Abstract
The negotiation of social order is intimately connected to the capacity to infer and track status relationships. Despite the foundational role of status in social cognition, we know little about how the brain constructs status from social interactions that display it. Although emerging cognitive neuroscience reveals that status judgments depend on the intraparietal sulcus, a brain region that supports the comparison of targets along a quantitative continuum, we present evidence that status judgments do not necessarily reduce to ranking targets along a quantitative continuum. The process of judging status also fits a social interdependence analysis. Consistent with third-party perceivers judging status by inferring whose goals are dictating the terms of the interaction and who is subordinating their desires to whom, status judgments were associated with increased recruitment of medial pFC and STS, brain regions implicated in mental state inference.
INTRODUCTION
Status is negotiated between individuals within their social interactions, but it is also conferred by third-party observers privy to those interactions (Ridgeway & Diekema, 1989). These judgments of relative social standing, by interaction partners and observers of their interactions, are essential to the status-sorting processes that occur during social interaction and thus serve in the negotiation of social order within groups (Anderson & Kilduff, 2009a; Willer, 2009; Tyler & Blader, 2003; Berger, Cohen, & Zelditch, 1972; Strodtbeck, James, & Hawkins, 1957; Goldhamer & Shils, 1939). When people have no prior knowledge of each other’s status, they interpret a variety of verbal and nonverbal behaviors in social interaction as indicating deference and dominance (Rule, Adams, Ambady, & Freeman, 2012; Fiske, 2011; Anderson & Kilduff, 2009b; Magee, 2009; Hall, Coats, & LeBeau, 2005; Mazur, 2005; Tiedens & Fragale, 2003; Morand, 1996; Dovidio, Brown, Heltman, Ellyson, & Keating, 1988; Stiles, 1978; Bales, 1970; Goffman, 1956). Although researchers understand which cues observers use to judge individuals’ social standing, comparatively little is known about how this information is processed to render such judgments. We aim to provide some insight into the process of judging status by examining the underlying neural apparatus involved in this ubiquitous feature of social cognition.
Neural Mechanisms of Status Judgments: Two Possibilities
Previous neuroscientific investigations have considered how people perceive status and related constructs (e.g., dominance) from isolated individuals’ facial features, body postures, and displays of emotion (e.g., Muscatell et al., 2012; Marsh, Blair, Jones, Soliman, & Blair, 2009; Chiao et al., 2008; Oosterhof & Todorov, 2008). These stimuli often obscure a relational dimension of status that is considered essential outside the neuroscientific literature (for a review, see Magee & Galinsky, 2008). Status is a property of social relationships, negotiated between interacting individuals and revealed through their behavior (e.g., Tiedens, 2001; Berger et al., 1972; Bales, 1970; Blau, 1964). Within a particular social exchange, judgments of the parties’ relative standing often involve deducing whose intentions are being prioritized and pursued in the interaction. Along the same lines, third-party observers infer status by determining whose goals govern the interaction and considering who adjusts their behavior to whom in pursuit of these goals (Mascaro & Csibra, 2012). That is, social interdependence helps define status for perceivers.
Observable behavior on its own can provide only ambiguous information about whose goals and intentions have priority. To understand how the behavior reflects the status structure that produced it, we argue that observers must engage in a process of mental state inference. Thus, one might expect status inferences to recruit areas of the brain that are involved in mentalizing (Dennett, 1987) or understanding others in terms of the intentions impelling their behavior.
The neural mechanisms people engage when attempting to understand others’ actions in terms of causal mental states — intentions, beliefs, desires, and needs — are well documented. When people assign psychological reasons to others’ behaviors, they recruit a distributed network of brain regions that includes the medial pFC (mPFC), TPJ, and posterior STS (pSTS; Lieberman, 2010; Mason, Banfield, & Macrae, 2004; Frith & Frith, 1999; Gallagher & Frith, 2003; Gallagher, Happé, Brunswick, Fletcher, Frith, & Frith, 2000; for reviews, see Mitchell, 2009; Mason & Macrae, 2008; Amodio & Frith, 2006). Activity in this mentalizing or “theory of mind” (ToM) network is enhanced when people reflect on a target’s perspective (e.g., Goel, Grafman, Sadato, & Hallett, 1995), infer the sentiments one person feels toward another (Mason, Magee, Kuwabara, & Nind, 2010), attempt to understand behavior in terms of underlying goals and motivations (e.g., Brunet, Sarfati, Hardy-Baylé, & Decety, 2000; Gallagher et al., 2000), or depend on the target (Ames & Fiske, 2013), consistent with interdependence motivating dispositional inferences (Erber & Fiske, 1984).
Previous research has considered the role of the ToM network in allocentric social hierarchy judgments. Recognizing the role of the STS in inferring intention (Gallagher et al., 2000), Allison, Puce, and McCarthy (2000) have suggested that specific regions within the STS might be central to the process of determining who is dominant and who is submissive within social interactions. Likewise, Karafin, Tranel, and Adolphs (2004) hypothesized that the ventral mPFC might mediate inferences about people’s relative standing in the social world. Although Karafin et al. (2004) found that patients with ventral mPFC lesions were not impaired at judging dominance from static pictures of faces and from actors interacting in film clips, ventral mPFC patients were less discriminating in their judgments (i.e., the range of dominance ratings used by ventral mPFC patients was restricted), which may reflect their relative insensitivity to status cues. As an example supporting their argument, Karafin and colleagues describe a patient with damage to bilateral aspects of the ventral mPFC who appeared oblivious to the status hierarchy in the research team and incapable of adjusting his behavior to show more deference to higher-ranking members (see Karafin et al., 2004, p. 1797).
