Abstract
OBJECTIVE
Symptomatic intracranial atherosclerotic disease (ICAD) has a high risk of recurrent stroke. Genetic polymorphisms in CYP2C19 and CES1 are associated with adverse outcomes in cardiovascular patients, but have not been studied in ICAD. The authors studied CYP2C19 and CES1 single-nucleotide polymorphisms (SNPs) in symptomatic ICAD patients.
METHODS
Genotype testing for CYP2C19*2, *3, *8, *17 and CES1 G143E was performed on 188 adult symptomatic ICAD patients from 3 medical centers who were medically managed with clopidogrel and aspirin. Testing was performed prospectively at 1 center, and retrospectively from a DNA sample biorepository at 2 centers. Multiple logistic regression and Cox regression analysis were performed to assess the association of these SNPs with the primary endpoint, which was a composite of transient ischemic attack (TIA), stroke, myocardial infarction, or death within 12 months.
RESULTS
The primary endpoint occurred in 14.9% of the 188 cases. In multiple logistic regression analysis, the presence of the CYP2C19 loss of function (LOF) alleles *2, *3, and *8 in the medically managed patients was associated with lower odds of primary endpoint compared with wild-type homozygotes (odds ratio [OR] 0.13, 95% CI 0.03–0.62, p = 0.0101). Cox regression analysis demonstrated the CYP2C19 LOF carriers had a lower risk for the primary endpoint, with hazard ratio (HR) of 0.27 (95% CI 0.08–0.95), p = 0.041. A sensitivity analysis of a secondary composite endpoint of TIA, stroke, or death demonstrated a significant trend in multiple logistic regression analysis of CYP2C19 variants, with lower odds of secondary endpoint in patients carrying at least 1 LOF allele (*2, *3, *8) than in wild-type homozygotes (OR 0.27, 95% CI 0.06–1.16, p = 0.078). Cox regression analysis demonstrated that the carriers of CYP2C19 LOF alleles had a lower risk for the secondary composite endpoint (HR 0.22, 95% CI 0.05–1.04, p = 0.056).
CONCLUSIONS
This is the first study examining genetic variants and their effects in symptomatic ICAD. Variant alleles of CYP2C19 (*2, *3, *8) were associated with lower odds of the primary and secondary composite endpoints. However, the direction of the association was opposite of what is expected based on this SNP. This may reflect an incomplete understanding of this genetic variation and its effect in symptomatic ICAD and warrants further investigations.
Keywords: antiplatelet, intracranial atherosclerotic disease, intracranial stenosis, stroke, pharmacogenomics, transient ischemic attack, vascular disorders
Symptomatic intracranial atherosclerotic disease (ICAD) is the most common cause of stroke worldwide and is associated with a high risk of recurrent stroke.3,8 The results of the recently completed Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial suggest that aggressive medical management alone is superior to percutaneous transluminal angioplasty and stenting (PTAS) plus aggressive medical management in preventing stroke or death in patients with symptomatic ICAD.2,5 Even though the risk of stroke or death in the SAMMPRIS trial was lower than seen in the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial, this risk in either the PTAS group or the medically managed group is still unacceptably high, 23% in the PTAS group and 15% in the medically managed group.2,3,5
In both the PTAS and the medical arms of the SAMMPRIS trial, patients were treated with aspirin and clopidogrel dual-antiplatelet therapy reflecting current management of symptomatic ICAD. Genetic polymorphisms in drug-metabolizing enzymes such as cytochrome P450 (CYP) 2C19 and carboxylesterase 1 (CES1) have been shown to affect the antiplatelet activity of clopidogrel11,14 and have been associated with increased risk of cardiovascular death, myocardial infarction (MI), or stroke in patients with acute coronary syndrome17 and patients undergoing percutaneous coronary interventions.15 It therefore seems possible that failure of aspirin and clopidogrel therapy in preventing stroke or death in symptomatic ICAD patients could be due to genetic polymorphisms that inhibit the antiplatelet activity of clopidogrel.
