with anti-TR3 antibody. (e) Telogen bulge keratinocyte colonies stained with anti-K15 antibody. (f) Hair matrix cell colonies stained with anti-K15 antibody. Bars indicate 25 μm. Representative images from three experiments are shown. H&E, hematoxylin and eosin.
DHT and T Inhibit Keratinocyte Growth
As bulge stem cell phenotypes are difficult to maintain in vitro, we used HaCaT cells and primary human keratinocytes for additional functional studies. Primary human keratinocytes were derived from neonatal foreskin as previously described.19 It has been shown that androgen receptor is expressed in HaCaT keratinocytes.22 To evaluate the effect of androgen on HaCaT keratinocytes, keratinocytes were serum starved and treated with DHT (100 nM) or T (100 nM) for 24 h, and cell viability assays were performed. As shown in Figure 3a, significant cell viability reduction (P<0.05) was observed in HaCaT cells treated with DHT (100 nM) or T (100 nM) compared with DMSO control. The inhibitory effects of DHT were more pronounced than T (Figure 3a). Similar inhibitory effects were also observed when primary keratinocytes were used (Figure 3b).
Figure 3.
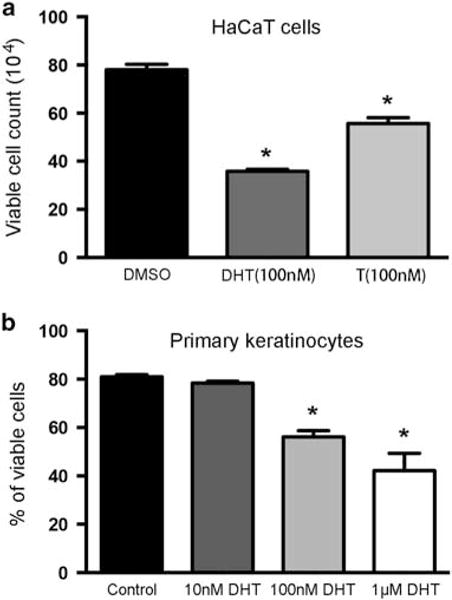
DHT and T inhibit keratinocyte growth. (a) Cell viability assay analysis of effects of DHT (100 nM) and T (100 nM) on HaCaT cells. (b) Cell viability assay analysis of effects of DHT (0, 10, 100, and 1000 nM) on cultured primary keratinocytes. *Statistical significance (P<0.05) compared with controls. Experiments were performed in triplicate at least three times.
TR3 Is Upregulated upon DHT or T Treatment
To study the potential role of TR3 in androgen-induced keratinocyte inhibition, we examined the RNA and protein expressions in serum-starved HaCaT cells and primary keratinocytes that were treated with DHT (10 or 100 nM) for 24 h. We found that DHT (10 or 100 nM) induced TR3 protein expression in both of these cell lines (Figure 4a and b). Previous studies have shown that TR3 interacts with the Bcl-2 family proteins and induces cell apoptosis. To further study the function of TR3 during apoptosis, we measured Bcl-2 and BAD expression in these cells. As shown in Figure 4a and b, Bcl-2 expression was decreased after DHT treatment, whereas BAD expression was increased (Figure 4a and b). We performed qRT-PCR analysis and the results further confirmed these observations. As shown in Figure 4c, in HaCaT cells, DHT induced dose-dependent increase of RNA expression of TR3 and BAD, and dose-dependent decrease of RNA expression of Bcl-2 significantly; similar results were observed in primary keratinocytes (Figure 4d).
TR3 Knockdown Blocks the Inhibitory Effect of DHT on Keratinocyte Proliferation
To test whether the effect of androgen on keratinocytes was mediated by TR3, we knocked down TR3 expression by siRNA using HaCaT cells. We employed two siRNAs and performed western blots to confirm that both siRNAs significantly decreased the expression of TR3 in transfected cells (Figure 5a). Cell proliferation was measured by MTT assays. As shown in Figure 5b, the growth rate of TR3 knockdown cells was similar to that of wild-type cells, but reduction of TR3 expression prevented DHT-medicated cell proliferation inhibition. These data indicate that TR3 mediates at least some of the inhibitory effects of androgen on keratinocytes.
Figure 5.