Combining this prior theorizing about the role of ToM brain regions in social hierarchy judgments with our notion that relative status can be interpreted through a process of inference about the intentions of interacting parties, we would expect status inferences that are based on an observed social exchange to be associated with enhanced activity in ToM brain regions, at least in situations where perceivers lack a priori knowledge about how the targets rank within a formal status hierarchy.
Another possibility is that status inferences can be reduced to an example of ranking targets — in this case, from low to high status. According to this view, status judgments are akin to quantity judgments and therefore depend on brain areas that support the comparison of targets along a quantitative continuum. This perspective is based primarily on evidence of the involvement of the intraparietal sulcus (IPS) in judgments of status (Chiao et al., 2009; see also Chiao, 2010). Across many studies employing a diversity of paradigms, researchers have demonstrated that bilateral aspects of the IPS are recruited when participants compare the magnitude of two or more quantities (e.g., Fias, Lammertyn, Reynvoet, Dupont, & Orban, 2003; Fulbright, Manson, Skudlarski, Lacadie, & Gore, 2003; Pinel, Dehaene, Riviere, & LeBihan, 2001; Chochon, Cohen, Moortele, & Dehaene, 1999; Faillenot, Decety, & Jeannerod, 1999; Dehaene, 1996). This body of evidence has led researchers to theorize that the IPS plays a crucial role in representing quantities spatially along a “mental number line” and that this region is engaged when people access and compare the location of items arranged on it (Dehaene, 2011; Dehaene, Dehaene-Lambertz, & Cohen, 1998).
Like comparisons of quantities, some relational properties of the social world can also be represented in terms of distance. For example, familiarity with a social target can be conceptualized as distance from the self (i.e., social distance). Parkinson and colleagues have pointed at that judgments of social distance activate regions of the right inferior parietal lobule similar to the regions activated by spatial distance judgments (Parkinson & Wheatley, 2013; Parkinson et al., submitted). Chiao (2010) similarly has argued for the notion that status judgments are akin to numeric distance judgments.
Consistent with the view that status is represented spatially along a mental number line that people access when judging social rank, Chiao et al. (2009) found that Naval Academy participants recruited the right IPS both when they compared the relative status of target individuals (e.g., “Is the target lower or higher in rank than a captain?”) and when they compared the relative magnitude of numbers (e.g., “Is the target number smaller or larger than 65?”). Cloutier, Ambady, Meagher, and Gabrieli (2012) also found greater activation in the right IPS when participants formed an impression of targets associated with low-status information, but only when this information was numerical (earning low income) and not when it was moral (working in a stigmatized occupation).
Furthermore, both Chiao et al. (2009) and Chiao, Bordeaux, and Ambady (2004) reported another property of status judgments that parallels judgments about numbers: Participants took longer to compare ranks that were close in status (e.g., Assistant Professor and Associate Professor) than ranks that were far apart in status (e.g., Graduate Student and Associate Professor). Chiao and colleagues explain these results in terms of the “numerical distance effect” whereby people take longer to compare numbers that are closer in quantity (e.g., 98 and 99) than numbers that are further apart in quantity (e.g., 11 and 99). Their results suggest knowledge of both social hierarchy and numbers is represented in a symbolic manner and that a critical feature of this symbolic representation is the property of numerical distance.
The existing neuroscience evidence might lead one to expect that status judgments as they occur in the real world depend on IPS mechanisms; however, the IPS may not necessarily always be involved. In some cases, status judgments can be based on the contents of declarative memory—for example, when one has prior experience with other people in their social roles. This kind of conceptual status judgment, however, best characterizes formal status hierarchies about which third-party observers have preexisting knowledge, such as the Navy hierarchy in Chiao and colleagues’ research (Chiao et al., 2004, 2009; see also Farrow et al., 2011). Many status judgments occur in novel contexts or in situations with ambiguous information about individuals’ social standing. The extent to which the IPS is involved in status inferences about targets embedded in these unfamiliar or informal status hierarchies is unclear, and the comparative operations of the IPS might seem to be useful to third-party observers, only insofar as target individuals occupy a ranked position already stored in observers’ memory and accessible in making their judgments.
Furthermore, the relational and contextual nature of status in most relationships implies that individuals’ relative status depends critically on the particular situation within which individuals are interacting. Whereas numbers have a fixed meaning in relation to each other, a target’s status varies from one interpersonal context to the next. Thus, the framework emphasizing the role of the IPS seems to apply most in situations in which the observer has a priori knowledge about each target’s rank within an established, formalized status hierarchy (i.e., when the judgment draws on episodic or semantic memory).
In summary, one possibility is that judgments of status require the same neural apparatus involved in comparative judgments of targets that can be arranged along a quantitative continuum. According to this logic, people represent status positions along a continuum, which they reference to make judgments of social targets’ relative status. This characterization of status judgments implies involvement of the IPS. Another possibility, and the one we favor for the context presented here, is that status judgments can involve inferring individuals’ intentions to determine whose goals dictate the terms of the interaction and who is subordinating their desires to whom. According to this reasoning, status judgments, as they typically occur in everyday life, can involve inferences about mental states based on observable social interaction. This characterization of status judgments implies the involvement of regions that support ToM reasoning—mPFC, pSTS, and TPJ.