We conducted this study to test our hypothesis that polymorphisms in CYP2C19 and CES1 affect the efficacy of aspirin and clopidogrel dual-antiplatelet therapy in patients with symptomatic ICAD. This is the first study of which we are aware that examines genetic variants and their effects on the efficacy of clopidogrel in symptomatic ICAD.
Methods
Symptomatic ICAD patients taking clopidogrel and aspirin were studied from 3 centers: the University of Florida, Vanderbilt University, and Marshfield Clinic. This study was approved by the institutional review boards of all 3 institutions. The following inclusion criteria were used to enroll/identify patients: age 18 years or older, stroke or transient ischemic attack (TIA) attributable to 50% or greater stenosis of a major intracranial artery, and treatment with aspirin and clopidogrel for 3 months or longer. Patients with moyamoya disease were excluded from this study.
At one of the 3 centers (University of Florida), patients were prospectively enrolled, and DNA samples were prospectively collected. At the other 2 centers (Vanderbilt University and Marshfield Clinic), patients were retrospectively identified from the electronic medical record using an ICD-9–based algorithm, followed by manual review of the electronic charts of each of the patients to verify accuracy, and DNA samples were obtained from a biorepository of stored patient samples.
Patients provided a sample of either saliva or blood. Patient samples from the University of Florida were genotyped at the University of Florida through the Sequenom, pyrosequencing (Qiagen) or Taqman (Life Technologies) genotyping method for the following variants: CYP2C19*2 (rs4244285, G > A), CYP2C19*3 (rs4986893, G > A), CYP2C19* 8 (rs41291556, T > C), CYP2C19*17 (rs12248560, G > A), and CES1 G143E (rs71647871, G > A).
Patient samples from Vanderbilt University and Marshfield clinic were genotyped at Vanderbilt University by the Vanderbilt Technologies for Advanced Genomics core with a combination of TaqMan Assay (rs12248560) and the Sequenom genotyping platform (rs4244285, rs4986893, rs41291556, rs71647871, rs12041331, rs662, rs854560). Genotyping efficiency was 100% in all samples, and genotypes conformed to expectations under Hardy-Weinberg equilibrium. Quality control also included inter- and intraplate replicate samples and HapMap controls showing 100% concordance and zero Mendelian errors.
The primary endpoint was a predefined composite of TIA, stroke, myocardial infarction, or death within 12 months of beginning clopidogrel. A sensitivity analysis of a secondary composite endpoint of TIA, stroke, or death within 12 months of beginning clopidogrel was also performed. Patients who were treated with PTAS and patients treated medically were analyzed separately, as we would expect a different rate of the primary endpoint between the 2 groups based on previous studies.2,5 After identification/enrollment of patients and collection of samples, the number of patients in the PTAS group with an event was too low to allow for meaningful conclusions. Therefore, PTAS-treated patients were excluded and only medically managed patients were included.
Statistical Analysis
Hardy-Weinberg equilibrium was evaluated within race/ethnicity using chi-square tests with 1 degree of freedom. CYP2C9 polymorphisms *2, *3, and *8 were combined for the number of loss of function (LOF) alleles (0, 1, or 2). Due to the low number of individuals with 2 copies of LOF alleles, patients with 1 or 2 copies of LOF alleles were combined as LOF allele carriers. Due to the functional basis of these variants, we performed the association analysis in the combined data set, adjusting for the race group. Multiple logistic regression was performed to assess the association of CYP2C19 polymorphisms or CES1 G143E genotype and the primary endpoint (the occurrence of TIA, stroke, myocardial infarction, or death within 12 months of initiation of clopidogrel therapy) and the secondary endpoint (the occurrence of TIA, stroke, or death within 12 months of initiation of clopidogrel therapy) adjusting for covariates: site, age, sex, race, hypertension, diabetes, taking lipid-lowering medication, and taking a proton pump inhibitor. A Kaplan-Meier curve was constructed by CYP2C19 LOF carrier status, and log-rank tests were performed to compare time to event by the carrier status. Multivariable Cox regression analysis was performed to assess the time to the primary and secondary endpoint. The covariates adjusted in the Cox regression analysis were the same as those used in the logistic regression analysis; p values < 0.025 were considered statistically significant because 2 genes were examined. All statistical analyses were performed in SAS 9.2.