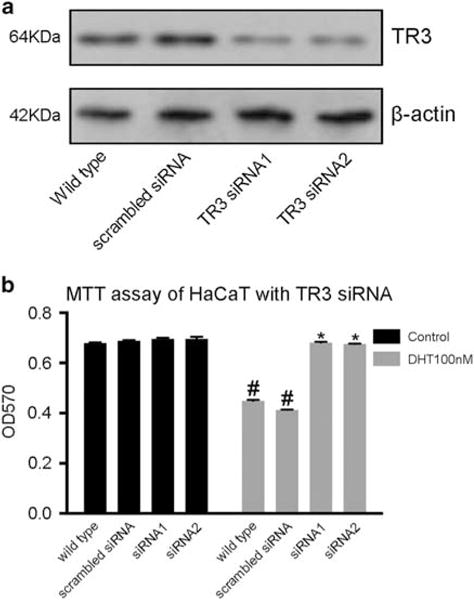
Knockdown of TR3 expression blocked the inhibitory effect of DHT on keratinocyte proliferation. (a) Western blot analyses of TR3 protein expression in control and TR3 knockdown cells. (b) Cell survival analysis of DHT-treated cells in control and TR3 knockdown cells. #P<0.05 comparing DHT-treated wild-type HaCaT cells with control; *P<0.05 comparing DHT-treated TR3 knockdown cells with DHT-treated wild-type cells. Experiments were performed in triplicate at least three times.
DISCUSSION
In this study, we demonstrate that TR3 is highly expressed in the bulge epithelial stem cell compartment of human HFs. DHT and T inhibit keratinocyte proliferation and induce TR3 expression. The effect of DHT on keratinocytes is prevented by knockdown of TR3 expression. These data suggest that TR3 plays a role in androgen-induced keratinocyte growth inhibition.
TR3 was first identified along with Nurr1 and Nor1 as an immediate early gene induced by nerve growth factor in PC12 cells.23 Although the precise physiological roles for TR3 are not fully understood, TR3 has been shown to have opposite biological functions in different types of cells: overexpression of TR3 leads to lung cancer cell proliferation;24 in contrast, in immature thymocytes, T-cell hybridomas,25 prostate,26 and stomach cancer cells,27 TR3 is required for apoptosis induction. However, the function of TR3 in human skin is poorly understood.
AGA is known to be driven by androgens, and DHT is believed to be the most important.28 DHT is generated from T by enzyme steroid 5α-reductase type I or type II. Levels of DHT, T, and DHT/T ratio in plasma from AGA patients are found to be higher than normal subjects.29 Plucked HFs and skin from bald scalps have been shown to contain higher levels of DHT than from normal scalps.29,30
In the present study, we show that TR3 is highly expressed in the suprabasal layer keratinocytes of epidermis, and its expression pattern is distinctively different from NURR1, suggesting that members of this family of proteins have different functions in keratinocytes. TR3 expression in the HFs coincides with K15, suggesting TR3 may have a physiological role in regulating bulge keratinocyte homeostasis. Short-term cultured bulge epithelial stem cells retain expression of K15 and TR3. Passaging of these cells in the culture results in loss of K15 expression with increased TR3 expression, and the result suggests that TR3 counteracts the proliferation in epithelial cells. As both suprabasal layer keratinocytes and bulge keratinocytes are nonproliferating cells in vivo, whereas induction of TR3 is known to be associated with apoptosis,26 we thus postulate that low-level expression of TR3 in the keratinocytes may be important in maintaining the nonproliferating state of keratinocytes.
We find that TR3 expression in HaCaT cells and primary keratinocytes is significantly induced by androgens, implying that TR3 mediates androgen-induced keratinocyte growth inhibition. This is supported by the results that knockdown of TR3 expression blocks the inhibitory effect of DHT on keratinocyte proliferation. In addition, we observe increased expression of BAD and decreased expression of Bcl-2 that coincide with TR3 upregulation upon DHT treatment. These data support the interaction of TR3 with Bcl-2 family proteins5 in keratinocytes, and suggest that an elevated level of TR3 inhibits keratinocyte growth. A recent study also shows that T and 5α-DHT induce apoptosis, and Bcl-2 protein expression is decreased in T-and 5α-DHT-treated human dermal papilla cells.31
In conclusion, we show for the first time that TR3 colocalizes with K15 in the bulge of human HFs and provide evidences that TR3 is involved in androgen-induced keratinocyte inhibition. TR3 may play a role in modulating keratinocyte proliferation and apoptosis. Further studies investigating interaction between androgen signaling pathway and TR3 may provide fresh insight into the development of AGA.