Overview
To test our hypothesis that observers of interacting social partners rely on ToM mechanisms to render status judgments, we employed a different experimental strategy than has been used in previous research on the neural underpinnings of status judgments. Three features in the experimental design distinguish our study from prior investigations.
First, we had participants judge either targets’ relative social status or relative physical weight. Whereas we expected status judgments to involve mentalistic representations of the targets, we selected weight as a comparison judgment because it requires no such mentalistic representation. Instead, weight is quantifiable and thus should depend on computations supported by the IPS.
Second, the stimuli depicted pairs of people engaged in the types of social interactions that reveal information about targets’ relative social standing (e.g., interactions in which one individual’s intentions govern both individuals’ behavior). Whereas previous neuroscientific investigations of status have measured neural activity while people judged isolated social targets (e.g., Cloutier et al., 2012; Muscatell et al., 2012; Farrow et al., 2011; Lindner, Hundhammer, Ciaramidaro, Linden, & Mussweiler, 2008), we measured neural activity while people judged status from social interactions where it was on display.
Third, the target individuals were unfamiliar to participants, unlike the celebrities in Farrow et al. (2011) and Lindner et al. (2008) or the naval ranks in Chiao et al. (2004). Using novel targets allowed us to determine how people infer status in the absence of prior knowledge about the targets.
METHODS
Participants (n = 19; 58% female; mean age = 21.7 years; 9 white, 3 Hispanic, 6 Asian, 1 black) completed the experiment for monetary compensation. All participants were strongly right-handed as measured by the Edinburgh handedness inventory (Raczkowski, Kalat, & Nebes, 1974), reported no significant abnormal neurological history, and had normal or corrected-to-normal visual acuity.
Stimulus Materials
We used stock photographs and Adobe Photoshop to create grayscale images of social interactions involving two people. The images were created in pairs. For each pair of images, a single individual (B) appeared in both images alongside different individuals (A and C). Person B was the heavier individual in one image and the lighter in the other image; similarly, B was in a high-status position in one image and a low-status position in the other (Figure 1).1
Figure 1.
Example stimuli.
For each image, two research assistants agreed on which individual was heavier and which was of higher status. These images were then pilot tested in the laboratory with 38 participants (47.6% female; mean age = 23 years) in exchange for monetary compensation. Participants were asked to judge which individual was of greater weight and which was of greater status in each image. Images for which three or more participants’ judgments did not match the research assistants’ judgments were excluded from the stimulus set.
Additional testing of our stimuli with a sample of 76 participants recruited through Amazon’s Mechanical Turk (39.5% female; mean age = 33.5 years) confirmed that participants relied on the apparent behavior in the interaction to render their status judgments and deemed a host of static characteristics of the interacting individuals (e.g., their age, gender, race/ethnicity, facial features, emotional expressions) irrelevant to their judgments.2 Finally, it is worth noting that the pictures were constructed such that there was no systematic relationship between weight and status. Forty-four percent of stimuli depicted the lighter person in the high status role; the remaining 56% of images depicted the heavier person in the high status role.
Procedure
The experimenter explained that the study was designed to investigate how people make a variety of person-related judgments. Participants were informed that they would see an image showing two figures on the screen and that their task was to indicate, via a key press, either which individual had higher status (status judgment) or which weighed more (weight judgment).
A trial consisted of the following sequence of events. The instruction either “weighs more” or “higher status” appeared at the center of the screen with the stimulus image. The image remained on the screen for 2500 msec. Participants registered their responses by pressing the left key to indicate “person on the left” and the right key to indicate “person on the right.”
Imaging
Participants were scanned in two event-related functional (EPI) runs. A total of 235 volumes were collected in each EPI run. Across the runs, participants completed 60 of each trial type for a total of 120 trials. Each trial lasted 1.5 repetition times (TR) or 3 sec in duration. The remaining EPI volumes were jittered catch trials (i.e., fixation symbols, “+”) used to optimize estimation of the event-related BOLD response. The stimuli were presented using Presentation (version 12.1) (Neurobehavioral Systems, Inc., Albany, CA) and back projected with an LCD projector onto a screen at the end of the magnet bore that participants viewed by way of a mirror mounted on the head coil. Pillow and foam cushions were placed within the head coil to minimize head movements. All images were collected using a GE scanner with standard eight-channel head coil. T1-weighted anatomical images were collected using a 3-D sequence (SPGR; 180 axial slices, TR = 19 msec, echo time = 5 msec, flip angle = 20°, field of view = 25.6 cm, slice thickness = 1 mm, matrix = 256 × 256). Functional images were collected with a gradient-echo EPI sequence (each volume comprised 27 slices; 4 mm thick, 0 mm skip; TR = 2000 msec, echo time = 35 msec, field of view = 19.2 cm, 64 × 64 matrix; 84° flip angle).
fMRI Analysis
fMRI data were analyzed using Statistical Parametric Mapping software (SPM8, Welcome Department of Cognitive Neurology, London, UK; Friston et al., 1995). For each functional run, data were preprocessed to remove sources of noise and artifact. Preprocessing included slice timing and motion correction, coregistration to each participant’s anatomical data, normalization to the ICBM 152 brain template (Montreal Neurological Institute), and spatial smoothing with an 8-mm (FWHM) Gaussian kernel. Analyses took place at two levels: formation of statistical images and regional analysis of hemodynamic responses. For each participant, a general linear model was specified. For each run, the model included regressors specifying the two conditions of interest (modeled with functions for the hemodynamic response and a temporal derivative), six motion-related regressors, a regressor for each of the first six brain volumes collected, and a regressor constant term that SPM automatically generates and includes in the model.