Results
A total of 188 medically managed patients were included in the study. Their baseline demographic characteristics are presented in Table 1. The patients had an average age of 67 years; 63.3% were males and more than 84% were white. Of the 188 patients, 92.6% had a history of hypertension, and 93.1% were treated with lipid-lowering medications, while about 58% were taking a proton pump inhibitor.
TABLE 1.
Baseline demographic characteristics of 188 patients treated with medical management in 3 centers
| Characteristics | Value |
|---|---|
| Age (years), mean ± SD | 67.0 ± 12.2 |
| Male sex | 119 (63.3) |
| Race/ethnicity | |
| White | 159 (84.6) |
| Black | 24 (12.8) |
| Other | 5 (2.7) |
| History of smoking | 109 (58) |
| History of hypertension | 174 (92.6) |
| Diabetes | 88 (46.8) |
| Taking a lipid-lowering drug | 175 (93.1) |
| Taking a proton pump inhibitor | 109 (58) |
Data represented as number (%) unless stated otherwise.
The primary endpoint (TIA, stroke, MI, death) occurred in 14.9% of medically treated patients (28/188) (Table 2). The secondary endpoint (TIA, stroke, death) occurred in 11.2% of medically treated patients (21/188) (Table 2). The remainder of the patients did not have an event within the first 12 months after initiation of clopidogrel treatment.
TABLE 2.
Events in 188 medically managed patients
| Event | UF (n = 28) | Vanderbilt (n = 120) | Marshfield (n = 40) | Total (n = 188) |
|---|---|---|---|---|
| TIA | 6 | 0 | 4 | 10 |
| Stroke | 2 | 4 | 1 | 7 |
| MI | 0 | 2* | 7 | 9 |
| Death | 2 | 2 | 2 | 6 |
| Composite of TIA, stroke, MI, death | 8 | 6* | 14 | 28 |
| Composite of TIA, stroke, death | 8 | 6 | 7 | 21 |
UF = University of Florida.
The 2 patients at Vanderbilt who had an MI also had stroke.
The genotype frequencies of the single-nucleotide polymorphisms (SNPs) did not deviate from Hardy-Weinberg equilibrium when tested with the patients stratified by race or as a single group (all p values > 0.05). The allele frequencies of the 5 SNPs are shown in Table 3. In all the studied patients, 1 heterozygote for CYP2C19*3 and 1 heterozygote for *8 were observed. The allele frequencies for CYP2C19*2, CYP2C19*17, and CES1 G143E were 14%, 21%, and 0.017%, respectively, consistent with those reported in the 1000 genomes project. Due to the low allele frequency of CYP2C19*3 and *8, the number of CYP2C19 LOF alleles was calculated, combining these 2 variants with the *2 variant. Twenty-seven percent of the participants had 1 or 2 LOF CYP2C19 alleles.
TABLE 3.
Minor allele frequencies of the SNPs tested
| Chromosome | SNP | rs No. | Alleles (major/minor) | Minor Allele Frequency | |||
|---|---|---|---|---|---|---|---|
| White | Black | Other | Overall | ||||
| 10 | CYP2C19*2 | rs4244285 | C/T | 0.14 | 0.14 | 0.43 | 0.14 |
| 10 | CYP2C19*3 | rs4986893 | G/A | 0 | 0.015 | 0 | 0.002 |
| 10 | CYP2C19*8 | rs41291556 | T/C | 0.0026 | 0 | 0 | 0.002 |
| 10 | CYP2C19*17 | rs12248560 | C/T | 0.21 | 0.14 | 0.14 | 0.21 |
| 16 | CES1 G143E | rs71647871 | G/A | 0.016 | 0.015 | 0.14 | 0.017 |
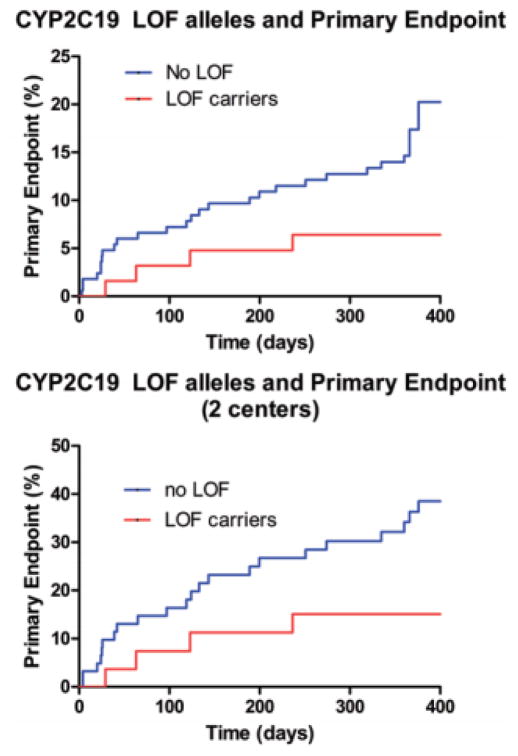