Acknowledgments
We thank Anatomic pathology test development laboratory in the Department of Pathology and Laboratory Medicine to provide histological assistance. This study was supported by the grants AR-054593 from the National Institute of Health to XX and research project no. 81271769 from the National Natural Science Foundation of China to MW.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 2.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 3.Lyle S, Christofidou-Solomidou M, Liu Y, et al. The C8/144b monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci. 1998;111(Pt 21):3179–3188. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- 4.Cotsarelis G, Kaur P, Dhouailly D, et al. Epithelial stem cells in the skin: definition, markers, localization and functions. Exp Dermatol. 1999;8:80–88. doi: 10.1111/j.1600-0625.1999.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 5.Botchkareva NV, Ahluwalia G, Shander D. Apoptosis in the hair follicle. J Invest Dermatol. 2006;126:258–264. doi: 10.1038/sj.jid.5700007. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs Y, Brown S, Gorenc T, et al. Sept4/ARTS regulates stem cell apoptosis and skin regeneration. Science. 2013;341:286–289. doi: 10.1126/science.1233029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller-Rover S, Rossiter H, Lindner G, et al. Hair follicle apoptosis and Bcl-2. J Investig Dermatol Symp Proc. 1999;4:272–277. doi: 10.1038/sj.jidsp.5640228. [DOI] [PubMed] [Google Scholar]
- 8.Garza LA, Yang CC, Zhao T, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121:613–622. doi: 10.1172/JCI44478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawaya ME, Price VH. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997;109:296–300. doi: 10.1111/1523-1747.ep12335779. [DOI] [PubMed] [Google Scholar]
- 10.Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964–973. doi: 10.1056/NEJM199909233411307. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa T, Matsuda K, Inui S, et al. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J Clin Endocrinol Metab. 2009;94:1288–1294. doi: 10.1210/jc.2008-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang T, Hoyer S, Yu R, et al. Immunocytochemical localization of androgen receptors in human skin using monoclonal antibodies against the androgen receptor. J Invest Dermatol. 1993;100:663–666. doi: 10.1111/1523-1747.ep12472330. [DOI] [PubMed] [Google Scholar]
- 13.Lachgar S, Charveron M, Sarraute J, et al. In vitro main pathways of steroid action in cultured hair follicle cells: vascular approach. J Investig Dermatol Symp Proc. 1999;4:290–295. doi: 10.1038/sj.jidsp.5640232. [DOI] [PubMed] [Google Scholar]
- 14.Uemura H, Mizokami A, Chang C. Identification of a new enhancer in the promoter region of human TR3 orphan receptor gene. A member of steroid receptor superfamily. J Biol Chem. 1995;270:5427–5433. doi: 10.1074/jbc.270.10.5427. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Yu H, Kumar SM, et al. Norcantharidin induces melanoma cell apoptosis through activation of TR3 dependent pathway. Cancer Biol Ther. 2011;12:1005–1014. doi: 10.4161/cbt.12.11.18380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin B, Kolluri SK, Lin F, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Lyle S, Liu Y, et al. Differential expression of cyclin D1 in the human hair follicle. Am J Pathol. 2003;163:969–978. doi: 10.1016/S0002-9440(10)63456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eming SA, Lee J, Snow RG, et al. Genetically modified human epidermis overexpressing PDGF-A directs the development of a cellular and vascular connective tissue stroma when transplanted to athymic mice– implications for the use of genetically modified keratinocytes to modulate dermal regeneration. J Invest Dermatol. 1995;105:756–763. doi: 10.1111/1523-1747.ep12325550. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen C, Thomas-Virnig C, Allen-Hoffmann BL. Classical human epidermal keratinocyte cell culture. Methods Mol Biol. 2013;945:161–175. doi: 10.1007/978-1-62703-125-7_11. [DOI] [PubMed] [Google Scholar]
- 20.Lebre MC, van der Aar AM, van Baarsen L, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 21.Guo Z, Draheim K, Lyle S. Isolation and culture of adult epithelial stem cells from human skin. J Vis Exp. 2011 doi: 10.3791/2561. pii: 2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J Invest Dermatol. 2001;116:793–800. doi: 10.1046/j.1523-1747.2001.01312.x. [DOI] [PubMed] [Google Scholar]
- 23.Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 24.Kolluri SK, Bruey-Sedano N, Cao X, et al. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol. 2003;23:8651–8667. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woronicz JD, Calnan B, Ngo V, et al. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Kolluri SK, Gu J, et al. Cytochrome C release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Liu S, Ye XF, et al. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis. 2002;23:1583–1592. doi: 10.1093/carcin/23.10.1583. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman KD. Androgens and alopecia. Mol Cell Endocrinol. 2002;198:89–95. doi: 10.1016/s0303-7207(02)00372-6. [DOI] [PubMed] [Google Scholar]
- 29.Bang HJ, Yang YJ, Lho DS, et al. Comparative studies on level of androgens in hair and plasma with premature male-pattern baldness. J Dermatol Sci. 2004;34:11–16. doi: 10.1016/j.jdermsci.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Schweikert HU, Wilson JD. Regulation of human hair growth by steroid hormones. I. Testerone metabolism in isolated hairs. J Clin Endocrinol Metab. 1974;38:811–819. doi: 10.1210/jcem-38-5-811. [DOI] [PubMed] [Google Scholar]
- 31.Winiarska A, Mandt N, Kamp H, et al. Effect of 5alpha-dihydrotestosterone and testosterone on apoptosis in human dermal papilla cells. Skin Pharmacol Physiol. 2006;19:311–321. doi: 10.1159/000095251. [DOI] [PubMed] [Google Scholar]