The general linear model was used to compute parameter estimates (β) and t contrast images for each comparison at each voxel. These individual contrast images were then submitted to a second-level, random-effects analysis to obtain mean t images. We applied a voxel-level threshold of p < .001, k = 10 and then corrected for multiple comparisons using a false discovery rate (FDR) corrected threshold of p = .05, applied at the cluster level (Chumbley, Worsley, Flandin, & Friston, 2010; Chumbley & Friston, 2009; Genovese, Lazar, & Nichols, 2002).
RESULTS
Behavioral
There was no difference in the speed with which participants made status (mean RT = 1694 msec, SD = 154 msec) and weight judgments (mean RT = 1680 msec, SD = 194 msec), t(18) = 0.48, p = .640. Accuracy across the two conditions was also similar; participants were accurate on 81.24% (SD = 10.04) of the weight trials and 77.17% (SD = 9.07) of the status trials, t(18) = 1.49, p = .154. These results suggest that the two types of judgments were not different in terms of difficulty, which attenuates a potential concern that any BOLD differences could be attributed to differences in the extent to which the tasks were cognitively demanding.
fMRI
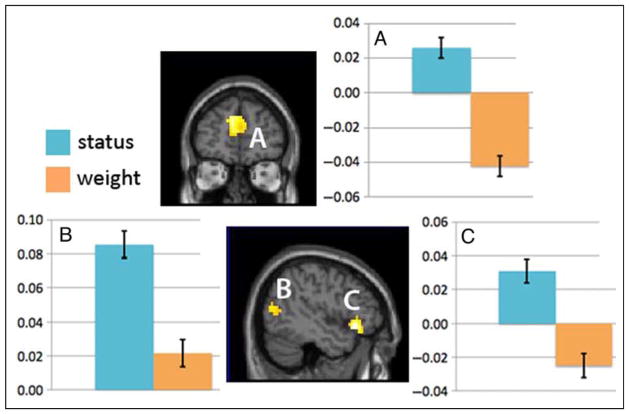
Consistent with the argument that observers of interacting social partners rely on ToM mechanisms to render status judgments and thus recruit brain regions that support mental state inference, bilateral aspects of the mPFC (BA 9/10; −6, 52, 18; k = 176) and a region of the left pSTS (BA 39; −53, −56, 8; k = 58) were significantly more active during status judgments than weight judgments (see Table 1 and Figure 2). A region of the right pSTS (BA 22; 48, −46, 10; k = 41) was also more active during status judgments (at a threshold of p < .001, k = 10, uncorrected). However, after applying a cluster-level FDR correction, the p value was marginally significant, p = .068. Status judgments were also associated with greater recruitment of the left ventrolateral pFCs (VLPFC; BA 47/11; −45, 22, −10; k = 67), a brain area that supports inhibition and cognitive control.
Table 1.
Peak coordinates of brain regions where there was greater activity during status relative to weight judgments, p < .001, k = 10, uncorrected (peak level) with a cluster level, FDR correction of p < .05 (k = 58)
| Cluster | BA | Region | Coordinates
|
Peak
|
Cluster
|
|||
|---|---|---|---|---|---|---|---|---|
| x | y | z | t | k | p | |||
| 1 | 47 | L. inferior frontal gyrus | −45 | 22 | −10 | 6.58 | 67 | .028 |
| 47 | L. inferior frontal gyrus | −39 | 30 | −13 | 5.51 | |||
| 2 | 9 | L. medial prefrontal | −6 | 52 | 18 | 6.05 | 176 | <.001 |
| 9 | R. medial prefrontal | 6 | 52 | 18 | 4.86 | |||
| 3 | 37 | L. middle temporal gyrus | −50 | −65 | 9 | 5.14 | 58 | .031 |
| 39 | L. superior temporal gyrus | −53 | −56 | 8 | 4.34 | |||
| 4a | 22 | R. superior temporal gyrus | 48 | −46 | 10 | 4.58 | 41 | .068 |
| 21 | R. middle temporal gyrus | 58 | −53 | 6 | 4.19 | |||
L. = left; R. = right; B. = bilateral; BA = Broadmann’s area.
An area that was significant at an FDR correction threshold of p < .07
Figure 2.
Regions that emerge from the direct comparison status judgment > weight judgment at a threshold of p < .05, corrected. (A) A bilateral cluster in the mPFC (−6, 52, 18). (B) A cluster that extends across the left middle and pSTS (−50, −65, 9). (C) A cluster in the VLPFC (−45, 22, −10). For each participant, the SPM ROI toolbox was used to estimate average signal change for both conditions (status judgment, weight judgment) at these 10 mm ROIs. Signal changes in ROIs were then averaged across participants, and mean signal was plotted. Error bars denote mean standard error exempted of between-subject differences (Cousineau, 2005).
A cluster in the right IPL (BA 40; 51, −31, 40; k = 21), a cortical area that we have discussed as supporting comparison of targets along a quantitative continuum, was significantly more active while participants made weight relative to status judgments (at a threshold of p < .001, k = 10, uncorrected); however, it did not survive the cluster-level FDR correction we applied to deal with issues of multiplicity. No other brain areas exhibited significantly greater activity while participants made weight judgments relative to status judgments at either the uncorrected or FDR-corrected threshold.