In the unadjusted analysis, none of the SNPs reached statistical significance (data not shown). After adjusting for covariates, in multiple logistic regression analysis, carriers of CYP2C19 LOF alleles *2, *3, *8 had lower odds of the primary endpoint in the medical group compared with wild-type homozygotes (OR 0.13, 95% CI 0.03–0.62, p = 0.01) (Table 4). A Kaplan-Meier curve by CYP2C19 LOF carrier status is shown in Fig. 1 (upper). The log-rank p value was 0.059.
TABLE 4.
Multiple logistic regression analysis results of the SNPs associated with the primary composite endpoint of TIA, stroke, MI, and death at all 3 centers*
| SNP | OR | 95% CI | p Value |
|---|---|---|---|
| Carrier of CYP2C19 LOF allele (*2, *3, or *8) | 0.13 | 0.03–0.62 | 0.01 |
| CYP2C19*17 | 0.94 | 0.39–2.28 | 0.89 |
| CES1 G143E | 5.54 | 0.63–49.03 | 0.12 |
The p values were adjusted for age, sex, race, site, hypertension, diabetes, taking lipid-lowering medication, and taking a proton pump inhibitor. The ORs in the PTAS group were not estimable due to low numbers.
Fig. 1.
Kaplan-Meier curves for the medically treated patients stratified by CYP2C19 loss of function (LOF) allele status in all 3 centers (upper) and 2 centers (lower).
Cox regression analysis showed that after adjusting for covariates, the risk for the primary endpoint was lower in the carriers of at least one CYP2C19 LOF allele compared with those with wild-type homozygotes, with a hazard ratio of 0.27 (95% CI 0.08–0.95, p = 0.041). The number of events in the PTSA group was too small to estimate the odds ratios and hazard ratios; therefore PTSA-treated patients were excluded from the analysis. A sensitivity analysis was performed of a secondary composite endpoint of TIA, stroke, or death. After adjusting for covariates, in multiple logistic regression analysis, carriers of CYP2C19 LOF alleles *2, *3, *8 had lower odds of the secondary composite endpoint in the medical group compared with wild-type homozygotes (OR 0.27, 95% CI 0.06–1.16, p = 0.078) (Table 5). Cox regression analysis demonstrated that the CYP2C19 LOF carriers had a lower risk for the secondary composite endpoint, with a hazard ratio of 0.22 (95% CI 0.05–1.04, p = 0.056).
TABLE 5.
Multiple logistic regression analysis results of the SNPs associated with the secondary composite endpoint of TIA, stroke, and death at all 3 centers
| SNP | OR | 95% CI | p |
|---|---|---|---|
| Carrier of CYP2C19 LOF allele (*2, *3, or *8) combined) | 0.27 | 0.06–1.16 | 0.078 |
| CYP2C19*17 | 1.95 | 0.77–4.93 | 0.16 |
| CES1 G143E | 8.25 | 0.94–72.08 | 0.056 |
A secondary analysis was performed excluding one of the retrospective centers (Vanderbilt) because the incidence of the endpoints at that center was disparate from the other 2 centers (University of Florida and Marshfield), which had very similar incidences of the endpoints. The secondary analysis included 93 patients, and the results were largely similar to the analysis that included all 3 centers. Multivariable logistic regression analysis demonstrated that the LOF variant alleles of CYP2C19 (*2, *3, *8) were associated with lower odds of primary endpoint in the medical group compared with wild-type homozygotes (OR 0.17, 95% CI 0.04–0.82, p = 0.0273). The Kaplan- Meier curve by CYP2C19 LOF allele carrier status is shown in Fig. 1 (lower). The log-rank p value was 0.0502. The adjusted hazard ratio for the primary endpoint was 0.23 (95% CI 0.06–0.85, p = 0.0268) for CYP2C19 LOF carriers compared with those with no LOF alleles.