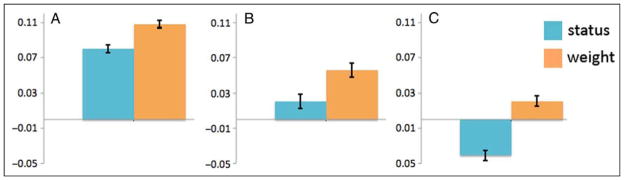
To further explore the possibility that status judgments depend on mechanisms supported by the IPS, we conducted supplementary ROI analyses. ROIs were defined using the two areas defined as the horizontal segments of the IPS by Dehaene, Piazza, Pinel, and Cohen’s (2003) meta-analysis and the aspect of the IPL that emerged from the direct comparison weight judgment > status judgment, p < .001, k = 10, uncorrected. All voxels within 10 mm of the peak were included in each ROI. For each participant, the SPM ROI toolbox (Brett, Anton, Valabregue, & Poline, 2002) was used to estimate average signal change for both conditions (status judgment, weight judgment) at these 3-D ROIs. Signal changes in ROIs were then averaged across participants, and mean signal was plotted (see Figure 3).
Figure 3.
Supplementary ROI analyses. (A and B) ROIs that were built based on Dehaene et al.’s (2003, p. 491) identification of the bilateral horizontal segments of the IPS involved in number processing. (A) The left IPS (−44, −48, 47) and (B) the right IPS (41, −47, 48). (C) The aspect of the IPL (51, −31, 40) that emerged from the direct comparison of weight judgment > status judgment, p < .001, uncorrected. For each participant, the SPM ROI toolbox was used to estimate average signal change for both conditions (status judgment, weight judgment) at these 10 mm ROIs. Signal changes in ROIs were then averaged across participants, and mean signal was plotted. Error bars denote mean standard error exempted of between-subject differences (Cousineau, 2005).
These supplementary ROI analyses revealed greater activity in the left IPS (−44, −48, 47) compared with rest both when participants were making weight judgments, t(18) = 5.01, p < .001, and when they were making status judgments, t(18) = 3.59, p = .002. Importantly, this region was significantly less active during status comparisons than it was during weight comparisons, t(18) = −3.15, p = .006. In contrast to the left IPS, the right IPS (41, −47, 48) exhibited increased activity (compared with rest) only when participants were making weight judgments, t(18) = 1.87, p = .078. As with the left IPS, the right IPS was significantly less active during status compared with weight judgments, t(18) = −2.30, p = .034. Finally, the ROI centered on the peak of the IPL cluster (51, −34, 42) that emerged from the weight > status contrast at a p < .001, k = 10, uncorrected threshold was significantly less active during status compared with weight judgments, t(18) = −5.31, p < .001. This region was not significantly more active during weight compared with rest, t(18) = .81, p = .428, but was marginally less active during status compared with rest, t(18) = −1.74, p = .099.
DISCUSSION
Our results provide evidence that both neural mechanisms— the IPS and ToM regions—underpin many status judgments. Lending support for our hypothesis that inferences of status from social interactions involves construing targets in terms of mental states, mPFC and pSTS were recruited more when observers made status judgments than weight judgments. Consistent with research by Chiao and colleagues (2004, 2009), results of our supplementary ROI analyses revealed that the IPS was active during both status and weight judgments, suggesting that the two types of judgments are not entirely distinct. These results present some important points of convergence with previous findings, but also nuance our understanding of how the brain renders status inferences.
The first point of convergence is with Chiao et al. (2009), as our results lend some tentative support to their theorized role of the IPS in status inferences. On the basis of the similar pattern of activity in the left IPS during status judgments and weight judgments, we found that aspects of status judgments overlap with judgments of quantifiable properties. One possibility is that the activity observed in the left IPS during both judgments reflects its general involvement in visual spatial processing rather than in making judgments of status and weight (cf., Colby & Goldberg, 1999); however, given that the visual information was held constant across the two conditions, the greater activity during weight judgments in all three intraparietal regions of the IPS examined here (see Figure 3) implies greater IPS involvement in comparing social targets along a physical dimension that is quantifiable (weight) versus a social one (status).
Although status judgments are associated with enhanced IPS activity, weight judgments seem to involve the IPS more extensively. This difference in brain processing seems due to a difference in mental representation of the two dimensions, status and weight. Whereas weight and many other quantifiable properties are invariant across situations, status is situationally and relationally determined and thus difficult to represent on a static mental number line. Several researchers have reported evidence consistent with this interpretation, finding no activation of the IPS when participants compare targets along nonnumerical scales (e.g., the relative ferocity of two animals; Lindner et al., 2008; Thioux, Pesenti, De Voider, & Seron, 2002; Le Clec’H et al., 2000; Pesenti, Thioux, Seron, & De Voider, 2000).
Although important, differences in IPS activity within our paradigm were subtle compared with the differences we found in recruitment of the ToM network for status judgments relative to weight judgments. Greater activity in mPFC and pSTS for status judgments lends further support to the notion that judging status from social interactions where it is on display is qualitatively distinct from judging status by referencing information that is represented on a mental number line.
In some previous research, a methodological feature might have prevented this result from materializing. In Chiao et al.’s (2009) research, for example, status was represented as an abstraction (e.g., through military symbols), divorced from the social interactions in which status can be negotiated and from which status judgments can be made. We recognize that status orderings sometimes can be inferred from abstract symbols or by observable characteristics that require no mental inference (e.g., age, race, gender). However, we contend that an analysis of social interdependence, involving inferences about parties’ mental states, often is required to derive a previously unknown status ordering. This process has received too little attention in research on the neuroscience of social hierarchy.