Discussion
This is the first study of which we are aware that examines genetic variants and their effects on the efficacy of clopidogrel in symptomatic ICAD. In our group of medically treated patients, the *2, *3, *8 variant alleles of CYP2C19 were associated with lower odds of the primary endpoint (TIA, stroke, myocardial infarction, or death within 12 months of beginning clopidogrel) and the secondary endpoint (TIA, stroke, or death within 12 months of beginning clopidogrel) in symptomatic ICAD patients treated with aspirin and clopidogrel. However, this finding is antithetical to what we would expect for these variant alleles, which are LOF alleles; the expected finding would be higher odds of the primary and secondary endpoint. The direction of this correlation was unexpected given the well-known association between CYP2C19 genetic variation and increased risk of cardiovascular death, MI, or stroke in acute coronary syndrome patients17 and percutaneous coronary intervention patients.15 In terms of cerebrovascular disease, CYP2C19 polymorphisms seem to correlate with 3-month and 6-month modified Rankin Scale outcomes in Chinese stroke patients, although ICAD and the risk of recurrent stroke have not been specifically examined until this present study.10 Additionally, there may be an association between CYP2C19 LOF genotypes and in-stent restenosis in Chinese patients treated with vertebral artery stenting.12 More than 25 variant alleles have been reported for the CYP2C19 gene. CYP2C19*2,*3, and *8 are all LOF alleles, and CYP2C19*17 is a gain-of-function allele. The CYP2C19*2 SNP (rs4244285, G681A) in exon 5 results in a splice defect; CYP2C19*3 (rs4986893, G > A) in exon 4 produces a stop codon; CYP2C19*8 (rs41291556, T > C), a change of Trp120Arg in exon 3 that affects the catalytic activity of the CYP2C19 enzyme; and the gain-of-function CYP2C19*17 (rs12248560, G > A), which affects the the promoter region, increases the CYP2C19 gene expression level.1,4,6,7,9
There are many possible explanations for our observed findings. First, the heterogeneity of the various ways ICAD can lead to stroke or TIA, in comparison with coronary atherosclerotic disease, could suggest that features of ICAD differ from coronary atherosclerosis, and we may not observe the same findings in the 2 disease states.13 Second, it is possible that clopidogrel is not efficacious in preventing stroke in ICAD, and that the observed results in medically managed patients are due to other interventions, such as statin therapy, blood pressure reduction, smoking cessation, and diabetes management. Other studies, such as those in patients with atrial fibrillation receiving clopidogrel, have shown a lack of the expected genetic effect when the overall efficacy of the drug is lacking.16 Finally, it is important to note that our sample size in this study is relatively small, and the observed findings could be due to chance and lack of power to detect a true genetic effect.
Our analysis adjusted for a number of covariates, including factors that are known to affect clopidogrel response, such as proton pump inhibitor use, and factors that affect event rate such as hypertension.
Our results did not show an association between genetic variants of CES1 and the primary endpoint. Carboxylesterase 1 is a serine esterase that is the primary enzyme responsible for metabolizing clopidogrel, its intermediate metabolite (2-oxo-clopidogrel), and the final bioactive thiol metabolite into biologically inactive carboxylic acid derivatives.11 The G > A (rs71647871) variation in exon 4 of CES1 results in a nonconservative substitution, p.Gly143Glu, and in vitro functional studies have shown that the catalytic function of p.Gly143Glu is substantially impaired, resulting in a complete loss of hydrolytic activity of carboxylesterase 1 toward methylphenidate.18 Thus, the expected effect of CES1 G143E (rs71647871, G > A) polymorphism would be that it would cause reduced degradation of clopidogrel, leading to more of the drug being available to go down the bioactivation pathway, leading to enhanced antiplatelet activity of clopidogrel.11 There have been no prior studies examining CES1 G143E (rs71647871, G > A) polymorphism and clopidogrel efficacy in cardiovascular or stroke patients, thus the non-association may reflect an incomplete understanding of the clinical effects of CES1 genetic variation in cardiovascular disease and stroke, and warrants further investigation. Nevertheless, due to the very low incidence of CES1 genetic variation, our study was unable to demonstrate an association with CES1 variants and the primary and secondary composite endpoints.