The perspective we adopt here is deeply informed by research on status in sociology and social psychology. Although we believe this marks an important step in a direction not previously explored by social neuroscientists, our approach has limitations. In particular, key features of our experimental design (e.g., images depicting multiple versus isolated individuals) preclude strengthening our case with supplementary brain–behavior analyses. Future research might look for ways to incorporate these into the study methodology.
Other researchers (e.g., Zink et al., 2008) have found mPFC activity during status judgments, but these results have been ambiguous as to whether participants were employing ToM regions in their judgments or engaging in self-referential processing (both of which involve mPFC). Unlike our method, which explicitly called on participants as third-party observers to make judgments about the status of two interacting individuals, these previous studies embedded participants within a hierarchy and asked them to make egocentric status judgments— judgments of others’ status relative to the self. pFC appears to play a crucial role in tasks that involve the assessment of relevance and value to the self (Schmitz & Johnson, 2007). Thus, the egocentric nature of the judgment, rather than the judgment of status per se, could have been responsible for mPFC activity in prior research. Further complicating interpretation of studies using egocentric status judgments, Muscatell et al. (2012) found that, when assessing their own standing in a social hierarchy, low-status individuals show greater activity in ToM regions, including the mPFC.
Our research paradigm clarifies the conflation of status judgments with self-referential processing by entirely removing the self from the status judgment. By making the judgments allocentric, we can infer that mPFC is recruited for status judgments even when those judgments are not self-relevant. Future research could ask participants to make both egocentric and allocentric status judgments to compare directly how much the mPFC activity is due to referencing the self versus inferring status (see Cloutier et al., 2012).
A study comparing egocentric and allocentric status judgments could also help resolve another open issue about the role of dorsolateral pFC (DLPFC) in the processing of social hierarchy. Although our results did not implicate DLPFC in status judgments, Zink and colleagues (2008) found that egocentric processing of higher-status individuals recruits DLPFC. However, we share the concerns expressed by Marsh et al. (2009) about how to interpret Zink et al.’s result. Fiske (1993) has argued that individuals direct more attentional resources toward high-status than to low-status individuals because high-status individuals have disproportionate control over their fate (see also Shepherd, Deaner, & Platt, 2006; Deaner, Khera, & Platt, 2005), and DLPFC is an important neural component of attentional control (Miller & Cohen, 2001; Desimone & Duncan, 1995). Thus, DLPFC activity might be observed in low-status individuals gazing “up” the hierarchy because of the attentional demands of the task, not because DLPFC is inherently involved in reasoning about status. Visual processing and reasoning about high-status individuals outside one’s own hierarchy would not require the same attentional control because those individuals’ status has no relevance or value to the self. This suggests that upward allocentric status judgments would not recruit DLPFC to the same extent as upward egocentric status judgments. Future research could be designed to resolve this issue.
CONCLUSION
We found that judgments of individuals’ relative status and weight require partially similar neural apparatuses. Consistent with prior research, both judgments involved the IPS, although weight judgments recruited the IPS more extensively. In addition to activity in the IPS region, noted for its role in quantitative comparisons, status judgments also involved mPFC and pSTS, which are implicated in understanding others’ goals and intentions. Although it is tempting to equate reasoning about all dimensions along which individuals can be rank ordered, as with status and weight, our findings illustrate that judgments about these dimensions are not necessarily equivalent. Some judgments are more social interactional than others, and the recruitment of a region of the ToM network suggests that making inferences of others’ social status from their social interactions is an inherently social process.
Footnotes
Each image depicted two women or two men interacting, never cross-gender interactions.
For each image, participants indicated the extent to which the following information about the people depicted in the photograph influenced their answer about which person has higher status (i.e., how relevant each of the following were in forming their impression), on a scale ranging from 1 = completely irrelevant to 5 = completely relevant: (i) their age, (ii) their race or ethnicity, (iii) the features of their faces, (iv) their apparent behavior, (v) what they appear to be doing, (vi) their emotional expressions, (vii) the actions they appear to be taking, (viii) their physical postures, and (ix) their clothing. As expected, participants indicated that they arrived at an understanding of the targets’ relative social standing based on the actions undertaken by the interaction partners (items v, vi, and viii). By contrast, they deemed the targets’ ages, races/ethnicities, features of their faces, and emotional expressions irrelevant to their status judgments. It is worth noting that participants indicated their status inferences were also influenced by (viii) the postures of the interacting parties and, to a lesser extent, by (ix) their clothing.
Contributor Information
Malia Mason, Columbia University, NY, USA.