Our study highlights the disparity in findings from a prospective study contrasted with a retrospective analysis from electronic medical records. One of the centers in this study (Vanderbilt), which conducted a retrospective analysis of data from electronic medical records, had incidences of the clinical endpoints that were significantly lower than expected for the natural history of the disease.2,3 The incidence of the clinical endpoints at this center was significantly different than at the other 2 centers, which had similar incidences for the endpoints. This was the reason we conducted a secondary analysis excluding this center. We believe this highlights a significant limitation of retrospective analysis for these types of studies. There has recently been great interest in genotypic and comparative analysis studies from electronic medical records. This enthusiasm for “big data” has led to significant interest in capturing clinical data from large electronic medical databases using billing and procedure codes. The advantage of these studies is the ability to capture large amounts of patient data across many centers. Many studies use an ICD-9-code–based query for the identification of patients and data, much like what was conducted at two of the centers in our study (Vanderbilt and Marshfield). In performing this study, however, we were clearly more confident in the ability in the prospective arm of the study to accurately identify patients who appropriately qualified according to all inclusion and exclusion criteria and the ability to accurately adjudicate clinical endpoints than in the retrospective arms of the study. We believe that this is a strong argument for funding and conducting prospective studies and a cause for cautionary concern regarding analysis of data from electronic medical records databases.
The combining of prospective data from 1 center with retrospective data from 2 centers was necessary to achieve an adequate sample size based on our prestudy power analysis. We took advantage of the biorepositories already established at two of the centers via the Pharmacogenomics Research Network (PGRN). However, there are obvious limitations of combining prospective data with retrospective data. Our finding of disparate incidences of the endpoints at one of the retrospective centers demonstrates some of the shortcomings of retrospective data collection and analysis. There has been great enthusiasm for comparative effectiveness research utilizing electronic medical records and “big data.” However, we feel that our study highlights the limitations of retrospective electronic capture of clinical data, which we believe does not match the scientific rigor of prospective enrollment and analysis. Therefore, we believe that it is critical to fund and implement prospective studies to address these questions.
In this study, we did not collect and analyze functional assays such as platelet function tests, platelet aggregation studies, thromboelastography, or P2Y12 receptor tests. The DNA samples from the 2 retrospective centers were collected from established biorepositories; thus the performance of platelet function assays was not possible. There are limitations of such platelet function tests such as the proper timing of these tests, variability among testing sites, and variability in interpretation. Nevertheless, in future investigations, it would be interesting to study the association of pharmacogenomic variations with these functional platelet assays.
Conclusions
While the findings of the recently completed SAMMPRIS trial2,5 suggest that medical management is superior to PTAS for patients with symptomatic ICAD, there are still subgroups of patients at unacceptably high risk of recurrent stroke despite aggressive medical management. Since the medical management of symptomatic ICAD is aspirin and clopidigrel dual antiplatelet therapy, it seems quite possible that patients with genetic variants predisposing to reduced antiplatelet efficacy of clopidogrel may be at higher risk of recurrent stroke. Defining these genetic variants and their association with the clinical efficacy of clopidogrel is important to the development of personalized therapeutic strategies for these high-risk patients. The findings of this study are a first step, and while we found that CYP2C19*2, *3, and *8 were associated with lower odds of the primary and secondary endpoints in the medical group compared with wild-type homozygotes, we do not completely understand this relationship or the mechanism of action. Due to the low incidence of CES1 genetic variation, our study was unable to demonstrate any association between CES1 variations and the primary and secondary endpoints. Further, larger-scale, prospective multicenter studies will be necessary to better elucidate these questions.
Acknowledgments
This study was funded by NIH U19 HL065962, NIH U01 GM074492, and the University of Florida Research Foundation.
ABBREVIATIONS
- CES1
carboxylesterase 1
- CYP
cytochrome P450
- ICAD
intracranial atherosclerotic disease
- LOF
loss of function
- MI
myocardial infarction
- PTAS
percutaneous transluminal angioplasty and stenting
- SAMMPRIS
Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis
- SNP
single-nucleotide polymorphism
- TIA
transient ischemic attack
- WASID
Warfarin-Aspirin Symptomatic Intracranial Disease
Footnotes
Disclosures
Dr. Mocco reports consultant relationships with Lazarus Effect, Reverse, Pulsar Edge Therapeutics, and Medina; an investor relationship with Blockade Medical; and being on the advisory board for Codman Neurovascular.
Author Contributions
Conception and design: Hoh, McDonough, Waters, Johnson. Acquisition of data: Hoh, Waters, Royster, Sheehan, Burkley, Mocco, Zuckerman, Mummareddy, Stephens, Ingram, Shaffer, Denny, Brilliant, Kitchner, Linneman, Roden, Johnson. Analysis and interpretation of data: Hoh, Gong, McDonough, Langaee, Johnson. Drafting the article: Hoh. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Hoh. Statistical analysis: Gong, Administrative/technical/material support: Hoh, Royster, Sheehan, Burkley, Ingram, Shaffer, Denny, Brilliant, Kitchner, Linneman, Roden, Johnson. Study supervision: Hoh.
References
- 1.Blaisdell J, Mohrenweiser H, Jackson J, Ferguson S, Coulter S, Chanas B, et al. Identification and functional characterization of new potentially defective alleles of human CYP2C19. Pharmacogenetics. 2002;12:703–711. doi: 10.1097/00008571-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 4.de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 5.Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383:333–341. doi: 10.1016/S0140-6736(13)62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41:913–958. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein JA, Blaisdell J. Genetic tests which identify the principal defects in CYP2C19 responsible for the polymorphism in mephenytoin metabolism. Methods Enzymol. 1996;272:210–218. doi: 10.1016/s0076-6879(96)72025-6. [DOI] [PubMed] [Google Scholar]
- 8.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 9.Ibeanu GC, Blaisdell J, Ferguson RJ, Ghanayem BI, Brosen K, Benhamou S, et al. A novel transversion in the intron 5 donor splice junction of CYP2C19 and a sequence polymorphism in exon 3 contribute to the poor metabolizer phenotype for the anticonvulsant drug S-mephenytoin. J Pharmacol Exp Ther. 1999;290:635–640. [PubMed] [Google Scholar]
- 10.Jia DM, Chen ZB, Zhang MJ, Yang WJ, Jin JL, Xia YQ, et al. CYP2C19 polymorphisms and antiplatelet effects of clopidogrel in acute ischemic stroke in China. Stroke. 2013;44:1717–1719. doi: 10.1161/STROKEAHA.113.000823. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JP, Horenstein RB, Ryan K, O’Connell JR, Gibson Q, Mitchell BD, et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genomics. 2013;23:1–8. doi: 10.1097/FPC.0b013e32835aa8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin YJ, Li JW, Zhang MJ, Qian L, Yang WJ, Zhang CL, et al. The association between CYP2C19 genotype and of in-stent restenosis among patients with vertebral artery stent treatment. CNS Neurosci Ther. 2014;20:125–130. doi: 10.1111/cns.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Cancio E, Matheus MG, Romano JG, Liebeskind DS, Prabhakaran S, Turan TN, et al. Infarct patterns, collaterals and likely causative mechanisms of stroke in symptomatic intracranial atherosclerosis. Cerebrovasc Dis. 2014;37:417–422. doi: 10.1159/000362922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 15.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paré G, Mehta SR, Yusuf S, Anand SS, Connolly SJ, Hirsh J, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 2010;363:1704–1714. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- 17.Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82:1241–1248. doi: 10.1016/j.ajhg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]