Joe C. Magee, New York University, NY, USA
Susan T. Fiske, Princeton University, NJ, USA
References
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Ames DL, Fiske ST. Intentional harms are worse, even when they’re not. Psychological Science. 2013;24:1755–1762. doi: 10.1177/0956797613480507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of the minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson C, Kilduff GJ. The pursuit of status in social groups. Current Directions in Psychological Science. 2009a;18:295–298. [Google Scholar]
- Anderson C, Kilduff GJ. Why do dominant personalities attain influence in face-to-face groups? The competence-signaling effects of trait dominance. Journal of Personality and Social Psychology. 2009b;96:491–503. doi: 10.1037/a0014201. [DOI] [PubMed] [Google Scholar]
- Bales RF. Personality and interpersonal behavior. New York: Holt, Rinehart, & Winston; 1970. [Google Scholar]
- Berger J, Cohen BP, Zelditch M. Status characteristics and social interaction. American Sociological Review. 1972;37:241–255. [Google Scholar]
- Blau PM. Exchange and power in social life. New York: Wiley; 1964. [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. 2002. Available on CD-ROM in NeuroImage. [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Baylé MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11:157–166. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Chiao JY. Neural basis of social status hierarchy across species. Current Opinion in Neurobiology. 2010;20:803–809. doi: 10.1016/j.conb.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Adams RB, Jr, Tse PU, Lowenthal WT, Richeson JA, Ambady N. Knowing who’s boss: fMRI and ERP investigations of social dominance perception. Group Processes and Intergroup Relations. 2008;11:201–214. doi: 10.1177/1368430207088038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Bordeaux AR, Ambady N. Mental representations of social status. Cognition. 2004;93:B49–B57. doi: 10.1016/j.cognition.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Oby ER, Li Z, Parrish T, Bridge DJ. Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia. 2009;47:354–363. doi: 10.1016/j.neuropsychologia.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Chochon F, Cohen L, Moortele PVD, Dehaene S. Differential contributions of the left and right inferior parietal lobules to number processing. Journal of Cognitive Neuroscience. 1999;11:617–630. doi: 10.1162/089892999563689. [DOI] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. Neuroimage. 2010;49:3057–3064. doi: 10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J, Ambady N, Meagher T, Gabrieli JDE. The neutral substrates of person perception: Spontaneous use of financial and moral status knowledge. Neuropsychologia. 2012;50:2371–2376. doi: 10.1016/j.neuropsychologia.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Colby CE, Goldberg ME. Space and attention in parietal cortex. Annual Review of Neuroscience. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology. 2005;1:4–45. [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Current Biology. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Dehaene S. The organization of brain activations in number comparison: Event-related potentials and the additive-factors methods. Journal of Cognitive Neuroscience. 1996;8:47–68. doi: 10.1162/jocn.1996.8.1.47. [DOI] [PubMed] [Google Scholar]
- Dehaene S. The number sense: How the mind creates mathematics: How the mind creates mathematics. New York: Oxford University; 2011. [Google Scholar]
- Dehaene S, Dehaene-Lambertz G, Cohen L. Abstract representations of numbers in the animal and human brain. Trends in Neurosciences. 1998;21:355–361. doi: 10.1016/s0166-2236(98)01263-6. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Dennett DC. The intentional stance. Cambridge, MA: MIT Press; 1987. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dovidio JF, Brown CE, Heltman K, Ellyson SL, Keating CF. Power displays between women and men in discussions of gender-linked tasks: A multichannel study. Journal of Personality and Social Psychology. 1988;55:580–587. doi: 10.1037//0022-3514.54.2.233. [DOI] [PubMed] [Google Scholar]
- Erber R, Fiske ST. Outcome dependency and attention to inconsistent information. Journal of Personality and Social Psychology. 1984;47:709–726. [Google Scholar]
- Faillenot I, Decety J, Jeannerod M. Human brain activity related to the perception of spatial features of objects. Neuroimage. 1999;10:114. doi: 10.1006/nimg.1999.0449. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Jones SC, Kaylor-Hughes CJ, Wilkinson ID, Woodruff PW, Hunter MD, et al. Higher or lower? The functional anatomy of perceived allocentric social hierarchies. Neuroimage. 2011;57:1552–1560. doi: 10.1016/j.neuroimage.2011.05.069. [DOI] [PubMed] [Google Scholar]
- Fias W, Lammertyn J, Reynvoet B, Dupont P, Orban GA. Parietal representation of symbolic and nonsymbolic magnitude. Journal of Cognitive Neuroscience. 2003;15:47–56. doi: 10.1162/089892903321107819. [DOI] [PubMed] [Google Scholar]
- Fiske ST. Controlling other people: The impact of power on stereotyping. American Psychologist. 1993;48:621–628. doi: 10.1037//0003-066x.48.6.621. [DOI] [PubMed] [Google Scholar]
- Fiske ST. Envy up, scorn down: How status divides us. New York: Russell Sage Foundation; 2011. [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SCR, Frackowiak RS, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds-A biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Manson SC, Skudlarski P, Lacadie CM, Gore JC. Quantity determination and the distance effect with letters, numbers, and shapes: A functional MR imaging study of number processing. American Journal of Neuroradiology. 2003;24:193–200. [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher P, Frith U, Frith CD. Reading the mind in cartoons and stories: An fMRI study of “theory of mind” in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J, Sadato N, Hallett M. Modeling other minds. NeuroReport-International Journal for Rapid Communications of Research in Neuroscience. 1995;6:1741–1746. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Goffman E. The nature of deference and demeanor. American Anthropologist. 1956;58:473–502. [Google Scholar]
- Goldhamer H, Shils EA. Types of power and status. American Journal of Sociology. 1939;45:171–182. [Google Scholar]
- Hall JA, Coats EJ, LeBeau LS. Nonverbal behavior and the vertical dimension of social relations: A meta-analysis. Psychological Bulletin. 2005;131:898–924. doi: 10.1037/0033-2909.131.6.898. [DOI] [PubMed] [Google Scholar]
- Karafin MS, Tranel D, Adolphs R. Dominance attributions following damage to the ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2004;16:1796–1804. doi: 10.1162/0898929042947856. [DOI] [PubMed] [Google Scholar]
- Le Clec’H G, Dehaene S, Cohen L, Mehler J, Dupoux E, Poline JB, et al. Distinct cortical areas for names of numbers and body parts independent of language and input modality. Neuroimage. 2000;12:381–391. doi: 10.1006/nimg.2000.0627. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of social psychology. 5. New York: McGraw-Hill; 2010. pp. 143–193. [Google Scholar]
- Lindner M, Hundhammer T, Ciaramidaro A, Linden DE, Mussweiler T. The neural substrates of person comparison-An fMRI study. Neuroimage. 2008;40:963–971. doi: 10.1016/j.neuroimage.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Magee JC. Seeing power in action: The roles of deliberation, implementation, and action in inferences of power. Journal of Experimental Social Psychology. 2009;45:1–14. [Google Scholar]
- Magee JC, Galinsky AD. Social hierarchy: The self-reinforcing nature of power and status. Academy of Management Annals. 2008;2:351–398. [Google Scholar]
- Marsh AA, Blair KS, Jones MM, Soliman N, Blair RJR. Dominance and submission: The ventrolateral prefrontal cortex and responses to status cues. Journal of Cognitive Neuroscience. 2009;21:713–724. doi: 10.1162/jocn.2009.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaro O, Csibra G. Representation of stable social dominance relations by human infants. Proceedings of the National Academy of Sciences. 2012;109:6862–6867. doi: 10.1073/pnas.1113194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Banfield JF, Macrae CN. Thinking about actions: The neural substrates of person knowledge. Cerebral Cortex. 2004;14:209–214. doi: 10.1093/cercor/bhg120. [DOI] [PubMed] [Google Scholar]
- Mason MF, Macrae CN. Perspective-taking from a social neuroscience standpoint. Group Processes & Intergroup Relations. 2008;11:215–232. [Google Scholar]
- Mason MF, Magee JC, Kuwabara K, Nind L. Specialization in relational reasoning: The efficiency, accuracy, and neural substrates of social versus nonsocial inferences. Social Psychological and Personality Science. 2010;1:318–326. [Google Scholar]
- Mazur A. Biosociology of dominance and deference. Lanham, MD: Rowman & Littlefield; 2005. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Philosophical Transactions of the Royal Society Biological Sciences. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand DA. Dominance, deference, and egalitarianism in organizational interaction: A sociolinguistic analysis of power and politeness. Organization Science. 1996;7:544–556. [Google Scholar]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, et al. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60:1771–1777. doi: 10.1016/j.neuroimage.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhof NN, Todorov A. The functional basis of face evaluation. Proceedings for the National Academy of Sciences. 2008;32:11087–11092. doi: 10.1073/pnas.0805664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson C, Liu S, Wheatley T. A common cortical metric for spatial, temporal and social distance. doi: 10.1523/JNEUROSCI.2159-13.2014. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson C, Wheatley T. Old cortex, new contexts: Repurposing spatial perception for social cognition. Frontiers in Human Neuroscience. 2013;7:645. doi: 10.3389/fnhum.2013.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesenti M, Thioux M, Seron X, De Voider A. Neuroanatomical substrates of arable number processing, numerical comparison, and simple addition: A PET study. Journal of Cognitive Neuroscience. 2000;12:461–479. doi: 10.1162/089892900562273. [DOI] [PubMed] [Google Scholar]
- Pinel P, Dehaene S, Riviere D, LeBihan D. Modulation of parietal activation by semantic distance in a number comparison task. Neuroimage. 2001;14:1013–1026. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Ridgeway C, Diekema D. Dominance and collective hierarchy formation in male and female task groups. American Sociological Review. 1989;54:79–93. [Google Scholar]
- Rule NO, Adams RB, Jr, Ambady N, Freeman JB. Perceptions of dominance following glimpses of faces and bodies. Perception. 2012;41:687–706. doi: 10.1068/p7023. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience & Biobehavioral Reviews. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Trends in Cognitive Science. 2006;4:138–147. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Stiles WB. Verbal response modes and dimensions of interpersonal roles: A method of discourse analysis. Journal of Personality and Social Psychology. 1978;36:693–703. [Google Scholar]
- Strodtbeck FL, James RM, Hawkins C. Social status in jury deliberations. American Sociological Review. 1957;22:713–719. [Google Scholar]
- Thioux M, Pesenti M, De Voider A, Seron X. Category-specific representation and processing of numbers and animal names across semantic tasks: A PET study. Neuroimage. 2002;13:S617. [Google Scholar]
- Tiedens LZ. Anger and advancement versus sadness and subjugation: The effect of negative emotion expressions on social status conferral. Journal of Personality and Social Psychology. 2001;80:86–94. [PubMed] [Google Scholar]
- Tiedens LZ, Fragale AR. Power moves: Complementarity in dominant and submissive nonverbal behavior. Journal of Personality and Social Psychology. 2003;84:558–568. doi: 10.1037//0022-3514.84.3.558. [DOI] [PubMed] [Google Scholar]
- Tyler TR, Blader SL. The group engagement model: Procedural justice, social identity, and cooperative behavior. Personality and Social Psychology Review. 2003;7:349–361. doi: 10.1207/S15327957PSPR0704_07. [DOI] [PubMed] [Google Scholar]
- Willer R. Groups reward individual sacrifice: The status solution to the collective action problem. American Sociological Review. 2009;74:23–43. [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: Neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]